Abstract
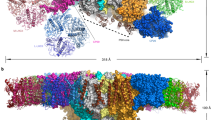
Chlorophyll biosynthesis, crucial to life on Earth, is tightly regulated because its precursors are phototoxic1. In flowering plants, the enzyme light-dependent protochlorophyllide oxidoreductase (LPOR) captures photons to catalyse the penultimate reaction: the reduction of a double bond within protochlorophyllide (Pchlide) to generate chlorophyllide (Chlide)2,3. In darkness, LPOR oligomerizes to facilitate photon energy transfer and catalysis4,5. However, the complete three-dimensional structure of LPOR, the higher-order architecture of LPOR oligomers and the implications of these self-assembled states for catalysis, including how LPOR positions Pchlide and the co-factor NADPH, remain unknown. Here, we report the atomic structure of LPOR assemblies by electron cryo-microscopy. LPOR polymerizes with its substrates into helical filaments around constricted lipid bilayer tubes. Portions of LPOR and Pchlide insert into the outer membrane leaflet, targeting the product, Chlide, to the membrane for the final reaction site of chlorophyll biosynthesis. In addition to its crucial photocatalytic role, we show that in darkness LPOR filaments directly shape membranes into high-curvature tubules with the spectral properties of the prolamellar body, whose light-triggered disassembly provides lipids for thylakoid assembly. Moreover, our structure of the catalytic site challenges previously proposed reaction mechanisms6. Together, our results reveal a new and unexpected synergy between photosynthetic membrane biogenesis and chlorophyll synthesis in plants, orchestrated by LPOR.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The atomic model and cryo-EM maps were deposited with PDB (no. 7JK9) and EMDB (no. EMD-22364). In this study the following additional data were used: LPOR structures from T. elongatus (PDB 6L1H) and Synechocystis sp. (PDB 6R48 or 6L1G); LPOR sequence database (Supplementary Data 1; https://doi.org/10.1042/BCJ20200323).
References
Schlicke, H. et al. Function of tetrapyrroles, regulation of tetrapyrrole metabolism and methods for analyses of tetrapyrroles. Procedia Chem. 14, 171–175 (2015).
Heyes, D. J. et al. The first catalytic step of the light-driven enzyme protochlorophyllide oxidoreductase proceeds via a charge transfer complex. J. Biol. Chem. 281, 26847–26853 (2006).
Gabruk, M. & Mysliwa-Kurdziel, B. J. Light-dependent protochlorophyllide oxidoreductase: phylogeny, regulation and catalytic properties. Biochemistry 54, 5255–5262 (2015).
Solymosi, K. & Schoefs, B. Etioplast and etio-chloroplast formation under natural conditions: the dark side of chlorophyll biosynthesis in angiosperms. Photosynth. Res. 105, 143–166 (2010).
Gabruk, M., Myśliwa-Kurdziel, B. & Kruk, J. MGDG, PG and SQDG regulate the activity of light-dependent protochlorophyllide oxidoreductase. Biochem. J. 474, 1307–1320 (2017).
Zhang, S. et al. Structural basis for enzymatic photocatalysis in chlorophyll biosynthesis. Nature 574, 722–725 (2019).
Otsuki, J. Supramolecular approach towards light-harvesting materials based on porphyrins and chlorophylls. J. Mater. Chem. A 6, 6710–6753 (2018).
Fujii, S., Wada, H. & Kobayashi, K. Role of galactolipids in plastid differentiation before and after light exposure. Plants 8, 357 (2019).
Kowalewska, L., Mazur, R., Suski, S., Garstka, M. & Mostowska, A. Three-dimensional visualization of the tubular-lamellar transformation of the internal plastid membrane network during runner bean chloroplast biogenesis. Plant Cell 28, 875–891 (2016).
Boudière, L. et al. Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim. Biophys. Acta Bioenerg. 1837, 470–480 (2014).
Selstam, E. & Sandelius, A. S. A comparison between prolamellar bodies and prothylakoid membranes of etioplasts of dark-grown wheat concerning lipid and polypeptide composition. Plant Physiol. 76, 1036–1040 (1984).
Böddi, B., Lindsten, A., Ryberg, M. & Sundqvist, C. On the aggregational states of protochlorophyllide and its protein complexes in wheat etioplasts. Physiol. Plant. 76, 135–143 (1989).
Nguyen, H. C. et al. Membrane constriction and thinning by sequential ESCRT-III polymerization. Nat. Struct. Mol. Biol. 27, 392–399 (2020).
Dong, C. et al. Crystal structures of cyanobacterial light-dependent protochlorophyllide oxidoreductase. Proc. Natl Acad. Sci. USA 117, 8455–8461 (2020).
Gabruk, M. & Mysliwa-Kurdziel, B. The origin, evolution and diversification of multiple isoforms of light-dependent protochlorophyllide oxidoreductase (LPOR): focus on angiosperms. Biochem. J. 477, 2221–2236 (2020).
Kahn, A., Boardman, N. K. & Thorne, S. W. Energy transfer between protochlorophyllide molecules: evidence for multiple chromophores in the photoactive protochlorophyllide-protein complex in vivo and in vitro. J. Mol. Biol. 48, 85–101 (1970).
Menon, B. R. K., Hardman, S. J. O., Scrutton, N. S. & Heyes, D. J. Multiple active site residues are important for photochemical efficiency in the light-activated enzyme protochlorophyllide oxidoreductase (POR). J. Photochem. Photobiol. B 161, 236–243 (2016).
Menon, B. R. K., Waltho, J. P., Scrutton, N. S. & Heyes, D. J. Cryogenic and laser photoexcitation studies identify multiple roles for active site residues in the light-driven enzyme protochlorophyllide oxidoreductase. J. Biol. Chem. 284, 18160–18166 (2009).
Gholami, S. et al. Theoretical model of the protochlorophyllide oxidoreductase from a hierarchy of protocols. J. Phys. Chem. B 122, 7668–7681 (2018).
Zhang, S. et al. Dual role of the active site ‘lid’ regions of protochlorophyllide oxidoreductase in photocatalysis and plant development. FEBS J. 288, 175–189 (2021).
Begley, T. P. & Young, H. Protochlorophyllide reductase. 1. Determination of the regiochemistry and the stereochemistry of the reduction of protochlorophyllide to chlorophyllide. J. Am. Chem. Soc. 111, 3095–3096 (1989).
Lebedev, N., Karginova, O., McIvor, W. & Timko, M. P. Tyr275 and Lys279 stabilize NADPH within the catalytic site of NADPH:protochlorophyllide oxidoreductase and are involved in the formation of the enzyme photoactive state. Biochemistry 40, 12562–12574 (2001).
Heyes, D. J., Sakuma, M., de Visser, S. P. & Scrutton, N. S. Nuclear quantum tunneling in the light-activated enzyme protochlorophyllide oxidoreductase. J. Biol. Chem. 284, 3762–3767 (2009).
Hoeven, R., Hardman, S. J. O., Heyes, D. J. & Scrutton, N. S. Cross-species analysis of protein dynamics associated with hydride and proton transfer in the catalytic cycle of the light-driven enzyme protochlorophyllide oxidoreductase. Biochemistry 55, 903–913 (2016).
Myśliwa-Kurdziel, B., Amirjani, M. R. M. R., Strzałka, K. & Sundqvist, C. Fluorescence lifetimes of protochlorophyllide in plants with different proportions of short-wavelength and long-wavelength protochlorophyllide spectral forms. Photochem. Photobiol. 78, 205–212 (2003).
Selstam, E., Schelin, J., Williams, W. P. & Brain, A. P. R. Structural organisation of prolamellar bodies (PLB) isolated from Zea mays. Parallel TEM, SAXS and absorption spectra measurements on samples subjected to freeze-thaw, reduced pH and high-salt perturbation. Biochim. Biophys. Acta Biomembr. 1768, 2235–2245 (2007).
Runge, S., Sperling, U., Frick, G., Apel, K. & Armstrong, G. A. Distinct roles for light-dependent NADPH:protochlorophyllide oxidoreductases (POR) A and B during greening in higher plants. Plant J. 9, 513–523 (1996).
Schoefs, B. & Franck, F. The photoenzymatic cycle of NADPH: protochlorophyllide oxidoreductase in primary bean leaves (Phaseolus vulgaris) during the first days of photoperiodic growth. Photosynth. Res. 96, 15–26 (2008).
Bastien, O. et al. New insights on thylakoid biogenesis in plant cells. Int. Rev. Cell Mol. Biol. 323, 1–30 (2016).
Rast, A., Heinz, S. & Nickelsen, J. Biogenesis of thylakoid membranes. Biochim. Biophys. Acta Bioenerg. 1847, 821–830 (2015).
Frick, G., Su, Q., Apel, K. & Armstrong, G. A. An Arabidopsis porB porC double mutant lacking light-dependent NADPH:protochlorophyllide oxidoreductases B and C is highly chlorophyll-deficient and developmentally arrested. Plant J. 35, 141–153 (2003).
Chiu, J., March, P. E., Lee, R. & Tillett, D. Site-directed, ligase-independent mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 32, e174 (2004).
Gabruk, M. et al. Photoactive protochlorophyllide-enzyme complexes reconstituted with PORA, PORB and PORC proteins of A. thaliana: fluorescence and catalytic properties. PLoS ONE 10, e0116990 (2015).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Wagner, T. & Raunser, S. The evolution of SPHIRE-crYOLO particle picking and its application in automated cryo-EM processing workflows. Commun. Biol. 3, 61 (2020).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
He, S. & Scheres, S. H. W. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Yang, J. et al. The I-TASSER suite: protein structure and function prediction. Nat. Methods 12, 7–8 (2015).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 9, 40 (2008).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Struct. Biol. 66, 486–501 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D. Struct. Biol. 74, 814–840 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Acknowledgements
We thank the Frost laboratory for helpful discussions and critical comments; P. Thomas, L. E. Miller-Vedam and M. Sun for computational assistance; N. Poweleit for discussions on helical processing; T. Borowski for generation of Pchlide ligand restraints; M. Braunfeld, D. Bulkley, M. Harrington, A. Myasnikov and Z. Yu of the UCSF Center for Advanced Cryo-EM for microscopy support; and J. Baker-LePain and the QB3 shared cluster (National Institutes of Health (NIH) grant no. 1S10OD021596-01) for computational support. Structural biology applications used in this project were compiled and configured by SBGrid. The Titan X Pascal used for this research was donated by the NVIDIA Corporation. This study was supported by a Bekker scholarship granted by the Polish National Agency for Academic Exchange (no. PPN/BEK/2018/1/00105), a START scholarship granted by the Foundation for Polish Science (no. 024.2018 to M.G) and NIH (no. 1DP2GM110772-01 to A.F). A.F. is further supported by a Faculty Scholar grant from Howard Hughes Medical Institute (HHMI) and is a Chan Zuckerberg Biohub investigator.
Author information
Authors and Affiliations
Contributions
M.G. and A.F. conceived the project. M.G., A.F. and H.C.N. designed experiments and analysed data. M.G. optimized and produced the samples and performed spectroscopy measurements and sequence alignment analysis. H.C.N. assisted in sample optimization and performed cryo-EM experiments and analysis. J.K. and M.G. purified Pchlide. A.A.M. prepared the animation video and assisted with cryo-EM data collection. H.C.N., A.F. and M.G. prepared the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Alexey Amunts, Benjamin Engel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Plant LPOR can form filaments of different sizes.
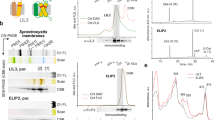
a, Negative stain electron microscopy (EM) micrographs of AtPORB filaments with different diameters formed from liposomes composed of 2.5 mol% MGDG / 50 mol% DGDG / 47.5 mol% PG (orange frame) or 50 mol% MGDG / 25 mol% DGDG / 25 mol% PG (black frames). Scale bar: 25 nm. b, Negative stain EM micrographs of AtPORB filaments produced from liposomes mimicking the lipid composition of PLBs (52 mol% MGDG / 32 mol% DGDG / 8 mol% PG, 8 mol% SQDG; orange frame, chloroplast inner membranes (50 mol% MGDG / 30 mol% DGDG / 8 mol% PG, 5 mol% SQDG, 7 mol% PC, cyan frame) and our optimized membrane composition (50 mol% MGDG / 35 mol% DGDG / 15 mol% PG, black frame). Scale bar: 25 nm. c, Low-temperature fluorescence emission spectra before (left panel) and after illumination (right panel) of the filaments produced with the liposomes mimicking the lipids of PLBs (orange), chloroplast inner membrane (cyan) and for our optimized membrane composition (black). Grey vertical lines indicate the emission maxima of: free Pchlide (632 nm), Pchlide within PLBs or the membrane-bound filaments (655 nm), and reduced Chlide (690 nm).
Extended Data Fig. 2 CryoEM validation of the AtPORB filament.
a, Representative fit of the cryoEM density to the atomic model. b, Angular distribution of the membrane-bound AtPORB cryoEM 3D reconstruction. c, Independent half-map and map-model Fourier shell correlation (FSC) of the reconstruction. d, Local resolution estimates.
Extended Data Fig. 3 A 4-start helix of plant LPOR.
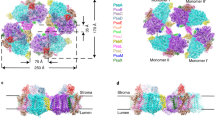
Exterior and top-down views of the 4-start PORB helix. Each start comprises a strand of antiparallel dimers, colored in orange, green, cyan, or magenta.
Extended Data Fig. 4 Comparison of plant and cyanobacterial LPORs.
a, Secondary structure elements of AtPORB annotated onto the tertiary structure. b, The comparison of the sequences of AtPORB, T. elongatus LPOR (Thermo), Synechocystis LPOR (Synech), and the consensus sequences of LPORs originating from plants and cyanobacteria (187 and 301 sequences, respectively). The size of the letter indicates the frequency of the given amino acid at the given position, while x indicates multiple amino acids with low frequency at a given position. Transit peptides of plant LPORs are excluded. Residues conserved in all sequences are grey. The secondary structure elements and Pchlide loop of AtPORB are marked. AtPORB residues known to play a role in oligomerization and substrate binding are highlighted with a pale red background. Orange/cyan/grey polygons indicate residues of NADPH/Pchlide/MGDG binding sites, respectively. Edges of the polygon highlighted in green indicate the oligomerization interfaces the given residue is involved in: bottom edge – antiparallel dimer, top edge – intra-strand interface, side edges – inter-strand interface. The previously proposed catalytic YxxxK motif is marked in red. Cysteine and serine residues that we suggest may be catalytically involved in the reaction are marked in green. c, Schematic representation of the LPOR interfaces for the polygons in b. d, Negative stain EM micrographs of AtPORB (left) and Synechocystis LPOR (right) filaments produced with our optimized membrane composition. Scale bar: 25 nm.
Extended Data Fig. 5 Comparison of the NADPH binding site across plants and cyanobacteria.
a, NADPH binding pocket of AtPORB. b, NADPH binding pocket of LPOR form T. elongatus (PDB: 6L1H). c, NADPH binding pocket of LPOR form Synechocystis (PDB: 6L1G). Hydrophobic interactions are shown as orange circles and hydrogen bonds are shown as dashed lines. Green dashed lines indicate the identical bonds between homologous residues. Resides in red indicate non-conserved residues. Waters are shown as small light blue circles.
Extended Data Fig. 6 Structural comparison of Pchlide binding motifs between Pchlide-bound plant and Pchlide-free cyanobacterial LPOR.
Superposition of Pchlide-bound AtPORB (dark green) with Pchlide-free LPOR from Synechocystis LPOR (a, dark purple, PDB: 6R48 or 6L1G), T. elongatus (b, PDB: 6RNW, orange), and T. elongatus (c, PDB: 6L1H, slate blue).
Extended Data Fig. 7 AtPORB S228A is less active upon illumination than WT, C309 mutants or Y276F mutant.
a, the spectra of the reactions before (blue) and after (orange) the illumination of AtPORB WT and selected mutants. b, proposed proton-relay path (green dashed lines) and hydride-relay path (yellow dashed line).
Supplementary information
Supplementary Information
Supplementary Table 1.
Supplementary Video 1
Structure of the membrane-bound LPOR filament with substrates bound.
Supplementary Video 2
Conformational changes following Pchlide binding.
Supplementary Video 3
Mechanistic model of substrate binding, polymerization and membrane remodelling by plant LPOR.
Rights and permissions
About this article
Cite this article
Nguyen, H.C., Melo, A.A., Kruk, J. et al. Photocatalytic LPOR forms helical lattices that shape membranes for chlorophyll synthesis. Nat. Plants 7, 437–444 (2021). https://doi.org/10.1038/s41477-021-00885-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00885-2
This article is cited by
-
Brominated lipid probes expose structural asymmetries in constricted membranes
Nature Structural & Molecular Biology (2023)
-
Cryo-electron tomography reveals structural insights into the membrane remodeling mode of dynamin-like EHD filaments
Nature Communications (2022)
-
Chlorophyll biogenesis sees the light
Nature Plants (2021)