Abstract
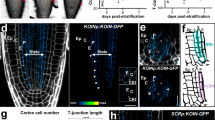
During lateral root initiation, lateral root founder cells undergo asymmetric cell divisions that generate daughter cells with different sizes and fates, a prerequisite for correct primordium organogenesis. An excess of the GLV6/RGF8 peptide disrupts these initial asymmetric cell divisions, resulting in more symmetric divisions and the failure to achieve lateral root organogenesis. Here, we show that loss-of-function GLV6 and its homologue GLV10 increase asymmetric cell divisions during lateral root initiation, and we identified three members of the RGF1 INSENSITIVE/RGF1 receptor subfamily as likely GLV receptors in this process. Through a suppressor screen, we found that MITOGEN-ACTIVATED PROTEIN KINASE6 is a downstream regulator of the GLV pathway. Our data indicate that GLV6 and GLV10 act as inhibitors of asymmetric cell divisions and signal through RGF1 INSENSITIVE receptors and MITOGEN-ACTIVATED PROTEIN KINASE6 to restrict the number of initial asymmetric cell divisions that take place during lateral root initiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings in this study are available from the corresponding author upon reasonable request.
References
Moreno-Risueno, M. A. et al. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329, 1306–1311 (2010).
Xuan, W. et al. Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science 351, 384–387 (2016).
De Smet, I. et al. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322, 594–597 (2008).
De Smet, I. et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl Acad. Sci. USA 107, 2705–2710 (2010).
De Rybel, B. et al. A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 20, 1697–1706 (2010).
Hofhuis, H. et al. Phyllotaxis and rhizotaxis in Arabidopsis are modified by three PLETHORA transcription factors. Curr. Biol. 23, 956–962 (2013).
Casimiro, I. et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852 (2001).
Lucas, M. et al. Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proc. Natl Acad. Sci. USA 110, 5229–5234 (2013).
von Wangenheim, D. et al. Rules and self-organizing properties of post-embryonic plant organ cell division patterns. Curr. Biol. 26, 439–449 (2016).
Meng, L., Buchanan, B. B., Feldman, L. J. & Luan, S. CLE-like (CLEL) peptides control the pattern of root growth and lateral root development in Arabidopsis. Proc. Natl Acad. Sci. USA 109, 1760–1765 (2012).
Fernandez, A. et al. Transcriptional and functional classification of the GOLVEN/ROOT GROWTH FACTOR/CLE-like signaling peptides reveals their role in lateral root and hair formation. Plant Physiol. 161, 954–970 (2013).
Fernandez, A. et al. The GLV6/RGF8/CLEL2 peptide regulates early pericycle divisions during lateral root initiation. J. Exp. Bot. 66, 5245–5256 (2015).
Peterson, B. A. et al. Genome-wide assessment of efficiency and specificity in CRISPR/Cas9 mediated multiple site targeting in Arabidopsis. PLoS ONE 11, e0162169 (2016).
Komori, R., Amano, Y., Ogawa-Ohnishi, M. & Matsubayashi, Y. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc. Natl Acad. Sci. USA 106, 15067–15072 (2009).
Zhou, W. et al. Arabidopsis tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell 22, 3692–3709 (2010).
Matsuzaki, Y., Ogawa-Ohnishi, M., Mori, A. & Matsubayashi, Y. Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science 329, 1065–1067 (2010).
Whitford, R. et al. GOLVEN secretory peptides regulate auxin carrier turnover during plant gravitropic responses. Dev. Cell 22, 678–685 (2012).
Wu, T. et al. An Arabidopsis thaliana copper-sensitive mutant suggests a role of phytosulfokine in ethylene production. J. Exp. Bot. 66, 3657–3667 (2015).
Lopez-Bucio, J. S. et al. Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. J. Exp. Bot. 65, 169–183 (2014).
Xu, J. & Zhang, S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20, 56–64 (2015).
Ditengou, F. A. et al. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 105, 18818–18823 (2008).
Laskowski, M. et al. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 6, e307 (2008).
Ortiz-Morea, F. A. et al. Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc. Natl Acad. Sci. USA 113, 11028–11033 (2016).
Song, W. et al. Signature motif-guided identification of receptors for peptide hormones essential for root meristem growth. Cell Res. 26, 674–685 (2016).
Ou, Y. et al. RGF1 INSENSITIVE 1 to 5, a group of LRR receptor-like kinases, are essential for the perception of root meristem growth factor 1 in Arabidopsis thaliana. Cell Res. 26, 686–698 (2016).
Shinohara, H., Mori, A., Yasue, N., Sumida, K. & Matsubayashi, Y. Identification of three LRR-RKs involved in perception of root meristem growth factor in Arabidopsis. Proc. Natl Acad. Sci. USA 113, 3897–3902 (2016).
Voss, U. et al. The circadian clock rephases during lateral root organ initiation in Arabidopsis thaliana. Nat. Commun. 6, 7641 (2015).
Peret, B. et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 14, 991–998 (2012).
Fukaki, H., Tameda, S., Masuda, H. & Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29, 153–168 (2002).
Okushima, Y. et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17, 444–463 (2005).
Benkova, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Dubrovsky, J. G. et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl Acad. Sci. USA 105, 8790–8794 (2008).
Cho, S. K. et al. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 105, 15629–15634 (2008).
Jewaria, P. K. et al. Differential effects of the peptides Stomagen, EPF1 and EPF2 on activation of MAP kinase MPK6 and the SPCH protein level. Plant Cell Physiol. 54, 1253–1262 (2013).
Turing, A. M. The chemical basis of morphogenesis. Phil. Trans. R. Soc. Lond. B 237, 37–72 (1952).
Torii, K. U. Two-dimensional spatial patterning in developmental systems. Trends Cell Biol. 22, 438–446 (2012).
Toyokura, K. et al. Lateral inhibition by a peptide hormone–receptor cascade during Arabidopsis lateral root founder cell formation. Dev. Cell 48, 64–75 (2019).
Kumpf, R. P. et al. Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proc. Natl Acad. Sci. USA 110, 5235–5240 (2013).
Zhu, Q. et al. A MAPK cascade downstream of IDA-HAE/HSL2 ligand–receptor pair in lateral root emergence. Nat. Plants 5, 414–423 (2019).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Barbez, E. et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485, 119–122 (2012).
Jun, J. et al. Comprehensive analysis of CLE polypeptide signaling gene expression and overexpression activity in Arabidopsis. Plant Physiol. 154, 1721–1736 (2010).
Schneeberger, K. et al. SHOREmap: simultaneous mapping and mutation identification by deep sequencing. Nat. Methods 6, 550–551 (2009).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schindelin, J., Arganda-Carreras, I. & Frise, E. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Malamy, J. E. & Benfey, P. N. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44 (1997).
Xuan, W., Opdenacker, D., Vanneste, S. & Beeckman, T. Long-term in vivo imaging of luciferase-based reporter gene expression in Arabidopsis roots. Methods Mol. Biol. 1761, 177–190 (2018).
Acknowledgements
This research was supported by FWO postdoctoral (A.F., grant no. 1293817N) and doctoral (J.J., grant no. 1168218N) fellowships, a China Scholarship Council grant (K.X., no. 201606350134) and a National Science Foundation Plant Genome Research Program Grant (Z.L.N., no. PGRP-1841917). We thank M. Njo for help with preparing the figures, V. Storme for guidance and assistance with the statistical analysis and D. Savatin for training with the MPK6 phosphorylation experiments.
Author information
Authors and Affiliations
Contributions
A.I.F. designed the project. A.I.F. generated the iGLV6 line, performed the EMS mutagenesis screen and analysed the EMS mutants’ identity with help from N.V., A.D., D.O. and T.M. A.I.F. and K.X. phenotypically characterized the CRISPR glv mutants, and generated and characterized the rgi mutants and reporter lines. N.V. generated and characterized the CRISPR glv6 mutants. N.V., K.X., J.J. and S.M.-H. phenotypically characterized the mpk6 mutants. J.J., S.M.-H. and H.D.G. characterized the cross-talk between the auxin and GLV pathways. Q.Y. generated the RGI4 reporter line. B. Parizot performed the in silico expression analysis. L.A.N.C., N.V. and E.R. performed the MPK6 phosphorylation experiments. B. Peterson and Z.L.N. generated the CRISPR glv mutants. K.H., W.V. and A.M. synthesized the peptides. J.J. and A.I.F. performed the statistical analysis. A.I.F., N.V. and T.B. wrote the manuscript with input from all authors. T.B. provided guidance and advice on the project, the experiments and the analysis of the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Melinka Butenko, Juan Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
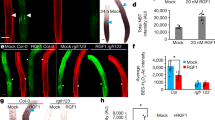
Extended Data Fig. 1 GLV6 and 10 act redundantly during LR initiation.
a–c, Phenotypic characterization of CRISPR glv6 mutants compared to wild type (8 dag, n = 12). Quantification of root length (a), all primordium stages density (b) and non-emerged primordia (NE) and emerged (E) LR density (c). d, Quantification of root length in the CRISPR glv mutant compared to wild type. e, Quantification of all primordium stages density in the glv6glv10 mutant germinated on MS or on 10 nM of GLV6p/GLV10p (8 dag). Charts show mean values ± s.d. (b, e) or s.e.m (c). Significant differences compared to wild type are shown and were determined using one-way ANOVA (a, d) or a GEE model (b-c, e). In e, only significant differences in stage I primordia are displayed. For full statistical analysis see Supplementary Table 2. n.s.: no significant differences were found between mutants and wild type. f, Example of nearby primordia frequently found in glv mutants. The lower picture shows a higher magnification image of the framed area in the upper picture for each genotype. Scale bars represent 50 μm.
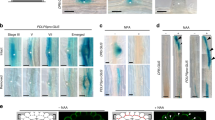
Extended Data Fig. 2 Suppression of the GLV6OE phenotype and LR defects in mpk6 mutants.
a, Suppression of the GLV6OE phenotype in mpk6 mutants after LR initiation was induced by gravistimulation of the primary root. This experiment was done three times with similar results. b, Quantification of all primordium stages in reported mpk6 mutants compared to wild type (8 dag). Chart represents mean values ± s.d. A GEE model was used. n.s.: no significant differences were found between mutants and wild type. For full statistical analysis see Supplementary Table 2. Scale bars represent 20 μm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Table 3 and methods.
Supplementary Tables 1 and 2
Table 1. Amino acid sequences predicted to be encoded in CRISPR glv6 or glv mutants. The GLV6 pre-propeptide is shown as a reference. The presumed mature GLV6 peptide sequence is highlighted in bold, and stop codons are depicted as asterisks. Amino acid sequences different from the corresponding GLV wild-type precursor are italicized in the mutants. Table 2. Statistical analysis of the primordia stages and LR density in glv and mpk6 mutants compared to controls. P values indicating significant differences are highlighted in green. See Methods for details on the statistical analysis.
Rights and permissions
About this article
Cite this article
Fernandez, A.I., Vangheluwe, N., Xu, K. et al. GOLVEN peptide signalling through RGI receptors and MPK6 restricts asymmetric cell division during lateral root initiation. Nat. Plants 6, 533–543 (2020). https://doi.org/10.1038/s41477-020-0645-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0645-z