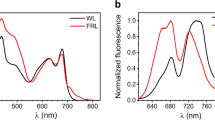
Abstract
Plants and cyanobacteria use the chlorophylls embedded in their photosystems to absorb photons and perform charge separation, the first step of converting solar energy to chemical energy. While oxygenic photosynthesis is primarily based on chlorophyll a photochemistry, which is powered by red light, a few cyanobacterial species can harness less energetic photons when growing in far-red light. Acclimatization to far-red light involves the incorporation of a small number of molecules of red-shifted chlorophyll f in the photosystems, whereas the most abundant pigment remains chlorophyll a. Due to its different energetics, chlorophyll f is expected to alter the excited-state dynamics of the photosynthetic units and, ultimately, their performances. Here we combined time-resolved fluorescence measurements on intact cells and isolated complexes to show that chlorophyll f insertion slows down the overall energy trapping in both photosystems. While this marginally affects the efficiency of photosystem I, it substantially decreases that of photosystem II. Nevertheless, we show that despite the lower energy output, the insertion of red-shifted chlorophylls in the photosystems remains advantageous in environments that are enriched in far-red light and therefore represents a viable strategy for extending the photosynthetically active spectrum in other organisms, including plants. However, careful design of the new photosynthetic units will be required to preserve their efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw data are available from the corresponding author (R.C.) upon reasonable request.
References
Bryant, D. A. & Frigaard, N. U. Prokaryotic photosynthesis and phototrophy illuminated. Trends Microbiol. 14, 488–496 (2006).
Björn, L. O., Papageorgiou, G. C., Blankenship, R. E. & Govindjee A viewpoint: why chlorophyll a? Photosynth. Res. 99, 85–98 (2009).
Tomo, T. et al. Characterization of highly purified photosystem I complexes from the chlorophyll d-dominated cyanobacterium Acaryochloris marina MBIC 11017. J. Biol. Chem. 283, 18198–18209 (2008).
Renger, T. & Schlodder, E. The primary electron donor of photosystem II of the cyanobacterium Acaryochloris marina is a chlorophyll d and the water oxidation is driven by a chlorophyll a/chlorophyll d heterodimer. J. Phys. Chem. B 112, 7351–7354 (2008).
Tomo, T., Allakhverdiev, S. I. & Mimuro, M. Constitution and energetics of photosystem I and photosystem II in the chlorophyll d-dominated cyanobacterium Acaryochloris marina. J. Photochem. Photobiol. B 104, 333–340 (2011).
Chen, M. et al. A red-shifted chlorophyll. Science 329, 1318–1320 (2010).
Gan, F. et al. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345, 1312–1317 (2014).
Chen, M., Li, Y., Birch, D. & Willows, R. D. A cyanobacterium that contains chlorophyll f—a red-absorbing photopigment. FEBS Lett. 586, 3249–3254 (2012).
Gan, F., Shen, G. & Bryant, D. A. Occurrence of far-red light photoacclimation (FaRLiP) in diverse cyanobacteria. Life 5, 4–24 (2015).
Nürnberg, D. J. et al. Photochemistry beyond the red limit in chlorophyll f-containing photosystems. Science 360, 1210–1213 (2018).
Ho, M. Y., Shen, G., Canniffe, D. P., Zhao, C. & Bryant, D. A. Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II. Science 353, aaf9178 (2016).
Ho, M. Y., Soulier, N. T., Canniffe, D. P., Shen, G. & Bryant, D. A. Light regulation of pigment and photosystem biosynthesis in cyanobacteria. Curr. Opin. Plant Biol. 37, 24–33 (2017).
Li, Y., Vella, N. & Chen, M. Characterization of isolated photosystem I from Halomicronema hongdechloris, a chlorophyll f-producing cyanobacterium. Photosynthetica 56, 306–315 (2018).
Kurashov, V. et al. Energy transfer from chlorophyll f to the trapping center in naturally occurring and engineered photosystem I complexes. Photosynth. Res. 141, 151–163 (2019).
Ho, M.-Y. et al. Extensive remodeling of the photosynthetic apparatus alters energy transfer among photosynthetic complexes when cyanobacteria acclimate to far-red light. Biochim. Biophys. Acta 1861, 148064 (2020).
Li, Y. et al. Characterization of red-shifted phycobilisomes isolated from the chlorophyll f-containing cyanobacterium Halomicronema hongdechloris. Biochim. Biophys. Acta 1857, 107–114 (2016).
Ho, M. Y., Gan, F., Shen, G. & Bryant, D. A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335. II. Characterization of phycobiliproteins produced during acclimation to far-red light. Photosynth. Res. 131, 187–202 (2017).
Loughlin, P., Lin, Y. & Chen, M. Chlorophyll d and Acaryochloris marina: current status. Photosynth. Res. 116, 277–293 (2013).
Zamzam, N., Kaucikas, M., Nurnberg, D., Rutherford, A. W. & van Thor, J. J. Femtosecond infrared spectroscopy of chlorophyll f-containing photosystem I. Phys. Chem. Chem. Phys. 21, 1224–1234 (2019).
Hastings, G. et al. Fourier transform visible and infrared difference spectroscopy for the study of P700 in photosystem I from Fischerella thermalis PCC 7521 cells grown under white light and far-red light: evidence that the A–1 cofactor is chlorophyll f. Biochim. Biophys. Acta 1860, 452–460 (2019).
Kato, K. et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 11, 238 (2020).
Gisriel, C. et al. The structure of photosystem I acclimated to far-red light illuminates an ecologically important acclimation process in photosynthesis. Sci. Adv. 6, eaay6415 (2020).
Itoh, S. et al. Harvesting far-red light by chlorophyll f in photosystems I and II of unicellular cyanobacterium strain KC1. Plant Cell Physiol. 56, 2024–2034 (2015).
Akimoto, S., Shinoda, T., Chen, M., Allakhverdiev, S. I. & Tomo, T. Energy transfer in the chlorophyll f-containing cyanobacterium, Halomicronema hongdechloris, analyzed by time-resolved fluorescence spectroscopies. Photosynth. Res. 125, 115–122 (2015).
Schmitt, F. J. et al. Photosynthesis supported by a chlorophyll f-dependent, entropy-driven uphill energy transfer in Halomicronema hongdechloris cells adapted to far-red light. Photosynth. Res. 139, 185–201 (2019).
Kaucikas, M., Nürnberg, D., Dorlhiac, G., Rutherford, A. W. & van Thor, J. J. Femtosecond visible transient absorption spectroscopy of chlorophyll f-containing photosystem I. Biophys. J. 112, 234–249 (2017).
Ho, M. Y., Gan, F., Shen, G., Zhao, C. & Bryant, D. A. Far-red light photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP gene expression. Photosynth. Res. 131, 173–186 (2017).
Holzwarth, A. R., Schatz, G., Brock, H. & Bittersmann, E. Picosecond transient absorption and fluorescence study of cyanobacterial photosystem I particles. Biophys. J. 64, 1813–1826 (1993).
Gobets, B. et al. Time-resolved fluorescence emission measurements of photosystem I particles of various cyanobacteria: a unified compartmental model. Biophys. J. 81, 407–424 (2001).
Tian, L., Farooq, S. & Van Amerongen, H. Probing the picosecond kinetics of the photosystem II core complex in vivo. Phys. Chem. Chem. Phys. 15, 3146–3154 (2013).
Vassiliev, S., Lee, C. I., Brudvig, G. W. & Bruce, D. Structure-based kinetic modeling of excited-state transfer and trapping in histidine-tagged photosystem II core complexes from Synechocystis. Biochemistry 41, 12236–12243 (2002).
Miloslavina, Y. et al. Charge separation kinetics in intact photosystem II core particles is trap-limited. A picosecond fluorescence study. Biochemistry 45, 2436–2442 (2006).
Szczepaniak, M. et al. Charge separation, stabilization, and protein relaxation in photosystem II core particles with closed reaction center. Biophys. J. 96, 621–631 (2009).
Niedzwiedzki, D. M., Liu, H., Chen, M. & Blankenship, R. E. Excited state properties of chlorophyll f in organic solvents at ambient and cryogenic temperatures. Photosynth. Res. 121, 25–34 (2014).
Krumova, S. B. et al. Monitoring photosynthesis in individual cells of Synechocystis sp. PCC 6803 on a picosecond timescale. Biophys. J. 99, 2006–2015 (2010).
Chukhutsina, V., Bersanini, L., Aro, E. M. & Van Amerongen, H. Cyanobacterial flv4-2 operon-encoded proteins optimize light harvesting and charge separation in photosystem II. Mol. Plant 8, 747–761 (2015).
Karapetyan, N. V., Holzwarth, A. R. & Rögner, M. The photosystem I trimer of cyanobacteria: molecular organization, excitation dynamics and physiological significance. FEBS Lett. 460, 395–400 (1999).
Gobets, B. & van Grondelle, R. Energy transfer and site of energy trapping in photosystem I. Biochim. Biophys. Acta 1507, 80–99 (2001).
Broess, K., Trinkunas, G., van Hoek, A., Croce, R. & van Amerongen, H. Determination of the excitation migration time in photosystem II. Biochim. Biophys. Acta 1777, 404–409 (2008).
Miyashita, H. et al. Pigment composition of a novel oxygenic photosynthetic prokaryote containing chlorophyll d as the major chlorophyll. Plant Cell Physiol. 38, 274–281 (1997).
Tomo, T. et al. Identification of the special pair of photosystem II in a chlorophyll d-dominated cyanobacterium. Proc. Natl Acad. Sci. USA 104, 7283–7288 (2007).
Itoh, S. et al. Function of chlorophyll d in reaction centers of photosystems I and II of the oxygenic photosynthesis of Acaryochloris marina. Biochemistry 46, 12473–12481 (2007).
Cser, K., Deák, Z., Telfer, A., Barber, J. & Vass, I. Energetics of photosystem II charge recombination in Acaryochloris marina studied by thermoluminescence and flash-induced chlorophyll fluorescence measurements. Photosynth. Res. 98, 131–140 (2008).
Schatz, G., Brock, H. & Holzwarth, A. R. Kinetic and energetic model for the primary processes in photosystem II. Biophys. J. 54, 397–405 (1988).
Van Grondelle, R., Dekker, J. P., Gillbro, T. & Sundström, V. Energy transfer and trapping in photosynthesis. Biochim. Biophys. Acta 1187, 1–65 (1994).
Gan, F. & Bryant, D. A. Adaptive and acclimative responses of cyanobacteria to far-red light. Environ. Microbiol. 17, 3450–3465 (2015).
Rippka, R., Deruelles, J. & Waterbury, J. B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111, 1–61 (1979).
Zhang, P., Allahverdiyeva, Y., Eisenhut, M. & Aro, E. M. Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by FIv2 and FIv4 in Synechocystis sp. PCC 6803. PLoS ONE 4, e5331 (2009).
Zhang, P. et al. Operon flv4–flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24, 1952–1971 (2012).
Van Oort, B. et al. Picosecond fluorescence of intact and dissolved PSI–LHCI crystals. Biophys. J. 95, 5851–5861 (2008).
van Stokkum, I. H. M., Larsen, D. S. & Van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 1657, 82–104 (2004).
Kuhl, H. et al. Towards structural determination of the water-splitting enzyme: purification, crystallization, and preliminary crystallographic studies of photosystem II from a thermophilic cyanobacterium. J. Biol. Chem. 275, 20652–20659 (2000).
Acknowledgements
We thank J. Schaefers (Vrije Universiteit Amsterdam) for helping with cell growth and pigment content determination and M. Tros for insightful discussions. This project was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 675006 and by the Netherlands Organization for Scientific Research (NWO) via a Top grant to R.C., and by the EMBO long-term fellowship (EMBO ALTF 292-2017) to L.B.
Author information
Authors and Affiliations
Contributions
L.B. and R.C. conceived the project. V.M. and L.B. performed the experiments. V.M. and L.B. analysed the data. The manuscript was written by V.M. with contributions by R.C. and L.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Robert Blankenship and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, tables and references.
Rights and permissions
About this article
Cite this article
Mascoli, V., Bersanini, L. & Croce, R. Far-red absorption and light-use efficiency trade-offs in chlorophyll f photosynthesis. Nat. Plants 6, 1044–1053 (2020). https://doi.org/10.1038/s41477-020-0718-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0718-z
This article is cited by
-
Computational dissection of genetic variation modulating the response of multiple photosynthetic phenotypes to the light environment
BMC Genomics (2024)
-
Far-red light modulates grapevine growth by increasing leaf photosynthesis efficiency and triggering organ-specific transcriptome remodelling
BMC Plant Biology (2024)
-
Chlorophyll triplet states in thylakoid membranes of Acaryochloris marina. Evidence for a triplet state sitting on the photosystem I primary donor populated by intersystem crossing
Photosynthesis Research (2024)
-
Exogenous ethylene application——an effective measure to alleviate waterlogging-induced stress on photosynthesis of Zanthoxylum armatum leaves
Plant Growth Regulation (2023)
-
The antenna of far-red absorbing cyanobacteria increases both absorption and quantum efficiency of Photosystem II
Nature Communications (2022)