Abstract
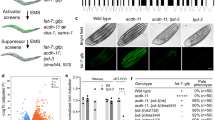
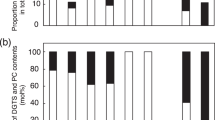
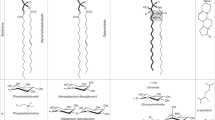
For plants, acclimation to low temperatures is fundamental to survival. This process involves the modification of lipids to maintain membrane fluidity. We previously identified a new cold-induced putative desaturase in Physcomitrium (Physcomitrella) patens. Lipid profiles of null mutants of this gene lack sphingolipids containing monounsaturated C24 fatty acids, classifying the new protein as sphingolipid fatty acid denaturase (PpSFD). PpSFD mutants showed a cold-sensitive phenotype as well as higher susceptibility to the oomycete Pythium, assigning functions in stress tolerance for PpSFD. Ectopic expression of PpSFD in the Atads2.1 (acyl coenzyme A desaturase-like 2) Arabidopsis thaliana mutant functionally complemented its cold-sensitive phenotype. While these two enzymes catalyse a similar reaction, their evolutionary origin is clearly different since AtADS2 is a methyl-end desaturase whereas PpSFD is a cytochrome b5 fusion desaturase. Altogether, we suggest that adjustment of membrane fluidity evolved independently in mosses and seed plants, which diverged more than 500 million years ago.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All moss mutants described here were deposited in the International Moss Stock Center (IMSC, http://www.moss-stock-center.org/). The IMSC numbers for the confirmed knockouts are 40561 (gKO25 3), 40568 (gKO25 10) and 40569 (gKO25 11). Source data are provided with this paper.
References
Smallwood, M. & Bowles, D. J. Plants in a cold climate. Philos. Trans. R. Soc. Lond. B 357, 831–847 (2002).
Barrero-Sicilia, C., Silvestre, S., Haslam, R. P. & Michaelson, L. V. Lipid remodelling: unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 263, 194–200 (2017).
Sperling, P., Ternes, P., Zank, T. K. & Heinz, E. The evolution of desaturases. Prostaglandins Leukot. Essent. Fatty Acids 68, 73–95 (2003).
Napier, J. A., Sayanova, O., Sperling, P. & Heinz, E. A growing family of cytochrome b5-domain fusion proteins. Trends Plant Sci. 4, 2–4 (1999).
Beike, A. K., Jaeger, C., Zink, F., Decker, E. & Reski, R. High contents of very long-chain polyunsaturated fatty acids in different moss species. Plant Cell Rep. 33, 245–254 (2014).
Mamode Cassim, A. et al. Plant lipids: key players of plasma membrane organization and function. Prog. Lipid Res. 73, 1–27 (2019).
Simon-Plas, F., Perraki, A., Bayer, E., Gerbeau-Pissot, P. & Mongrand, S. An update on plant membrane rafts. Curr. Opin. Plant Biol. 14, 642–649 (2011).
Nagano, M. et al. Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice. Plant Cell 28, 1966–1983 (2016).
Lenarčič, T. et al. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 358, 1431–1434 (2017).
Beike, A. K. et al. Insights from the cold transcriptome of Physcomitrella patens: global specialization pattern of conserved transcriptional regulators and identification of orphan genes involved in cold acclimation. New Phytol. 205, 869–881 (2015).
Lang, D. et al. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 93, 515–533 (2018).
Kajikawa, M. et al. Isolation and characterization of Δ6-desaturase, an ELO-like enzyme and Δ5-desaturase from the liverwort Marchantia polymorpha and production of arachidonic and eicosapentaenoic acids in the methylotrophic yeast Pichia pastoris. Plant Mol. Biol. 54, 335–352 (2004).
Chen, M., Markham, J. E. & Cahoon, E. B. Sphingolipid Δ8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis. Plant J. 69, 769–781 (2012).
Kaewsuwan, S. et al. Identification and functional characterization of the moss Physcomitrella patens Δ5-desaturase gene involved in arachidonic and eicosapentaenoic acid biosynthesis. J. Biol. Chem. 281, 21988–21997 (2006).
Hohe, A. et al. An improved and highly standardised transformation procedure allows efficient production of single and multiple targeted gene-knockouts in a moss, Physcomitrella patens. Curr. Genet. 44, 339–347 (2004).
Horst, N. A. et al. A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nat. Plants 2, 15209 (2016).
Tarazona, P., Feussner, K. & Feussner, I. An enhanced plant lipidomics method based on multiplexed liquid chromatography–mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling. Plant J. 84, 621–633 (2015).
Stukey, J. E., McDonough, V. M. & Martin, C. E. The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265, 20144–20149 (1990).
Shanklin, J. & Cahoon, E. B. Desaturation and related modifications of fatty acids. Ann. Rev. Plant Physiol. Plant Mol. Biol. 49, 611–641 (1998).
Francis, G. W. & Veland, K. Alkylthiolation for the determination of double-bond positions in linear alkenes. J. Chromatogr. A 219, 379–384 (1981).
Heilmann, I., Mekhedov, S., King, B., Browse, J. & Shanklin, J. Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol Δ7-desaturase gene FAD5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol. 136, 4237–4245 (2004).
Chen, M. & Thelen, J. J. ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 25, 1430–1444 (2013).
Smith, M. A. et al. Involvement of Arabidopsis ACYL-COENZYME A DESATURASE-LIKE2 (At2g31360) in the biosynthesis of the very-long-chain monounsaturated fatty acid components of membrane lipids. Plant Physiol. 161, 81–96 (2013).
Martin, F. N. & Loper, J. E. Soilborne plant diseases caused by Pythium spp.: ecology, epidemiology, and prospects for biological control. Crit. Rev. Plant Sci. 18, 111–181 (1999).
Mittag, J., Gabrielyan, A. & Ludwig-Müller, J. Knockout of GH3 genes in the moss Physcomitrella patens leads to increased IAA levels at elevated temperature and in darkness. Plant Physiol. Biochem. 97, 339–349 (2015).
Ponce de León, I. & Montesano, M. Adaptation mechanisms in the evolution of moss defenses to microbes. Front. Plant Sci. 8, 366 (2017).
Oliver, J. et al. Pythium infection activates conserved plant defense responses in mosses. Planta 230, 569–579 (2009).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2014).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Bhat, R. & Panstruga, R. Lipid rafts in plants. Planta 223, 5–19 (2005).
Degenkolbe, T. et al. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant J. 72, 972–982 (2012).
Goncalves, E. C., Wilkie, A. C., Kirst, M. & Rathinasabapathi, B. Metabolic regulation of triacylglycerol accumulation in the green algae: identification of potential targets for engineering to improve oil yield. Plant Biotechnol. J. 14, 1649–1660 (2016).
Benveniste, P. Biosynthesis and accumulation of sterols. Annu. Rev. Plant Biol. 55, 429–457 (2004).
Meesapyodsuk, D. & Qiu, X. The front-end desaturase: structure, function, evolution and biotechnological use. Lipids 47, 227–237 (2012).
Berkey, R., Bendigeri, D. & Xiao, S. Sphingolipids and plant defense/disease: the ‘death’ connection and beyond. Front. Plant Sci. 3, 68 (2012).
Sperling, P. & Heinz, E. Desaturases fused to their electron donor. Eur. J. Lipid Sci. Technol. 103, 158–180 (2001).
Burki, F., Roger, A. J., Brown, M. W. & Simpson, A. G. B. The new tree of eukaryotes. Trends Ecol. Evol. 35, 43–55 (2020).
Reski, R. Enabling the water-to-land transition. Nat. Plants 4, 67–68 (2018).
Roads, E., Longton, R. E. & Convey, P. Millennial timescale regeneration in a moss from Antarctica. Curr. Biol. 24, R222–R223 (2014).
Reski, R. & Abel, W. O. Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165, 354–358 (1985).
Knight, C. D., Cove, D. J., Cuming, A. C. & Quatrano, R. S. Moss gene technology. Mol. Plant Biol. 2, 285–301 (2002).
Hohe, A., Rensing, S. A., Mildner, M., Lang, D. & Reski, R. Day length and temperature strongly influence sexual reproduction and expression of a novel MADS-box gene in the moss Physcomitrella patens. Plant Biol. 4, 595–602 (2002).
Markham, N. R. & Zuker, M. in Bioinformatics: Structure, Function and Applications (ed. Keith, J. M.) 3–31 (Humana Press, 2008).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Markham, J. E., Li, J., Cahoon, E. B. & Jaworski, J. G. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 281, 22684–22694 (2006).
Buré, C. et al. Fast screening of highly glycosylated plant sphingolipids by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 25, 3131–3145 (2011).
Ternes, P. et al. Two pathways of sphingolipid biosynthesis are separated in the yeast Pichia pastoris. J. Biol. Chem. 286, 11401–11414 (2011).
Dunn, T. M., Lynch, D. V., Michaelson, L. V. & Napier, J. A. A post-genomic approach to understanding sphingolipid metabolism in Arabidopsis thaliana. Ann. Bot. 93, 483–497 (2004).
Barnes, J. D., Balaguer, L., Manrique, E., Elvira, S. & Davison, A. W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 32, 85–100 (1992).
Potter, S. C. et al. HMMER web server: 2018 update. Nucleic Acids Res. 46, W200–W204 (2018).
The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 (2016).
Li, F.-W. et al. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat. Plants 6, 259–272 (2020).
Lamesch, P. et al. The Arabidopsis information resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 40, D1202–D1210 (2011).
Li, F.-W. et al. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat. Plants 4, 460–472 (2018).
Moreau, H. et al. Gene functionalities and genome structure in Bathycoccus prasinos reflect cellular specializations at the base of the green lineage. Genome Biol. 13, R74 (2012).
Nishiyama, T. et al. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell 174, 448–464 (2018).
Merchant, S. S. et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 (2007).
Wang, S. et al. Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nat. Plants 6, 95–106 (2020).
Collén, J. et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl Acad. Sci. USA 110, 5247–5252 (2013).
Blanc, G. et al. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 13, R39 (2012).
Mock, T. et al. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 541, 536–540 (2017).
Schönknecht, G. et al. Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 339, 1207–1210 (2013).
Hori, K. et al. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 5, 3978 (2014).
Bowman, J. L. et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171, 287–304 (2017).
Cheng, S. et al. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 179, 1057–1067 (2019).
Ouyang, S. et al. The TIGR rice genome annotation resource: improvements and new features. Nucleic Acids Res. 35, D883–D887 (2006).
Palenik, B. et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc. Natl Acad. Sci. USA 104, 7705–7710 (2007).
Bowler, C. et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456, 239–244 (2008).
Nystedt, B. et al. The Norway spruce genome sequence and conifer genome evolution. Nature 497, 579–584 (2013).
Bhattacharya, D. et al. Genome of the red alga Porphyridium purpureum. Nat. Commun. 4, 1941 (2013).
Banks, J. A. et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332, 960–963 (2011).
Lommer, M. et al. Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genome Biol. 13, R66 (2012).
Armbrust, E. V. et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306, 79–86 (2004).
De Clerck, O. et al. Insights into the evolution of multicellularity from the sea lettuce genome. Curr. Biol. 28, 2921–2933 (2018).
Prochnik, S. E. et al. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329, 223–226 (2010).
Acknowledgements
We thank P. Meyer and S. Freitag (Göttingen) for technical assistance, D. Trautmann (Freiburg) for help with carotinoid profiling, A. Hartenhauer (Dresden) for help with the infection experiments, L. Mohnike (Göttingen) for help with the art work and T. Haslam (Göttingen) for critical reading of the manuscript. H.C.R. and J.G. were supported by the Microbiology and Biochemistry programme of Göttingen Graduate Center for Neurosciences, Biophysics, and Molecular Biosciences (GGNB). I.F. acknowledges funding through the German Research Foundation (DFG: INST 186/822-1, INST 186/1167-1 and ZUK 45/2010). R.R. acknowledges funding from the Excellence Initiative of the German Federal and State Governments (EXC 294 and CIBSS – EXC-2189 – Project ID 390939984). J.d.V. is supported through funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant no. 852725; ERC Starting Grant ‘TerreStriAL’).
Author information
Authors and Affiliations
Contributions
R.R., J. Markham, J.L.M. and I.F. designed the study. H.C.R., C.H., K.F., E.H., A.K.O. and J. Mittag undertook the experiments. H.C.R., C.H., K.F., J.L.M., J.G., J. Markham, J. Mittag, N.v.G., J.d.V., R.R. and I.F. analysed the data. H.C.R., K.F., J.d.V., R.R. and I.F. wrote the paper. All authors read, commented on and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Sebastien Mongrand, Jay Thelen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Tables 1 and 2.
Supplementary Data
Supplementary Fig. 2, uncropped and unprocessed full scans; Supplementary Fig. 4, growth rate in liquid media; Supplementary Fig. 5, fatty acid profile; Supplementary Fig. 6, carotenoid profiles from gametophores of P. patens; Supplementary Fig. 7, ceramide profile; Supplementary Figs. 8–12, detected lipid species (relative peak areas of all detected molecular lipid species); Supplementary Fig. 13, relative transcript levels of genes AtADS2 and PpSFD in different cold-stressed A. thaliana complementation lines; Supplementary Fig. 14, relative peak areas of all detected lipid molecular species of A. thaliana plants.
Source data
Source Data Fig. 2
Data sheet for a,b, detected lipid species (relative peak areas of all detected molecular lipid species); data sheet for c, Cer profiles of yeasts (ceramide profiles of transgenic S. cerevisiae lines).
Source Data Fig. 3
Data sheet for a, total lipid peak areas (absolute total peak areas of lipid class [cps] → fold changes); data sheet for b, detected lipid species (relative peak areas of all detected molecular lipid species → relative lipid amount regarding fatty acid composition).
Source Data Fig. 4
Chlorophyll content.
Source Data Fig. 5
Data sheet for a, cold-stress photos (images and weight of A. thaliana plants before and after cold stress.); data sheet for b, relative transcript levels of genes AtADS2 and PpSFD in different cold-stressed A. thaliana complementation lines; data sheet for c, SF 5.2 cold-stress lipid data (relative peak areas of all detected lipid molecular species of A. thaliana plants).
Source Data Fig. 6
Pythium infection.
Rights and permissions
About this article
Cite this article
Resemann, H.C., Herrfurth, C., Feussner, K. et al. Convergence of sphingolipid desaturation across over 500 million years of plant evolution. Nat. Plants 7, 219–232 (2021). https://doi.org/10.1038/s41477-020-00844-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-00844-3
This article is cited by
-
A deeply conserved protease, acylamino acid-releasing enzyme (AARE), acts in ageing in Physcomitrella and Arabidopsis
Communications Biology (2023)
-
Autopolyploidization affects transcript patterns and gene targeting frequencies in Physcomitrella
Plant Cell Reports (2022)
-
Effects of extraction parameters on lipid profiling of mosses using UPLC-ESI-QTOF-MS and multivariate data analysis
Metabolomics (2021)