Abstract
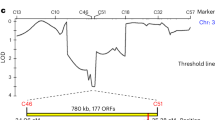
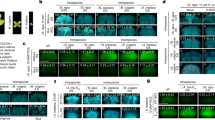
Pre-zygotic interspecies incompatibility in angiosperms is a male–female relationship that inhibits the formation of hybrids between two species. Here, we report on the identification of STIGMATIC PRIVACY 1 (SPRI1), an interspecies barrier gene in Arabidopsis thaliana. We show that the rejection activity of this stigma-specific plasma membrane protein is effective against distantly related Brassicaceae pollen tubes and is independent of self-incompatibility. Point-mutation experiments and functional tests of synthesized hypothetical ancestral forms of SPRI1 suggest evolutionary decay of SPRI1-controlled interspecies incompatibility in self-compatible A. thaliana. Hetero-pollination experiments indicate that SPRI1 ensures intraspecific fertilization in the pistil when pollen from other species are present. Our study supports the idea that SPRI1 functions as a barrier mechanism that permits entrance of pollen with an intrinsic signal from self species.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Sequence data can be found at The Arabidopsis Information Resource database (https://www.arabidopsis.org/) or in the 1001 genomes website (1001genomes.org). Raw phenotype data used for the GWAS has been deposited in the GWA-Portal (https://gwas.gmi.oeaw.ac.at/#/study/4205/overview) and Arapheno (https://doi.org/10.21958/study:37). Raw data for pollen tube count was deposited to Mendeley (https://doi.org/10.17632/yzy85dtwk3.1). All other data are available in the article or in the Supplementary information.
References
de Nettancourt, D. Incompatibility and Incongruity in Wild and Cultivated Plants (Springer, 2001).
Hogenboom, N. G. & Mather, K. Incompatibility and incongruity: two different mechanisms for the non-functioning of intimate partner relationships. Proc. Roy. Soc. B 188, 361–375 (1975).
Okuda, S. et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458, 357–361 (2009).
Takeuchi, H. & Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531, 245–248 (2016).
Boehm, T. Quality control in self/nonself discrimination. Cell 125, 845–858 (2006).
Igic, B., Lande, R. & Kohn, J. R. Loss of self-incompatibility and its evolutionary consequences. Int. J. Plant Sci. 169, 93–104 (2008).
Fujii, S., Kubo, K. & Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2, 16130 (2016).
Harada, Y. et al. Mechanism of self-sterility in a hermaphroditic chordate. Science 320, 548–550 (2008).
Kubo, K. et al. Collaborative non-self recognition system in S-RNase-based self-incompatibility. Science 330, 796–799 (2010).
Takayama, S. et al. Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413, 534–538 (2001).
Wheeler, M. J. et al. Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature 459, 992–995 (2009).
Wilkins, K. A. et al. Self-incompatibility-induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiol. 167, 766–779 (2015).
Thomas, S. G. & Franklin-Tong, V. E. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 429, 305–309 (2004).
Lewis, D. & Crowe, L. K. Unilateral interspecific incompatibility in flowering plants. Heredity 12, 233–256 (1958).
Lewis, D. Incompatibility in flowering plants. Biol. Rev. 24, 472–496 (1949).
Hiscock, S. J. & Dickinson, H. G. Unilateral incompatibility within the Brassicaceae: further evidence for the involvement of the self-incompatibility (S)-locus. Theor. Appl. Genet. 86, 744–753 (1993).
Li, W. & Chetelat, R. T. A pollen factor linking inter- and intraspecific pollen rejection in tomato. Science 330, 1827–1830 (2010).
Murfett, J. et al. S RNase and interspecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8, 943–958 (1996).
Li, W. & Chetelat, R. T. Unilateral incompatibility gene ui1.1 encodes an S-locus F-box protein expressed in pollen of Solanum species. Proc. Natl Acad. Sci. USA 112, 4417–4422 (2015).
Tovar-Méndez, A. et al. Restoring pistil-side self-incompatibility factors recapitulates an interspecific reproductive barrier between tomato species. Plant J. 77, 727–736 (2014).
Burdfield-Steel, E. R. & Shuker, D. M. Reproductive interference. Curr. Biol. 21, R450–R451 (2011).
Tsuchimatsu, T. et al. Evolution of self-compatibility in Arabidopsis by a mutation in the male specificity gene. Nature 464, 1342–1346 (2010).
Seren, U. et al. GWAPP: A web application for genome-wide association mapping in Arabidopsis. Plant Cell 24, 4793–4805 (2012).
Hooper, C. M. et al. SUBAcon: a consensus algorithm for unifying the subcellular localization data of the Arabidopsis proteome. Bioinformatics 30, 3356–3364 (2014).
Iwano, M. et al. Calcium signalling mediates self-incompatibility response in the Brassicaceae. Nat. Plants 1, 15128 (2015).
Klepikova, A. V., Kasianov, A. S., Gerasimov, E. S., Logacheva, M. D. & Penin, A. A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 88, 1058–1070 (2016).
Novikova, P. Y. et al. Sequencing of the genus Arabidopsis identifies a complex history of nonbifurcating speciation and abundant trans-specific polymorphism. Nat. Genet. 48, 1077–1082 (2016).
Murat, F. et al. Understanding Brassicaceae evolution through ancestral genome reconstruction. Genome Biol. 16, 1–17 (2015).
Mizukami, A. G. et al. The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr. Biol. 26, 1091–1097 (2016).
Hemler, M. E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811 (2005).
Miyado, K. et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321–324 (2000).
Boavida, L. C., Qin, P., Broz, M., Becker, J. D. & McCormick, S. Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo- and heterodimers when expressed in yeast. Plant Physiol. 163, 696–712 (2013).
Tsuchiya, T. in Sexual Reproduction in Animals and Plants (eds Sawada, H. et al.) 305–325 (Springer, 2014).
Vanacker, H., Lu, H., Rate, D. N. & Greenberg, J. T. A role for salicylic acid and NPR1 in regulating cell growth in Arabidopsis. Plant J. 28, 209–216 (2001).
Doughty, J., Wong, H. Y. & Dickinson, H. G. Cysteine-rich pollen coat proteins (PCPs) and their Interactions with stigmatic S (incompatibility) and S-related proteins in Brassica: putative roles in SI and pollination. Ann. Bot. 85, 161–169 (2000).
Takada, Y. et al. Duplicated pollen–pistil recognition loci control intraspecific unilateral incompatibility in Brassica rapa. Nat. Plants 3, 17096 (2017).
Zhang, Z. et al. A pectin methylesterase gene at the maize Ga1 locus confers male function in unilateral cross-incompatibility. Nat. Commun. 9, 3678 (2018).
Tsuchimatsu, T. et al. Patterns of polymorphism at the self-incompatibility Locus in 1,083 Arabidopsis thaliana genomes. Mol. Biol. Evol. 34, 1878–1889 (2017).
Platt, A. et al. The scale of population structure in Arabidopsis thaliana. PLoS Genet. 6, e1000843 (2010).
Shimizu, K. K., Kudoh, H. & Kobayashi, M. J. Plant sexual reproduction during climate change: gene function in natura studied by ecological and evolutionary systems biology. Ann. Bot. 108, 777–787 (2011).
Saito, K. et al. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 3, 1262 (2012).
Rawat, V. et al. Improving the annotation of Arabidopsis lyrata using RNA-seq data. PLoS ONE 10, e0137391 (2015).
Wang, Z.-P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015).
Iwano, M. et al. Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 150, 1322–1334 (2009).
Shiba, H. et al. Alteration of the self-incompatibility phenotype in Brassica by transformation of the antisense SLG gene. Biosci. Biotechnol. Biochem. 64, 1016–1024 (2000).
Hasegawa, J. et al. Three-dimensional imaging of plant organs using a simple and rapid transparency technique. Plant Cell Physiol. 57, 462–472 (2016).
Seren, Ü. GWA-Portal: Genome-wide association studies made easy. Methods Mol. Biol. 1761, 303–319 (2018).
The 1001 Genomes Consortium 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166, 481–491 (2016).
Bandelt, H.-J., Forster, P. & Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 16, 37–48 (1999).
South, A. rworldmap: A new R package for mapping global data. R Journal 3, 35–43 (2011).
Durvasula, A. et al. African genomes illuminate the early history and transition to selfing in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 114, 5213–5218 (2017).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Hofmann, K. & Stoffel, W. TMBASE-A database of membrane spanning protein segments. Biol. Chem. 374, 166 (1993).
Käll, L., Krogh, A. & Sonnhammer, E. L. L. Advantages of combined transmembrane topology and signal peptide prediction-the Phobius web server. Nucleic Acids Res. 35, W429–W432 (2007).
Tsirigos, K. D., Peters, C., Shu, N., Käll, L. & Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 43, W401–W407 (2015).
Hirokawa, T., Boon-Chieng, S. & Mitaku, S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14, 378–379 (1998).
Juretić, D., Zoranić, L. & Zucić, D. Basic charge clusters and predictions of membrane protein topology. J. Chem. Inf. Comput. Sci. 42, 620–632 (2002).
Haudry, A. et al. An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nat. Genet. 45, 891–898 (2013).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Ashkenazy, H. et al. FastML: a web server for probabilistic reconstruction of ancestral sequences. Nucleic Acids Res. 40, W580–W584 (2012).
Slotte, T. et al. The Capsella rubella genome and the genomic consequences of rapid mating system evolution. Nat. Genet. 45, 831–835 (2013).
Rice, P., Longden, I. & Bleasby, A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16, 276–277 (2000).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Couvreur, T. L. P. et al. Molecular phylogenetics, temporal diversification, and principles of evolution in the mustard family (Brassicaceae). Mol. Biol. Evol. 27, 55–71 (2010).
Talavera, G. & Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577 (2007).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Acknowledgements
We thank M. Okamura, M. Nara, T. Manabe, Y. Yamamoto, M. Niidome and M. Ishii for their technical assistance and A. Kawabe and Y. Kato for helpful discussions. This work was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas (23113002, 16H06467 and 16H06464 to S. Takayama; 16H01467 and 18H04776 to S. Fujii; 17H05833 and 18H04813 to T.T. and 16H06469 to K.K.S.), Grants-in-Aid for Scientific Research (25252021 and 16H06380 to S.Takayama and 18H02456 to S. Fujii), Grant-in-Aid for Challenging Exploratory Research (15K14626 to S. Fujii) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), Swiss National Science Foundation to K.K.S. and Japan Science and Technology Agency (JST) PRESTO programme (JPMJPR16Q8) to S. Fujii.
Author information
Authors and Affiliations
Contributions
S. Fujii, K.K.S. and S. Takayama conceived the study. S. Fujii, T.T., K.K.S. and S. Takayama wrote the manuscript. S. Fujii conducted most of the experiments and data analysis. T.T. conducted the GWAS and the geographical analysis. H.S.-A., S. Furukawa, W.I. and Y.W. contributed to phenotyping in the GWAS. Y.K. performed the fluorescent pollen experiment. S.I. contributed to the transgenic experiments. S. Tangpranomkorn contributed to the sequence analysis. M.I. performed the microarray analysis and W.I. performed the real-time PCR.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Plants thanks Daphne Goring and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Supplementary Tables 1–5, and titles and legends for Supplementary Videos 1 and 2.
Supplementary Video 1
Time-lapse imaging analysis of a M. littorea pollen grain attached on a Col-0 stigmatic papilla cell (left) and on a spri1-1 stigmatic papilla cell (right).
Supplementary Video 2
Time-lapse imaging analysis of Col-0 and M. littorea pollen grains adjacently attached on a spri1-2 + SPRI1A stigmatic papilla cell.
Rights and permissions
About this article
Cite this article
Fujii, S., Tsuchimatsu, T., Kimura, Y. et al. A stigmatic gene confers interspecies incompatibility in the Brassicaceae. Nat. Plants 5, 731–741 (2019). https://doi.org/10.1038/s41477-019-0444-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0444-6
This article is cited by
-
A pollen selection system links self and interspecific incompatibility in the Brassicaceae
Nature Ecology & Evolution (2024)
-
FERONIA and reactive oxygen species: regulators in the self-incompatibility response and in interspecific pollination
Molecular Horticulture (2023)
-
SHI family transcription factors regulate an interspecific barrier
Nature Plants (2023)
-
Insights into pollen–stigma recognition: self-incompatibility mechanisms serve as interspecies barriers in Brassicaceae?
aBIOTECH (2023)
-
Live imaging-based assay for visualising species-specific interactions in gamete adhesion molecules
Scientific Reports (2022)