Abstract
While rhodopsin-based optogenetics has revolutionized neuroscience1,2, poor expression of opsins and the absence of the essential cofactor all-trans-retinal has complicated the application of rhodopsins in plants. Here, we demonstrate retinal production in plants and improved rhodopsin targeting for green light manipulation of plant cells using the Guillardia theta light-gated anion channelrhodopsin GtACR13. Green light induces a massive increase in anion permeability and pronounced membrane potential changes when GtACR1 is expressed, enabling non-invasive manipulation of plant growth and leaf development. Using light-driven anion loss, we could mimic drought conditions and bring about leaf wilting despite sufficient water supply. Expressed in pollen tubes, global GtACR1 activation triggers membrane potential depolarizations due to large anion currents. While global illumination was associated with a reversible growth arrest, local GtACR1 activation at the flanks of the apical dome steers growth direction away from the side with increased anion conductance. These results suggest a crucial role of anion permeability for the guidance of pollen tube tip growth. This plant optogenetic approach could be expanded to create an entire pallet of rhodopsin-based tools4, greatly facilitating dissection of plant ion-signalling pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The source data and DNA sequences of this study are provided as supplementary data files. Source data are provided with this paper.
References
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Govorunova, E. G., Sineshchekov, O. A., Janz, R., Liu, X. & Spudich, J. L. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650 (2015).
Govorunova, E. G., Sineshchekov, O. A., Li, H. & Spudich, J. L. Microbial rhodopsins: diversity, mechanisms, and optogenetic applications. Annu. Rev. Biochem. 86, 845–872 (2017).
Hedrich, R. & Marten, I. 30-year progress of membrane transport in plants. Planta 224, 725–739 (2006).
Palmgren, M. G. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 817–845 (2001).
Hedrich, R. Ion channels in plants. Physiol. Rev. 92, 1777–1811 (2012).
Fromm, J. & Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 30, 249–257 (2007).
Hedrich, R., Salvador-Recatala, V. & Dreyer, I. Electrical wiring and long-distance plant communication. Trends Plant Sci. 21, 376–387 (2016).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA 100, 13940–13945 (2003).
Reyer, A. et al. Channelrhodopsin-mediated optogenetics highlights a central role of depolarization-dependent plant proton pumps. Proc. Natl Acad. Sci. USA 117, 20920–20925 (2020).
Cosentino, C. et al. Engineering of a light-gated potassium channel. Science 348, 707–710 (2015).
Papanatsiou, M. et al. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 363, 1456–1459 (2019).
Ullrich, S., Gueta, R. & Nagel, G. Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants. Biol. Chem. 394, 271–280 (2013).
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006).
Dawydow, A. et al. Channelrhodopsin-2–XXL, a powerful optogenetic tool for low-light applications. Proc. Natl Acad. Sci. USA 111, 13972–13977 (2014).
Kim, Y. S., Kim, N. H., Yeom, S. J., Kim, S. W. & Oh, D. K. In vitro characterization of a recombinant Blh protein from an uncultured marine bacterium as a β-carotene 15,15′-dioxygenase. J. Biol. Chem. 284, 15781–15793 (2009).
Shen, B. R. et al. An optimized transit peptide for effective targeting of diverse foreign proteins into chloroplasts in rice. Sci. Rep. 7, 46231 (2017).
Raven, J. A. Chloride: essential micronutrient and multifunctional beneficial ion. J. Exp. Bot. 68, 359–367 (2016).
Roelfsema, M. R. G., Hedrich, R. & Geiger, D. Anion channels: master switches of stress responses. Trends Plant Sci. 17, 221–229 (2012).
Shepard, B. D., Natarajan, N., Protzko, R. J., Acres, O. W. & Pluznick, J. L. A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS ONE 8, e68758 (2013).
Battle, M. W., Vegliani, F. & Jones, M. A. Shades of green: untying the knots of green photoperception. J. Exp. Bot. 71, 5764–5770 (2020).
Smith, H. L., McAusland, L. & Murchie, E. H. Don’t ignore the green light: exploring diverse roles in plant processes. J. Exp. Bot. 68, 2099–2110 (2017).
Battle, M. W. & Jones, M. A. Cryptochromes integrate green light signals into the circadian system. Plant Cell Environ. 43, 16–27 (2020).
Kumari, A., Chetelat, A., Nguyen, C. T. & Farmer, E. E. Arabidopsis H+-ATPase AHA1 controls slow wave potential duration and wound-response jasmonate pathway activation. Proc. Natl Acad. Sci. USA 116, 20226–20231 (2019).
Seo, P. J. et al. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23, 1138–1152 (2011).
Gutermuth, T. et al. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 25, 4525–4543 (2013).
Gutermuth, T. et al. Tip-localized Ca2+-permeable channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation. N. Phytol. 218, 1089–1105 (2018).
Michard, E., Simon, A. A., Tavares, B., Wudick, M. M. & Feijo, J. A. Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol. 173, 91–111 (2017).
Tang, L. Y. et al. Visualization of plastids in pollen grains: involvement of FtsZ1 in pollen plastid division. Plant Cell Physiol. 50, 904–908 (2009).
Schulte, F., Mader, J., Kroh, L. W., Panne, U. & Kneipp, J. Characterization of pollen carotenoids with in situ and high-performance thin-layer chromatography supported resonant Raman spectroscopy. Anal. Chem. 81, 8426–8433 (2009).
Zonia, L., Cordeiro, S., Tupy, J. & Feijó, J. A. Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. Plant Cell 14, 2233–2249 (2002).
Müller, K. et al. A red light-controlled synthetic gene expression switch for plant systems. Mol. Biosyst. 10, 1679–1688 (2014).
Ochoa-Fernandez, R. et al. Optogenetic control of gene expression in plants in the presence of ambient white light. Nat. Methods 17, 717–725 (2020).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Twell, D., Yamaguchi, J. & McCormick, S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109, 705–713 (1990).
Acknowledgements
This study is funded by the Deutsche Forschungsgemeinschaft (DFG) Projektnummer 374031971 TRR 240 A04 and 417451587. G.N. acknowledges the support provided by the Prix Louis-Jeantet. Financial support by the Deutsche Forschungsgemeinschaft is accredited to K.R.K. (DFG KO3657/2-3). Y.Z., M.D. and X.D. are supported by the Chinese Scholarship Council. We acknowledge A. Reyer for help with plant cell impalement.
Author information
Authors and Affiliations
Contributions
G.N., S.G., M.K., M.J.M., R.H. and K.R.K. conceived the project. S.G., K.R.K. and G.N. designed and supervised the experiments. Y.Z., M.D., J.Y.-S. and S.G. performed the plant experiments and analysed the data. J.Y.-S. and X.D. performed the Xenopus oocyte experiments. M.K. performed the retinal and carotenoids analysis. J.L. and M.R. performed the leaf cuticular wax analysis. S.G., K.R.K. and G.N. wrote the paper. All authors edited and approved the final version of the manuscript to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Toshinori Kinoshita, Matias Zurbriggen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–8, legends for Videos and DNA sequences used in this study.
Supplementary Video 1
Transient expression of RC2–eYFP in pollen tubes. Pollen transiently transformed with RC2–eYFP were pre-incubated in semi-solid pollen germination medium for 5–7 h at room temperature in the dark. Real time is indicated in the top left corner. eYFP fluorescence is indicated by yellow colour. Scale bar, 10 μm.
Supplementary Video 2
Growths of WT, LeLAT52::Ret–eYFP and LeLAT52::Ret–ACR1 2.0 pollen tubes in response to the global illumination. WT, LeLAT52::Ret–eYFP #1 and LeLAT52::Ret–ACR1 2.0 #1 transgenic expressed pollen were pre-incubated in semi-solid pollen germination medium for 5–7 h at room temperature in the dark. Red light was used to perform the bright-field imaging. Pollen tubes growing in 10 min dark, 10 min green light illumination and then 20 min dark were captured. Both WT and Ret–eYFP-expressing pollen tubes showed no response to green light (532 nm, 2 mW mm−2 (9,600 μmol m−2 s−1)) illumination. Global illumination had a negative effect on the growth of Ret–ACR1 2.0-expressing transgenic pollen tubes. Real time (min:s) is indicated in the top left corner.
Supplementary Video 3
Growth of LeLAT52::Ret–ACR1 2.0 #1-1 pollen tubes in response to local illumination.
Supplementary Video 4
Growth of LeLAT52::Ret–ACR1 2.0 #1-2 pollen tubes in response to local illumination.
Supplementary Video 5
Growth of LeLAT52::Ret–ACR1 2.0 #1-3 pollen tubes in response to local illumination.
Supplementary Video 6
Growth of LeLAT52::Ret–ACR1 2.0 #2-1 pollen tubes in response to local illumination.
Supplementary Video 7
Growth of LeLAT52::Ret–ACR1 2.0 #2-2 pollen tubes in response to local illumination.
Supplementary Video 8
Growth of LeLAT52::Ret–ACR1 2.0 #2-3 pollen tubes in response to local illumination.
Supplementary Video 9
Growth of WT pollen tubes in response to local illumination #1.
Supplementary Video 10
Growth of WT pollen tubes in response to local illumination #2.
Supplementary Video 11
Growth of WT pollen tubes in response to local illumination #3.
Source data
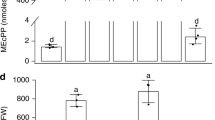
Source Data Fig. 1
Statistical source data.
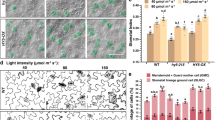
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhou, Y., Ding, M., Gao, S. et al. Optogenetic control of plant growth by a microbial rhodopsin. Nat. Plants 7, 144–151 (2021). https://doi.org/10.1038/s41477-021-00853-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00853-w
This article is cited by
-
Combining different ion-selective channelrhodopsins to control water flux by light
Pflügers Archiv - European Journal of Physiology (2023)
-
Visual function restoration with a highly sensitive and fast Channelrhodopsin in blind mice
Signal Transduction and Targeted Therapy (2022)
-
Optogenetics for light control of biological systems
Nature Reviews Methods Primers (2022)
-
Biofortifying green optogenetics
Nature Plants (2021)