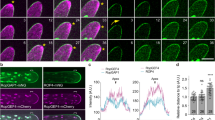
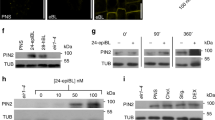
Abstract
Polar growth requires the precise tuning of Rho GTPase signalling at distinct plasma membrane domains. The activity of Rho of plant (ROP) GTPases is regulated by the opposing action of guanine nucleotide-exchange factors (GEFs) and GTPase-activating proteins (GAPs). Whereas plant-specific ROPGEFs have been shown to be embedded in higher-level regulatory mechanisms involving membrane-bound receptor-like kinases, the regulation of GAPs has remained enigmatic. Here, we show that three Arabidopsis ARMADILLO REPEAT ONLY (ARO) proteins are essential for the stabilization of growth sites in root hair cells and trichomes. AROs interact with ROP1 enhancer GAPs (RENGAPs) and bind to the plasma membrane via a conserved polybasic region at the ARO amino terminus. The ectopic spreading of ROP2 in aro2/3/4 mutant root hair cells and the preferential interaction of AROs with active ROPs and anionic phospholipids suggests that AROs recruit RENGAPs into complexes with ROPs to confine ROP signalling to distinct membrane regions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All of the main data supporting the findings of this study are available within the article and its Supplementary Information files. Materials used in this study are available on request from the corresponding author. The Arabidopsis gene identifiers for the genes described here are as follows: At4g34940 (ARO1), At5g66200 (ARO2), At4g36030 (ARO3), At3g26600 (ARO4), At4g24580 (REN1), At5g12150 (REN2/PHGAP1), At5g19390 (REN3/PHGAP2), At3g51300 (ROP1), At1G20090 (ROP2), At5G62880 (ROP11) and At3g53750 (ACT3). The M. polymorpha gene identifier for MpoARO is Mapoly0055s0122.1. A three-dimensional model of the microscopy chamber designed and used here can be found at https://www.thingiverse.com/thing:3710588. Source data are provided with this paper.
Change history
06 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41477-020-00841-6
References
Feiguelman, G., Fu, Y. & Yalovsky, S. ROP GTPases structure–function and signaling pathways. Plant Physiol. 176, 57–79 (2018).
Jaffe, A. B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Wu, G., Li, H. & Yang, Z. Arabidopsis RopGAPs are a novel family of Rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for Rop-specific GTPase stimulation. Plant Physiol. 124, 1625–1636 (2000).
Berken, A., Thomas, C. & Wittinghofer, A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 436, 1176–1180 (2005).
Carol, R. J. et al. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 438, 1013–1016 (2005).
Gu, Y. et al. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 169, 127–138 (2005).
Klahre, U. & Kost, B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 18, 3033–3046 (2006).
Zhang, Y. & McCormick, S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 18830–18835 (2007).
Hwang, J.-U., Vernoud, V., Szumlanski, A., Nielsen, E. & Yang, Z. A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol. 18, 1907–1916 (2008).
Cherfils, J. & Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 (2013).
Zhang, D. et al. The pollen receptor kinase LePRK2 mediates growth-promoting signals and positively regulates pollen germination and tube growth. Plant Physiol. 148, 1368–1379 (2008).
Duan, Q., Kita, D., Li, C., Cheung, A. Y. & Wu, H.-M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl Acad. Sci. USA 107, 17821–17826 (2010).
Chang, F., Gu, Y., Ma, H. & Yang, Z. AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol. Plant 6, 1187–1201 (2013).
Takeuchi, H. & Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531, 245–248 (2016).
Kost, B. in Integrated G Proteins Signaling in Plants (eds Yalovsky S. et al.) 27–48 (Springer, 2010).
Stöckle, D. et al. Putative RopGAPs impact division plane selection and interact with kinesin-12 POK1. Nat. Plants 2, 16120 (2016).
Gebert, M., Dresselhaus, T. & Sprunck, S. F-actin organization and pollen tube tip growth in Arabidopsis are dependent on the gametophyte-specific Armadillo repeat protein ARO1. Plant Cell 20, 2798–2814 (2008).
Marks, M. D. et al. Transcriptome analysis of Arabidopsis wild-type and gl3–sst sim trichomes identifies four additional genes required for trichome development. Mol. Plant 2, 803–822 (2009).
Schwab, B., Folkers, U., Ilgenfritz, H. & Hülskamp, M. Trichome morphogenesis in Arabidopsis. Phil. Trans. R. Soc. Lond. B Biol. Sci. 355, 879–883 (2000).
Bowman, J. L. et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171, 287–304 (2017).
Heo, W. D. et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 (2006).
Williams, C. L. The polybasic region of Ras and Rho family small GTPases: a regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell. Signal. 15, 1071–1080 (2003).
Arabidopsis Interactome Mapping Consortium Evidence for network evolution in an Arabidopsis interactome map. Science 333, 601–607 (2011).
Altmann, M., Altmann, S., Falter, C. & Falter-Braun, P. High-quality yeast-2-hybrid interaction network mapping. Curr. Protoc. Plant Biol. 3, e20067 (2018).
Molendijk, A. J. et al. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20, 2779–2788 (2001).
Jones, M. A. et al. The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14, 763–776 (2002).
Denninger, P. et al. Distinct RopGEFs successively drive polarization and outgrowth of root hairs. Curr. Biol. 29, 1854–1865 (2019).
Lan, P., Li, W. & Schmidt, W. Genome-wide co-expression analysis predicts protein kinases as important regulators of phosphate deficiency-induced root hair remodeling in Arabidopsis. BMC Genomics 14, 210 (2013).
Luo, N. et al. Exocytosis-coordinated mechanisms for tip growth underlie pollen tube growth guidance. Nat. Commun. 8, 1687 (2017).
Lemmon, M. A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9, 99–111 (2008).
Yanagisawa, M., Alonso, J. M. & Szymanski, D. B. Microtubule-dependent confinement of a cell signaling and actin polymerization control module regulates polarized cell growth. Curr. Biol. 28, 2459–2466 (2018).
Guo, J. & Yang, Z. Exocytosis and endocytosis: coordinating and fine-tuning the polar tip growth domain in pollen tubes. J. Exp. Bot. 71, 2428–2438 (2020).
Hirano, T. et al. PtdIns(3,5)P2 mediates root hair shank hardening in Arabidopsis. Nat. Plants 4, 888–897 (2018).
Platre, M. P. et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 364, 57–62 (2019).
Good, M. C., Zalatan, J. G. & Lim, W. A. Scaffold proteins: hubs for controlling the flow of cellular information. Science 332, 680–686 (2011).
Garbett, D. & Bretscher, A. The surprising dynamics of scaffolding proteins. Mol. Biol. Cell 25, 2315–2319 (2014).
Kourtidis, A., Ngok, S. P. & Anastasiadis, P. Z. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog. Mol. Biol. Transl. Sci. 116, 409–432 (2013).
Hiwatashi, T. et al. The RopGEF KARAPPO is essential for the initiation of vegetative reproduction in Marchantia polymorpha. Curr. Biol. 29, 3525–3531 (2019).
Ishizaki, K., Nishihama, R., Yamato, K. T. & Kohchi, T. Molecular genetic tools and techniques for Marchantia polymorpha research. Plant Cell Physiol. 57, 262–270 (2016).
Fu, Y., Xu, T., Zhu, L., Wen, M. & Yang, Z. A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr. Biol. 19, 1827–1832 (2009).
Gendre, D. et al. Rho-of-plant activated root hair formation requires Arabidopsis YIP4a/b gene function. Development 146, dev168559 (2019).
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003).
Sessions, A. et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell 14, 2985–2994 (2002).
Twell, D., Yamaguchi, J. & McCormick, S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109, 705–713 (1990).
Vogler, F., Schmalzl, C., Englhart, M., Bircheneder, M. & Sprunck, S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod. 27, 153–167 (2014).
Curtis, M. D. & Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003).
Karimi, M., Inzé, D. & Depicker, A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002).
Karimi, M., De Meyer, B. & Hilson, P. Modular cloning in plant cells. Trends Plant Sci. 10, 103–105 (2005).
Karimi, M., Depicker, A. & Hilson, P. Recombinational cloning with plant gateway vectors. Plant Physiol. 145, 1144–1154 (2007).
Mylle, E., Codreanu, M.-C., Boruc, J. & Russinova, E. Emission spectra profiling of fluorescent proteins in living plant cells. Plant Methods 9, 10 (2013).
Nallamsetty, S., Austin, B. P., Penrose, K. J. & Waugh, D. S. Gateway vectors for the production of combinatorially-tagged His6-MBP fusion proteins in the cytoplasm and periplasm of Escherichia coli. Protein Sci. 14, 2964–2971 (2005).
Bleckmann, A., Weidtkamp-Peters, S., Seidel, C. A. M. & Simon, R. Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiol. 152, 166–176 (2010).
Lampropoulos, A. et al. GreenGate—a novel, versatile, and efficient cloning system for plant transgenesis. PLoS ONE 8, e83043 (2013).
Dreze, M. et al. High-quality binary interactome mapping. Methods Enzymol. 470, 281–315 (2010).
Marks, M. D. et al. A new method for isolating large quantities of Arabidopsis trichomes for transcriptome, cell wall and other types of analyses. Plant J. 56, 483–492 (2008).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Julkowska, M. M., Rankenberg, J. M. & Testerink, C.Liposome-binding assays to assess specificity and affinity of phospholipid–protein interactions. Methods Mol. Biol. 1009, 261–271 (2013).
Lavy, M. et al. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 17, 947–952 (2007).
Goodstein, D. M. et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186 (2012).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Guindon, S. & Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 (2003).
Platre, M. P. et al. A combinatorial lipid code shapes the electrostatic landscape of plant endomembranes. Dev. Cell 45, 465–480 (2018).
Acknowledgements
We thank U. Hammes, G. Grossmann and C. Schwechheimer for sharing materials. The Nottingham Arabidopsis Stock Centre is acknowledged for providing seed stocks of T-DNA insertion mutants. We thank M. Kammerer for plant culture and genotyping and A. Alkofer for Y2H support. We are grateful to V. Neděla for help with scanning electron microscopy. This work was supported by a research fellowship from the Alexander von Humboldt Foundation (to I.K.). Funding was provided by the German Research Foundation (SP686/1-2 to S.S., SFB960 to S.S. and T.D. and SFB924 to S.S. and P.F.-B.).
Author information
Authors and Affiliations
Contributions
I.K., S.S., F.V., A.B., T.S. and P.F.-B. designed the experiments. S.S. conceived and supervised the project. I.K., F.V., A.B., P.C., T.S., I.F., M.L., S.S., L.K. and J.S. performed the experiments. I.K., F.V., S.S., A.B., L.K. and T.S. analysed the data. T.D., G.L., P.F.-B. and J.B. contributed reagents, materials or analysis tools. I.K., M.L., S.S., F.V. and T.S. prepared the figures. S.S. and I.K. wrote the manuscript with input from F.V., A.B., T.S. and P.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Dolf Weijers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quantification of root hair phenotypes and trichoblast viability.
a, Propidium iodide staining of root hairs. Representative images of the different root hair (RH) and trichoblast phenotypes observed in the aro2-1 aro3-1 (aro2/3) double and the aro2/3/4 triple mutant are shown. Propidium iodide stains pectins in the cell walls of living cells and the contents of dead cells, as it does not pass through the intact cell membranes of living cells. b, Quantification of root hair phenotypes and trichoblast viability in comparison to wild type (Col-0) root hairs. Note that the graph shows percentages of root hairs in different non-exclusive categories. Trichoblasts were classified as dead when a strong staining of collapsed cytoplasm and nucleus was observed. Every data point represents the average data from 30 to 60 trichoblasts counted on a single root. Root hairs were analyzed for n = 8 (Col-0, aro2/3/4) and 6 (aro2/3) individual seedlings, respectively. Error bars = s.d.
Extended Data Fig. 2 Mature aro2/3/4 trichomes show variable phenotypes.
a, ARO promoter-driven NLS3xGFP reporter fluorescence in the large nuclei of mature trichomes (arrows). Note that in ARO2p:NLS3xGFP and ARO3p:NLS3xGFP the reporter is also visible in the much smaller nuclei of leaf epidermal cells. Inset in ARO4p:NLS3xGFP shows a single optical section with magnified trichome nucleus as a merged bright field and fluorescence image, to discriminate between nuclear GFP and the autofluorescence of the trichome cell wall. b, Bright field images of developing trichomes that burst either during stalk formation (left), or during branching (right). c, Typical phenotypes of the few fully matured aro2/3/4 leaf trichomes in comparison to those of Col-0. d, Number of branches per mature trichome (in %). Total number of trichomes is displayed at the bottom of each column. e, Scatter dot plot, showing data from stalk and branch length measurements of mature wild-type (Col-0) and aro2/3/4 trichomes. The total number of trichomes is shown below each plot. Bold, horizontal line represents the mean. Error bars = s.d. Scale bars, 20 μm in a,b and 100 μm in c.
Extended Data Fig. 3 ARO2, ARO3 and Marchantia polymorpha ARO fully restore root hair formation in aro2/3/4.
a, Quantification of root hair phenotypes in propidium iodide-stained roots of aro2/3/4, complemented either with one of the Arabidopsis AROs, or with Marchantia polymorpha ARO (MpoARO). All AROs were expressed as mCHERRY fusion proteins under control of the ARO2 promoter. Roots from the wild type (Col-0), aro2-1 aro3-1 (aro2/3) and uncomplemented aro2/3/4 served as controls. The graph shows percentages of root hair phenotypes in different non-exclusive categories. Note that ARO2, ARO3 and MpARO fully restored aro2/3/4 root hair growth, whereas ARO1 and ARO4 were less effective. Furthermore, the percentage of swollen root hairs in ARO4-complemented lines was comparable to that in aro2/3. b, Quantification of trichoblast viability in propidium iodide-stained roots. Note that all AROs restored the viability of aro2/3/4 trichoblasts. For a, b, each data point represents the average percentage calculated from 30 to 60 trichoblasts of a single seedling (n = 6 seedlings for aro2/3, 8 seedlings for all other genotypes). Error bars = s.d.
Extended Data Fig. 4 Localization of ARO-GFP fusion proteins in the differentiation zone of the root.
a, ARO-GFP fusion proteins, expressed under control of their native promoters. Overall view of the differentiation zone (left) and progressive developmental stages of trichoblasts during bulging and tip growth of root hairs (RH) (right). Magnified root hair tips are shown in the insets. Live imaging of ARO-GFP fusion proteins in growing root hairs is shown in Supplementary Movie 1. b, Both mCherry(mCH)-ARO2 and mCH-ARO2-PBR accumulate in Brefeldin A (BFA)-induced compartments (arrowheads). Note that mCH-ARO2 still localizes to the plasma membrane of BFA-treated cells (arrows). CLSM images of root epidermal cells, 1 hour after adding either control solution (0.1% DMSO), or 50 μm BFA in 0.1% DMSO are shown. Scale bars, 40 μm in a, 20 μm in b.
Extended Data Fig. 5 Verification of ARO-RENGAP and ARO-ROP interactions.
a, GST-pulldowns show that all RENGAPs interact with all AROs. GST-tagged AROs, or free GST (control), were added to E. coli lysates with His-MBP-tagged REN1, REN2, or REN3 (input). Magnetic glutathione-agarose beads were used to pull down GST-AROs (arrowheads). b, GST-pulldowns reveal interaction of ROP1 with ARO1, ARO2 and ARO3, respectively. c, Fraction bound normalized data from MicroScale Thermophoresis (MST) indicate that ROP1 interacts with ARO1, ARO2 and ARO3 but not with ARO4. Recombinant His-MBP-ROP1 was serially diluted and mixed with the respective NT647-labelled GST-tagged ARO protein. ROP1 MST data were normalized to REN3 binding. Mean values from two technical repeats of the same capillary are shown. 0 = unbound, 1 = bound. Note that ligand-binding measurements with ROP1 were performed in the absence of GTP. d, Guanine nucleotide-dependent pulldown assays suggest that pulldown of ROP2 by AROs is enhanced by including GTP in the pulldown reaction. Lower panels show comparable bait usage (GST-ARO2; GST control). Relative pull-down efficiencies (normalized by the GST bait) are shown on the right. e, Guanine nucleotide-dependent pulldown assays for ROP11 show interaction with GST-ARO1, GST-ARO3 and GST-ARO4 (arrowhead), with GTP positively affecting the pull-down efficiencies. Lower panel shows comparable bait usage (GST-ARO2; GST control). Relative pull-down efficiencies, normalized by the GST bait, are shown on the right. Input for d,e, was His-MBP-ROP protein, pre-treated for 2 hours with 10 mM GDP, or GTP, respectively.
Extended Data Fig. 6 Loss of ROP2 polarity in aro2/3/4 root hair bulges and trichomes is accompanied by the loss of REN1 plasma membrane localization.
a, Lateral spreading of GFP-ROP2 in aro2/3/4 root hair bulges, stabilized by 0.2 M mannitol. Polarity index of 1 = identical apical:lateral signal intensities. Scatter dot plot with bold, horizontal line representing the mean. Error bars = s.d. Statistically significant difference was determined by two-sample unequal variance t-test (n = 50). b, Time lapse imaging of Col-0 root hair expressing mCH-REN1, showing that it is enriched at the subapical plasma membrane (PM; arrowhead) and spreads to the tip when growth ceases (arrow). c, Representative in vitro-germinated pollen tubes from ren1-2/+ plants, showing that ARO1-GFP locates to the PM (n >10 pollen tubes per genotype). Strong ARO1-GFP signals in the vesicle-rich clear zone of wild type-like pollen tubes often hampered the detection of PM-derived signals (top panel), which became only apparent in pollen tubes with a sparse number of tip-localized vesicles (middle panel). Colored arrows depict the lines used for measuring the signal intensity profiles shown on the right. Black arrows indicate PM. d, REN3 does not bind to phosphatidylserine (PS, red arrow). PIP strips™ with immobilized phospholipids were incubated with 0.5 µg/ml His-MBP-REN3 or GST-tagged PLCδ1, respectively. MBP and GST were used as controls. Phospholipid species are shown on the left (see Supplementary Materials for further details). e, Developing trichomes, co-expressing mCH-REN1 and GFP-ROP2. Blue arrows = area for plotting fluorescence intensity profiles shown in (f). f, Fluorescence intensity profiles revealed that mCH-REN1 does not co-localize with GFP-ROP2 at the PM of aro2/3/4 trichome branches. Scale bars, 100 µm in a, 10 µm in b, 20 µm in c,e.
Extended Data Fig. 7 A model summarizing the data on ARO proteins in the context of previous findings in tip-growing cells.
a, Tip-growing wild type (WT) cell. ROP GTPases in the PM are switched “on” by GEFs (not shown), which, in turn, may be activated by membrane-bound RLKs upon extracellular stimuli8,11,12,13,14. ROP-controlled downstream signaling regulates polar exocytosis by reorganizing the cytoskeleton and recruiting the exocyst through its effectors1,6,32. Secretory vesicles accumulate in the apical inverted-cone shaped region (shaded in yellow). Tip-focused secretion supplies cell wall and membrane material to the growth site, while excess material is recycled via lateral endocytosis (broken arrows). ROP-dependent polarized exocytosis establishes positive feedforward and negative feedback mechanisms, as it delivers proteins involved in the regulation of ROP activity and also ROPs to the plasma membrane29,32,41. In pollen tubes, the PH-domain containing GAP REN1 is a key negative regulator of ROP19,29. REN1 is associated with secretory vesicles and must be targeted to the apical PM by exocytosis to confine ROP1 activity to the pollen tube tip. After ROP1 is switched “off”, REN1 appears to be removed from the pollen tube PM without localizing to endocytic compartments9. However, contrary to tobacco pollen tubes transiently co-expressing REN1 and ROP19, stably transformed root hairs display mCherry-REN1 signals at their lateral PM. GDIs (not shown) sequester ROPs from the PM to the cytosol5,15. ARO proteins interact with RENs and ROPGTP, accumulate on secretory vesicles and bind to the PM via their polybasic region (PBR), which mediates interaction with anionic phospholipids such as phosphatidylserine (PS) and phosphatidic acid (PA). In Arabidopsis root epidermis cells genetically encoded sensors for PA accumulate at the PM cytosolic leaflet, whereas PS sensors mark the PM cytosolic leaflet and PM-derived organelles62. Contrary to ARO2, REN3 does not bind PS, which was reported to stabilize ROP6 in PM nanodomains of root cells34. AROs are likely associated with the trans-Golgi network (TGN)/early endosome (EE), as ARO2 and ARO2-PBR both localized to BFA-induced bodies. b, In aro2/3/4 trichoblasts, co-recruitment of active ROP and its negative regulator RENGAP into the same PM-localized scaffolded complex cannot be achieved. Consequently, RENGAPs do not confine ROP signaling to the polar growth site that has been established during the root hair initiation phase27 but ROP spreads laterally, which abrogates focal exocytosis of vesicles delivering components of the positive feedforward mechanism (e.g., RLKs and ROPs). A growth arrested wide and flat bulge is formed, or the bulge collapses shortly after emergence, similar to root hair bulges of feronia mutants with reduced levels of active ROPs12. Likewise, the tip growth phase cannot be initiated in the absence of ARO scaffolds. REN1 is not detectable at the PM and ROP2 is not confined to the tip but spreads along the PM of the aro2/3/4 root hair bulge. Whether PM-localized ROPs remain active in aro2/3/4 trichoblasts and whether RENGAPs are still capable of binding to exocytic vesicles without ARO proteins remains to be shown.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1–3.
Supplementary Video 1
Localization of ARO–GFP fusion proteins in growing root hairs, related to Fig. 4b and Extended Data Fig. 4a. Seven-day-old seedlings were placed in the imaging chamber with half-strength Murashige and Skoog and 1% agarose. Imaging by confocal laser scanning microscopy started after 1 h. Single focal planes are displayed.
Supplementary Video 2
TIRF/VAEM microscopy of ARO–GFP in growing root hairs, related to Fig. 4c. Seven-day-old seedlings were placed in the imaging chamber with half-strength Murashige and Skoog and 1% agarose. Imaging started after 1 h.
Supplementary Video 3
Side-by-side live imaging of GFP–ROP2 in the differentiation zone of two aro2/3/4 roots, where one root is complemented with mCH–ARO2, related to Fig. 6a,b. Two 7-d-old seedlings were placed side by side in the imaging chamber with half-strength Murashige and Skoog and 1% agarose. After 1 h, imaging by spinning disc microscopy started (180 min; one z stack per 2 min). A projection of the z series is displayed.
Supplementary Data 1
Source data for Supplementary Fig. 1.
Supplementary Data 2
Source data for Supplementary Fig. 2.
Supplementary Data 3
Source data for Supplementary Fig. 5.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and gels.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed gels.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Kulich, I., Vogler, F., Bleckmann, A. et al. ARMADILLO REPEAT ONLY proteins confine Rho GTPase signalling to polar growth sites. Nat. Plants 6, 1275–1288 (2020). https://doi.org/10.1038/s41477-020-00781-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-00781-1
This article is cited by
-
Two subtypes of GTPase-activating proteins coordinate tip growth and cell size regulation in Physcomitrium patens
Nature Communications (2023)
-
ARMADILLOs delimit Rho signalling
Nature Plants (2020)