Abstract
Photosynthesis provides food, fibre and fuel that support our society; understanding the mechanisms controlling dynamic changes in this process helps identify new options to improve photosynthesis. Photosynthesis shows diel changes, which have been largely attributed to external light/dark conditions, as well as internal gene expression and the post-translational modification of critical enzymes. Here we report diel fluctuations of magnesium (Mg) in rice (Oryza sativa) chloroplasts, which may function as a rhythm regulator contributing to the post-translational regulation of photosynthetic CO2 assimilation in rice. We found that a chloroplast-localized Mg2+ transporter gene, OsMGT3, which is rhythmically expressed in leaf mesophyll cells, partly modulates Mg fluctuations in rice chloroplasts. Knockout of OsMGT3 substantially reduced Mg2+ uptake, as well as the amplitude of free Mg2+ fluctuations in chloroplasts, which was closely associated with a decrease in ribulose 1,5-bisphosphate carboxylase activity in vivo and a consequent decline in the photosynthetic rate. In addition, the mesophyll-specific overexpression of OsMGT3 remarkably improved photosynthetic efficiency and growth performance in rice. Taken together, these observations demonstrate that OsMGT3-dependent diel Mg fluctuations in chloroplasts may contribute to Mg-dependent enzyme activities for photosynthesis over the daily cycle. Enhancing Mg2+ input to chloroplasts could be a potential approach to improving photosynthetic efficiency in plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article, and any source data can be obtained from the corresponding author on request.
References
Long, S. P., Marshall-Colon, A. & Zhu, X. G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66 (2015).
Zhu, X. G., Long, S. P. & Ort, D. R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 (2010).
Shen, B. R. et al. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 12, 199–214 (2019).
South, P. F., Cavanagh, A. P., Liu, H. W. & Ort, D. R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363, eaat9077 (2019).
Herdean, A. Ion Transport in Chloroplasts with Role in Regulation of Photosynthesis (Department of Biological and Environmental Sciences, Univ. of Gothenburg, 2015).
Dodd, A. N., Kusakina, J., Hall, A., Gould, P. D. & Hanaoka, M. The circadian regulation of photosynthesis. Photosynth. Res. 119, 181–190 (2014).
Dodd, A. N. et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 (2005).
Hennessey, T. L. & Field, C. B. Circadian rhythms in photosynthesis oscillations in carbon assimilation and stomatal conductance under constant conditions. Plant Physiol. 96, 831–836 (1991).
Nassoury, N., Fritz, L. & Morse, D. Circadian changes in ribulose-1,5-bisphosphate carboxylase/oxygenase distribution inside individual chloroplasts can account for the rhythm in dinoflagellate carbon fixation. Plant Cell 13, 923–934 (2001).
Haydon, M. J., Roman, A. & Arshad, W. Nutrient homeostasis within the plant circadian network. Front. Plant Sci. 6, 299 (2015).
Feeney, K. A. et al. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532, 375–379 (2016).
Ruiz, M. C. M. et al. Circadian oscillations of cytosolic free calcium regulate the Arabidopsis circadian clock. Nat. Plants 4, 690–698 (2018).
Covington, M. F., Maloof, J. N., Straume, M., Kay, S. A. & Harmer, S. L. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 9, R130 (2008).
Wang, G. Y. et al. Circadian clock-regulated phosphate transporter PHT4;1 plays an important role in Arabidopsis defense. Mol. Plant 4, 516–526 (2011).
Chen, Z. C., Peng, W. T., Li, J. & Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 74, 142–152 (2018).
Farhat, N. et al. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 38, 145 (2016).
Stec, B. Structural mechanism of Rubisco activation by carbamylation of the active site lysine. Proc. Natl Acad. Sci. USA 109, 18785–18790 (2012).
Hermans, C., Conn, S. J., Chen, J., Xiao, Q. & Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 5, 1170–1183 (2013).
Li, L., Tutone, A. F., Drummond, R. S., Gardner, R. C. & Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 13, 2761–2775 (2001).
Saito, T. et al. Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol. 54, 1673–1683 (2013).
Sun, Y., Yang, R., Li, L. & Huang, J. The magnesium transporter MGT10 is essential for chloroplast development and photosynthesis in Arabidopsis thaliana. Mol. Plant 10, 1584–1587 (2017).
Chen, Z. C., Yamaji, N., Motoyama, R., Nagamura, Y. & Ma, J. F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 159, 1624–1633 (2012).
Chen, Z. C. et al. A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiol. 174, 1837–1849 (2017).
Zhang, L. D., Peng, Y. Y., Li, J., Tian, X. Y. & Chen, Z. C. OsMGT1 confers resistance to magnesium deficiency by enhancing the import of Mg in rice. Int. J. Mol. Sci. 20, 207 (2019).
Cakmak, I. & Kirkby, E. A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 133, 692–704 (2008).
Peng, Y. Y. et al. Magnesium deficiency triggers SGR-mediated chlorophyll degradation for magnesium remobilization. Plant Physiol. 181, 262–275 (2019).
Kucharski, L. M., Lubbe, W. J. & Maguire, M. E. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 275, 16767–16773 (2000).
Li, H. et al. Molecular and functional characterization of the magnesium transporter gene ZmMGT12 in maize. Gene 665, 167–173 (2018).
Maeshima, K. et al. A transient rise in free Mg2+ ions released from ATP–Mg hydrolysis contributes to mitotic chromosome condensation. Curr. Biol. 28, 444–451 (2018).
Finazzi, G. et al. Ions channels/transporters and chloroplast regulation. Cell Calcium 58, 86–97 (2014).
Kikuchi, S. et al. Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301, 376–379 (2003).
Izumi, M. et al. Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation. Plant Physiol. 167, 1307–1320 (2015).
Greenham, K. & McClung, C. R. Integrating circadian dynamics with physiological processes in plants. Nat. Rev. Genet. 16, 598–610 (2015).
Ishijima, S., Uchibori, A., Takagi, H., Maki, R. & Ohnishi, M. Light-induced increase in free Mg2+ concentration in spinach chloroplasts: measurement of free Mg2+ by using a fluorescent probe and necessity of stromal alkalinization. Arch. Biochem. Biophys. 412, 126–132 (2003).
Martí Ruiz, M. C., Jung, H. J. & Webb, A. A. R. Circadian gating of dark-induced increases in chloroplast- and cytosolic-free calcium in Arabidopsis. New Phytol. 225, 1993–2005 (2019).
Pottosin, I. & Shabala, S. Transport across chloroplast membranes: optimizing photosynthesis for adverse environmental conditions. Mol. Plant 9, 356–370 (2016).
Schneider, A. et al. The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 28, 892–910 (2016).
Zhang, B. et al. Inner envelope CHLOROPLAST MANGANESE TRANSPORTER 1 supports manganese homeostasis and phototrophic growth in Arabidopsis. Mol. Plant 11, 943–954 (2018).
Yamagami, R., Bingaman, J. L., Frankel, E. A. & Bevilacqua, P. C. Cellular conditions of weakly chelated magnesium ions strongly promote RNA stability and catalysis. Nat. Commun. 9, 2149 (2018).
Vothknecht, U. C. & Soll, J. Chloroplast membrane transport: interplay of prokaryotic and eukaryotic traits. Gene 354, 99–109 (2005).
Parry, M. A. et al. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 64, 717–730 (2013).
Kubis, A. & Bar-Even, A. Synthetic biology approaches for improving photosynthesis. J. Exp. Bot. 70, 1425–1433 (2019).
Ma, X. et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 8, 1274–1284 (2015).
Yamaji, N. & Ma, J. F. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 143, 1306–1313 (2007).
Long, S. P. & Bernacchi, C. J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 54, 2393–2401 (2003).
Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Andrews, J. R., Bredenkamp, G. J. & Baker, N. R. Evaluation of the role of state transitions in determining the efficiency of light utilisation for CO2 assimilation in leaves. Photosynth. Res. 38, 15–26 (1993).
Nelson, D. L. & Kennedy, E. P. Magnesium transport in Escherichia coli inhibition by cobaltous ion. J. Biol. Chem. 246, 3042–3049 (1971).
Yokosho, K., Yamaji, N. & Ma, J. F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 68, 1061–1069 (2011).
Abramoff, M. D., Magalhaes, P. J. & Ram, S. J. Image processing with ImageJ. Biophotonics Intern. 11, 36–42 (2004).
Field, A. Discovering Statistics Using IBM SPSS Statistics 4th edn (SAGE Publications Ltd., 2013).
Kunst, L. Preparation of physiologically active chloroplasts from Arabidopsis. Methods Mol. Biol. 82, 43–48 (1998).
Lan, Y. & Mott, K. A. Determination of apparent K m values for ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase using the spectrophotometric assay of Rubisco activity. Plant Physiol. 95, 604–609 (1991).
Kalderon, D., Richardson, W. D., Markham, A. F. & Smith, A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 311, 33–38 (1984).
Acknowledgements
We thank L. Li for providing the MM281 strain and the pTrc99A and AtMGT10-pTrc99A vectors. We thank T. Nagai for providing MARIO. We also thank R. Gardner for providing the CM66 strain and Y. Liu for providing pYLsgRNA-U6a. We further thank M. Qu for help with guiding the ΔpH measurements, and T. Walk and Golden Fidelity LLC for language editing. This work is financially supported by the China National Key Program for Research and Development (grant no. 2016YFD0100700 to Z.C.C. and H.L.) and the National Natural Science Foundation of China (grant no. 31672218 to Z.C.C.), and by a Grant-in-Aid for Specially Promoted Research (JSPS KAKENHI grant no. 16H06296 to J.F.M.).
Author information
Authors and Affiliations
Contributions
J.F.M. and Z.C.C. conceived and designed the experiments. J.L., K.Y., S.L., H.R.C., N.Y., J.F.M. and Z.C.C. performed the experiments. J.L., K.Y., S.L. and Z.C.C. analysed the data. J.L., J.F.M. and Z.C.C. wrote the article. X.G.Z. and H.L. gave critical comments and revisions to the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
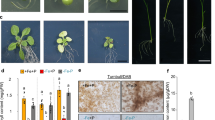
Extended Data Fig. 1 Photosynthetic parameters of rice in response to Mg depletion.
a, Net photosynthetic rate (An). b, Maximum rates of Rubisco carboxylation (Vc,max). c, Electron transport rate. d, Fv / Fm. e, SPAD value. 38-d-old seedlings of Dongjin (WT1) were grown in hydroponics with (250 μM) or without (0 μM) MgCl2 for 15 d, and photosynthetic parameters of the youngest fully-expanded leaves were measured dynamically by a portable photosynthesis system (LI-6800, Li-Cor). The SPAD value was measured using a chlorophyll meter (SPAD-502 Plus). Data are means ± s.d. Biologically independent replicates (n) are shown in each figure.
Extended Data Fig. 2 Inhibition of chloroplast 25Mg uptake by CHA.
a, Chloroplast 25Mg uptake in response to Mg depletion. Intact chloroplasts were isolated from WT (cv. Dongjin) after grown with or without Mg for 12 d, and exposed to 500 μM 25Mg with (+) or without (-) 1 mM CHA at 30 °C for 30 min. b, Comparison of chloroplast 25Mg uptake in WT and mutant. Intact chloroplasts from WT1 and osmgt3-1 were exposed to 500 μM 25Mg with (+) or without (−) 1 mM CHA at 30 °C for 30 min. 25Mg was determined by ICP-MS. Data are means ± s.d. n = 3 biologically independent replicates. Different letters indicate significant differences (P ≤ 0.05) using Tukey’s test.
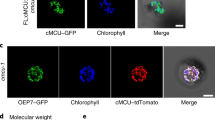
Extended Data Fig. 3 Gene expression, structure and protein expression of OsMGT3.
a, Rhythmical expression of a marker gene (Os03g0586500) encoding a chloroplast post-illumination chlorophyll fluorescence increase protein in leaf blade. b, Expression of OsMGT9 (MRS2-5; Os03g0137700) in leaf blade over daily cycle. c, Gene structure of OsMGT3. T-DNA insertion site and CRIPSR/Cas9 editing site were indicated with triangles. d, Expression of OsMGT3 in the leaf blade of the wild-type rice (WT1) and knockout line (osmgt3-1). Leaf blades were sampled every 6 h for 2 d. e, Immunoblot assay in rice chloroplasts. Chloroplasts at time point 0, 6, 12, 18 (refer to annotation in a) from pOsMGT3:OsMGT3-GFP transgenic lines were harvested after growing with 250 μM Mg for 50 d. RbcL, Rubisco large subunit. A representative result of 3 repeated tests is shown. f, Relative protein quantification at time point 0, 6, 12 and 18 by Image J software. Data are means ± s.d. n = 3 biologically independent seedlings.
Extended Data Fig. 4 Elemental analysis in rice chloroplasts and Mg concentration in rice leaves.
a, Percentage difference [(element content in mutant - element content in WT) / element content in WT] of 9 elements in chloroplasts of osmgt3-1 compared with WT1, and osmgt3-2 compared with WT2. The tracer elements Rb (10 μM) and Sr (10 μM) were added into the nutrient solution at one day before sampling. b, Mg concentration in leaf blade. 50-d-old seedlings of WTs and mutants were harvested every 6 h for 2 d under light (12 h) / dark (12 h) conditions. The intact chloroplasts in each time point were isolated, washed and digested for elemental determination. The leaf blades in each time point were harvested, dried and digested for Mg determination. All elements were determined by ICP-MS. Data are means ± s.d. n = 3 biologically independent replicates. Asterisks indicate significant differences compared with WTs. 0.001 < **P ≤ 0.01, ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Extended Data Fig. 5 FRET-based Mg2+ imaging in rice protoplast.
a, b, Responses of stroma (a) or nucleus (b) targeted MARIO to dawn and dusk. Bars = 10 μm. c-d, Intensity ratio of Venus to ECFP. The boxes indicate the first and third quartiles and the whiskers indicate the minimum to maximum values, and the lines within the boxes indicate the median values. n = 15 independent protoplasts, and the representative results are shown in a-b. 14-d-old WT2 (cv. Nipponbare) and osmgt3-2 mutant seedlings grown in +Mg (250 μM) conditions were used for protoplast isolation. For signal observation at dawn (9 o’clock), protoplasts were isolated and introduced with sMARIO (stroma-targeted) or nMARIO (nucleus-targeted) at 18 o’clock one day ahead. For signal observation at dusk (21 o’clock), protoplasts were isolated and introduced with sMARIO or nMARIO at 6 o’clock the same day. For FRET imaging, the 458 nm argon laser was used for excitation of ECFP (480 ± 12.5 nm for emission) and VENUS (535 ± 12.5 nm for emission). Chlorophyll signals were observed at 633 nm. Asterisks in (c) indicate significant differences. ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Extended Data Fig. 6 Phenotypic analysis in osmgt3 mutants.
a, Assay of a pH difference (ΔpH) across thylakoid membrane in osmgt3 mutants at day time. b, Assay of ΔpH at night time. 38-d-old seedlings of WTs and mutants were grown in hydroponics with (250 μM) or without (0 μM) MgCl2 for 12 d. After dark adaptation overnight, the youngest fully-expanded leaves were used for ΔpH measurement using a chlorophyll fluorometer (DUAL-PAM 100). c, Dry weight. d, Tiller number. e, Plant height. f, SPAD value. g, Fv / Fm. h, Stomatal conductance. i, Transpiration rate. 50-d-old seedlings of both WTs and osmgt3 mutants in hydroponics were harvested. The tiller number was counted and the plant height was measured by a ruler. The SPAD value of the youngest fully-expanded leaf was measured using a chlorophyll meter (SPAD-502 Plus). The plant dry weight was measured after drying in a 65 °C oven for 1 week. Fv / Fm, stomatal conductance, and transpiration rate were measured by a portable photosynthesis system (LI-6800, Li-Cor). Data are means ± s.d. Biologically independent replicates (n) are shown in each bar graph. Asterisks in b-e and g indicate significant differences compared with WTs. 0.01 < *P ≤ 0.05, 0.001 < **P ≤ 0.01, ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Extended Data Fig. 7 Complementation test of osmgt3 mutant.
a, Growth of 50-d-old rice seedlings of WT2, osmgt3-2 mutant and three independent complementation lines. Bars = 10 cm. b, Dry weight. c, Net photosynthetic rate (An). d, Maximum rate of Rubisco carboxylation (Vc,max). An and Vc,max was determined in the youngest fully-expanded leaves by a portable photosynthesis system (LI-6800, Li-Cor). e, Δ25Mg uptake in chloroplasts. Intact chloroplasts were exposed to 500 μM 25Mg at 30 °C for 30 min. Biologically independent replicates (n) are shown in each bar graph. 25Mg was determined by ICP-MS. Data are means ± s.d. Asterisks in (b-e) indicate significant differences compared with WT. 0.01 < *P ≤ 0.05, 0.001 < **P ≤ 0.01, ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Extended Data Fig. 8 Phenotypic analysis of mesophyll−specific overexpression lines.
a, Mg content in rice chloroplasts. 50-d-old seedlings of WTs and three overexpression lines grown in sufficient Mg (250 μM) conditions were harvested every 6 h for 2 d under 12 h light / 12 h dark conditions. Mg content in chloroplasts of the youngest fully-expanded leaves were measured. Data are means ± s.d. b, Relative gene expression of OsMGT3 at dusk. Expression relative to WT is shown. The relative expression level was determined by real-time RT-PCR. c, Maximum rate of Rubisco carboxylation (Vc,max) at dusk. d, Fv/Fm. e, Transpiration rate. f, Stomatal conductance. The boxes in b–f indicate the first and third quartiles and the whiskers indicate the minimum to maximum values, and the lines within the boxes indicate the median values. Biologically independent replicates (n) are shown in each figure. All parameters were measured in the youngest fully-expanded leaves of WT and overexpression lines by a portable photosynthesis system (LI-6800, Li-Cor). Asterisks in (b, f) indicate significant differences compared with WT. 0.01 < *P ≤ 0.05, ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Extended Data Fig. 9 Photosynthetic enhancement in pOsRbcS2-OsMGT3 overexpression lines.
a–c, Improved growth of rice seedlings by overexpressing OsMGT3 in mesophyll cells. a, Growth of three independent overexpression lines and their corresponding WT (NC, negative control) with sufficient Mg (250 μM) supply. NC1, OE1, OE2 are Dongjin background. NC2 and OE3 are Nipponbare background. Bars = 10 cm. b, Total fresh weight. c, Relative gene expression of OsMGT3. Expression relative to WT is shown. The relative expression level was determined by real-time RT-PCR. d, Net photosynthetic rate (An). e, f, Maximum rate of Rubisco carboxylation (Vc,max) at day (e) and night (f) time. g, Fv/Fm. h, Transpiration rate. i, Stomatal conductance. The boxes in b-i indicate the first and third quartiles and the whiskers indicate the minimum to maximum values, and the lines within the boxes indicate the median values. Biologically independent replicates (n) are shown in each figure. All parameters were measured in the youngest fully-expanded leaves of WT and overexpression lines by a portable photosynthesis system (LI-6800, Li-Cor). Asterisks in (b-e, h-i) indicate significant differences compared with WTs. 0.01 < *P ≤ 0.05, 0.001 < **P ≤ 0.01, ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Extended Data Fig. 10 Photosynthetic efficiency affected by Mg availably.
a–l, Net photosynthetic rate (An). 21-d-old rice seedlings of WTs and osmgt3 mutants, as well as pOsPsaE-OsMGT3 overexpression lines and their corresponding WT were treated with 10 (a–c), 20 (d–f), 250 (g–i) or 1000 (j–l) μM Mg for 22 days. An was recorded at day 0, 7, 10, 14, 18, and 22 by a portable photosynthesis system (LI-6800, Li-Cor). m, Total fresh weight at day 22. Data are means ± s.d. Biologically independent replicates (n) are shown in each figure. Asterisks in (m) indicate significant differences compared with their respective WT. 0.01 < *P ≤ 0.05, 0.001 < **P ≤ 0.01, ***P ≤ 0.001 by Student’s t-test, two-tailed t-test.
Supplementary information
Supplementary Table 1
List of primers used in this study.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Li, J., Yokosho, K., Liu, S. et al. Diel magnesium fluctuations in chloroplasts contribute to photosynthesis in rice. Nat. Plants 6, 848–859 (2020). https://doi.org/10.1038/s41477-020-0686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0686-3
This article is cited by
-
Metabolite interactions in the bacterial Calvin cycle and implications for flux regulation
Communications Biology (2023)
-
Effects of magnesium application on the arbuscular mycorrhizal symbiosis in tomato
Symbiosis (2023)
-
New insights into comprehensive analysis of magnesium transporter (MGT) gene family in rice (Oryza sativa L.)
3 Biotech (2023)
-
Conserved mechanism for vacuolar magnesium sequestration in yeast and plant cells
Nature Plants (2022)
-
The circadian clock ticks in plant stress responses
Stress Biology (2022)