Abstract
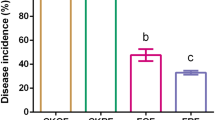
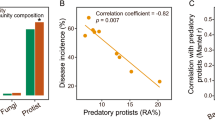
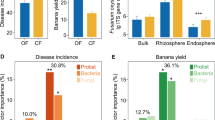
Reduced insect pest populations found on long-term organic farms have mostly been attributed to increased biodiversity and abundance of beneficial predators, as well as to changes in plant nutrient content. However, the role of plant resistance has largely been ignored. Here, we determine whether host plant resistance mediates decreased pest populations in organic systems and identify potential underpinning mechanisms. We demonstrate that fewer numbers of leafhoppers (Circulifer tenellus) settle on tomatoes (Solanum lycopersicum) grown using organic management as compared to conventional. We present multiple lines of evidence, including rhizosphere soil microbiome sequencing, chemical analysis and transgenic approaches, to demonstrate that changes in leafhopper settling between organically and conventionally grown tomatoes are dependent on salicylic acid accumulation in plants and mediated by rhizosphere microbial communities. These results suggest that organically managed soils and microbial communities may play an unappreciated role in reducing plant attractiveness to pests by increasing plant resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support these findings are available from C.L.C., A.G. and R.L.V. upon request. The raw sequencing dataset is available at the NCBI SRA data repository under the project accession number PRJNA539989.
References
Verbruggen, E. et al. Positive effects of organic farming on below-ground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 186, 968–979 (2010).
Lori, M., Symnaczik, S., Mäder, P., De Deyn, G. & Gattinger, A. Organic farming enhances soil microbial abundance and activity—a meta-analysis and meta-regression. PLoS ONE 12, e0180442 (2017).
Lupatini, M., Korthals, G. W., de Hollander, M., Janssens, T. K. S. & Kuramae, E. E. Soil microbiome is more heterogeneous in organic than in conventional farming system. Front. Microbiol. 7, 2064 (2016).
Gosling, P., Hodge, A., Goodlass, G. & Bending, G. D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 113, 17–35 (2006).
Reganold, J. P. & Wachter, J. M. Organic agriculture in the twenty-first century. Nat. Plants 2, 15221 (2016).
Shennan, C. et al. Organic and conventional agriculture: a useful framing? Annu. Rev. Environ. Resour. 42, 317–346 (2017).
Seufert, V. & Ramankutty, N. Many shades of gray—the context-dependent performance of organic agriculture. Sci. Adv. 3, e1602638 (2017).
Crowder, D. W., Northfield, T. D., Strand, M. R. & Snyder, W. E. Organic agriculture promotes evenness and natural pest control. Nature 466, 109–112 (2010).
Lichtenberg, E. M. et al. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob. Change Biol. 23, 4946–4957 (2017).
Muneret, L. et al. Evidence that organic farming promotes pest control. Nat. Sustain. 1, 361–368 (2018).
Garratt, M. P. D., Wright, D. J. & Leather, S. R. The effects of farming system and fertilisers on pests and natural enemies: a synthesis of current research. Agric. Ecosyst. Environ. 141, 261–270 (2011).
Drinkwater, L., Letourneau, D., Workneh, F., van Bruggen, A. & Shennan, C. Fundamental differences between conventional and organic tomato agroecosystems in California. Ecol. Appl. 5, 1098–1112 (1995).
Hole, D. G. et al. Does organic farming benefit biodiversity? Biol. Conserv. 122, 113–130 (2005).
Mattson, W. J. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11, 119–161 (1980).
Megali, L., Glauser, G. & Rasmann, S. Fertilization with beneficial microorganisms decreases tomato defenses against insect pests. Agron. Sustain. Dev. 34, 649–656 (2014).
Hartmann, M., Frey, B., Mayer, J., Mäder, P. & Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 9, 1177–1194 (2015).
Schmidt, J. E. et al. Effects of Agricultural Management on Rhizosphere Microbial Structure and Function in Processing Tomato Plants. Appl. Environ. Microbiol. 85, e01064-19 (2019).
Vannette, R. L. & Hunter, M. D. Mycorrhizal fungi as mediators of defence against insect pests in agricultural systems. Agric. Entomol. 11, 351–358 (2009).
Pineda, A., Zheng, S.-J., van Loon, J. J. A., Pieterse, C. M. J. & Dicke, M. Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci. 15, 507–514 (2010).
Berendsen, R. L., Pieterse, C. M. J. & Bakker, P. A. H. M. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486 (2012).
Pozo, M. J. & Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 10, 393–398 (2007).
Vannette, R. L. & Hunter, M. D. Plant defence theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. J. Ecol. 99, 66–76 (2010).
Fritz, M., Jakobsen, I., Lyngkjær, M. F., Thordal-Christensen, H. & Pons-Kühnemann, J. Arbuscular mycorrhiza reduces susceptibility of tomato to Alternaria solani. Mycorrhiza 16, 413–419 (2006).
Kempel, A., Schmidt, A. K., Brandl, R. & Schädler, M. Support from the underground: induced plant resistance depends on arbuscular mycorrhizal fungi. Funct. Ecol. 24, 293–300 (2010).
Pineda, A., Kaplan, I. & Bezemer, T. M. Steering soil microbiomes to suppress aboveground insect pests. Trends Plant Sci. 22, 770–778 (2017).
Pangesti, N., Pineda, A., Pieterse, C. M. J., Dicke, M. & Van Loon, J. J. A. Two-way plant mediated interactions between root-associated microbes and insects: from ecology to mechanisms. Front. Plant Sci. 4, 414 (2013).
Katayama, N., Zhang, Z. Q. & Ohgushi, T. Community-wide effects of below-ground rhizobia on above-ground arthropods. Ecol. Entomol. 36, 43–51 (2011).
De Souza, R., Ambrosini, A. & Passaglia, L. M. P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38, 401–419 (2015).
Chen, L.-F., Batuman, O., Aegerter, B. J., Willems, J. & Gilbertson, R. L. First report of curly top disease of pepper and tomato in California caused by the spinach curly top strain of beet curly top virus. Plant Dis. 101, 1334 (2017).
Erb, M., Meldau, S. & Howe, G. A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17, 250–259 (2012).
Bari, R. & Jones, J. D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488 (2009).
Prudic, K. L., Oliver, J. C. & Bowers, M. D. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143, 578–587 (2005).
Lu, Z., Yu, X., Heong, K. & Hu, C. Effect of nitrogen fertilizer on herbivores and its stimulation to major insect pests in rice. Rice Sci. 14, 56–66 (2007).
Pieterse, C. M. J. et al. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375 (2014).
Wolf, K. M. et al. The century experiment: the first twenty years of UC Davis’ Mediterranean agroecological experiment. Ecology 99, 503 (2018).
Walling, L. L. Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol. 146, 859–866 (2008).
Kaloshian, I. & Walling, L. L. Hemipterans as plant pathogens. Annu. Rev. Phytopathol. 43, 491–521 (2005).
Rodríguez-Álvarez, C., López-Climent, M. F., Gómez-Cadenas, A., Kaloshian, I. & Nombela, G. Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull. Entomol. Res. 105, 574–582 (2015).
Ellis, C., Karafyllidis, L. & Turner, J. G. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant–Microbe Interact. 15, 1025–1030 (2002).
Kloth, K. J. et al. AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J. Exp. Bot. 67, 3383–3396 (2016).
Cui, J. et al. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl Acad. Sci. USA 102, 1791–1796 (2005).
Barber, N. A., Kiers, E. T., Theis, N., Hazzard, R. V. & Adler, L. S. Linking agricultural practices, mycorrhizal fungi, and traits mediating plant–insect interactions. Ecol. Appl. 23, 1519–1530 (2013).
Nakano, M. & Mukaihara, T. Ralstonia solanacearum type III effector RipAL targets chloroplasts and induces jasmonic acid production to suppress salicylic acid-mediated defense responses in plants. Plant Cell Physiol. 59, 2576–2589 (2018).
Baichoo, Z. & Jaufeerally-Fakim, Y. Ralstonia solanacearum upregulates marker genes of the salicylic acid and ethylene signaling pathways but not those of the jasmonic acid pathway in leaflets of Solanum lines during early stage of infection. Eur. J. Plant Pathol. 147, 615–625 (2017).
Berg, M. & Koskella, B. Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 28, 2487–2492 (2018).
Blubaugh, C. K., Carpenter-Boggs, L., Reganold, J. P., Schaeffer, R. N. & Snyder, W. E. Bacteria and competing herbivores weaken top-down and bottom-up aphid suppression. Front. Plant Sci. 9, 1239 (2018).
Heinen, R. et al. Species-specific plant–soil feedbacks alter herbivore-induced gene expression and defense chemistry in Plantago lanceolata. Oecologia 188, 801–811 (2018).
Bastías, D. A. et al. Jasmonic acid regulation of the anti-herbivory mechanism conferred by fungal endophytes in grasses. J. Ecol. 106, 2365–2379 (2018).
Gilbert, L. & Johnson, D. Plant-mediated ‘apparent effects’ between mycorrhiza and insect herbivores. Curr. Opin. Plant Biol. 26, 100–105 (2015).
Howard, M. M., Kao-Kniffin, J. & Kessler, A. Shifts in plant–microbe interactions over community succession and their effects on plant resistance to herbivores. New Phytol. 226, 1144–1157 (2020).
Brading, P. A., Hammond-Kosack, K. E., Parr, A. & Jones, J. D. G. Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J. 23, 305–318 (2000).
Lightner, J., Pearce, G., Ryan, C. A. & Browse, J. Isoaltion of signalling mutants of tomato (Lycopersicon esculentum). Mol. Genet. Genomics 241, 595–601 (1993).
Casteel, C. L. et al. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 169, 209–218 (2015).
Microchemical Determination of Carbon, Hydrogen, and Nitrogen AOAC 972.43-1975 (AOAC Official Method, 2006).
Knepel, K. Determination of nitrate in 2M KCl soil extracts by flow injection analysis. QuikChem Method 12, 107 (2003).
Olsen, S. R. & Sommers, L. E. in Methods of Soil Analysis. Part 2 Chemical and Microbiological Properties 2nd edn (eds Page, A. et al.) 403–427 (ASA and SSSA, 1982).
Nelson, D.W. & Sommers, L.E. in Methods of Soil Analysis. Part 3. Chemical Methods (eds Sparks, D. L. et al.) 961–1010 (SSSA and ASA, 1996).
Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis (CRC Press, 2001).
Walters, W. et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1, e00009-15 (2016).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Glöckner, F. O. et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J. Biotechnol. 261, 169–176 (2017).
Kõljalg, U. et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 22, 5271–5277 (2013).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Oksanen, J. et al. VEGAN: Community Ecology Package v.2.5-4 (2018).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550–571 (2014).
RStudio Team RStudio: Integrated Development for R v.1.0.136 (2015).
Acknowledgements
We thank F. Bender, J. Richardy, G. Hall, S. Tookey, University of California Cooperative Extension (UCCE) farm advisors, Russell Ranch staff and growers for participating in this study and assisting with sampling. This work was supported by start-up funds from UC Davis to C.L.C., A.G. and R.L.V.; the California Tomato Research Institute to A.G., C.L.C. and R.L.V.; the California Potato Research Advisory Board to C.L.C. and the USDA-NIFA, Agricultural Experiment Station Projects no. CA-D-PPA-2297-H to C.L.C., no. CA-D-PLS-2332-H to A.G. and no. CA-D-ENM-2354-RR to R.L.V.
Author information
Authors and Affiliations
Contributions
R.B., A.L.C., J.E.S, C.L.C, R.L.V. and A.I. conducted most of the experiments and analysis. C.L.C., A.G. and R.L.V. designed all experiments and directed the project. R.B., C.L.C., A.G. and R.L.V. wrote the paper with comments and input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental Information
Supplementary Tables 1–5 and Figs. 1–3.
Rights and permissions
About this article
Cite this article
Blundell, R., Schmidt, J.E., Igwe, A. et al. Organic management promotes natural pest control through altered plant resistance to insects. Nat. Plants 6, 483–491 (2020). https://doi.org/10.1038/s41477-020-0656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0656-9
This article is cited by
-
Soil microbes from conservation agriculture systems reduce growth of Bt-resistant western corn rootworm larvae
Journal of Pest Science (2024)
-
Intercropping-induced leaf metabolic changes increase plant resistance to herbivory
Plant and Soil (2024)
-
Feeding experience on hairy vetch induces a loss of host-specific adaptation to maize and changes in the gut microbiota communities of the fall armyworm
Journal of Pest Science (2024)
-
Soil conditions and the plant microbiome boost the accumulation of monoterpenes in the fruit of Citrus reticulata ‘Chachi’
Microbiome (2023)
-
Plant pathogen resistance is mediated by recruitment of specific rhizosphere fungi
The ISME Journal (2023)