Abstract
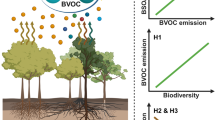
Plant-emitted volatile organic compounds (VOCs) play fundamental roles in atmospheric chemistry and ecological processes by contributing to aerosol formation1 and mediating species interactions2. Rising temperatures and the associated shifts in vegetation composition have been shown to be the primary drivers of plant VOC emissions in Arctic ecosystems3. Although herbivorous insects also strongly alter plant VOC emissions2, no studies have addressed the impact of herbivory on plant VOC emissions in the Arctic. Here we show that warming dramatically increases the amount, and alters the blend, of VOCs released in response to herbivory. We observed that a tundra ecosystem subjected to warming, by open-top chambers, for 8 or 18 years showed a fourfold increase in leaf area eaten by insect herbivores. Herbivory by autumnal moth (Epirrita autumnata) larvae, and herbivory-mimicking methyl jasmonate application, on the widespread circumpolar dwarf birch (Betula nana) both substantially increased emissions of terpenoids. The long-term warming treatments and mimicked herbivory caused, on average, a two- and fourfold increase in monoterpene emissions, respectively. When combined, emissions increased 11-fold, revealing a strong synergy between warming and herbivory. The synergistic effect was even more pronounced for homoterpene emissions. These findings suggest that, in the rapidly warming Arctic, insect herbivory may be a primary determinant of VOC emissions during periods of active herbivore feeding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All VOC and background herbivory data that support the findings of this study are available in Figshare with the data DOI identifier https://doi.org/10.6084/m9.figshare.7879340.
References
Jokinen, T. et al. Production of extremely low volatile organic compounds from biogenic emissions: measured yields and atmospheric implications. Proc. Natl Acad. Sci. USA 112, 7123–7128 (2015).
Turlings, T. C. J. & Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 63, 433–452 (2018).
Kramshoj, M. et al. Large increases in Arctic biogenic volatile emissions are a direct effect of warming. Nat. Geosci. 9, 349–352 (2016).
Kesselmeier, J. et al. Volatile organic compound emissions in relation to plant carbon fixation and the terrestrial carbon budget. Glob. Biogeochem. Cycles 16, 73-1–73-9 (2002).
Dicke, M., van Loon, J. J. A. & Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 5, 317–324 (2009).
Vickers, C. E., Gershenzon, J., Lerdau, M. T . & Loreto, F.A. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 5, 283–291 (2009).
Peñuelas, J. & Staudt, M. BVOCs and global change. Trends Plant Sci. 15, 133–144 (2010).
Kessler, A. & Baldwin, I. T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144 (2001).
Holopainen, J. K., Kivimäenpää, M. & Nizkorodov, S. A. Plant-derived secondary organic material in the air and ecosystems. Trends Plant Sci. 22, 744–753 (2017).
Ehn, M. et al. A large source of low-volatility secondary organic aerosol. Nature 506, 476–479 (2014).
IPCC Climate Change 2014: Synthesis Report (eds Pachauri, R. K. et al.) (Cambridge Univ. Press, 2014).
Potosnak, M. J. et al. Isoprene emissions from a tundra ecosystem. Biogeosciences 10, 871–889 (2013).
Valolahti, H., Kivimäenpää, M., Faubert, P., Michelsen, A. & Rinnan, R. Climate change-induced vegetation change as a driver of increased subarctic biogenic volatile organic compound emissions. Glob. Change Biol. 21, 3478–3488 (2015).
Tang, J., Valolahti, H., Kivimäenpää, M., Michelsen, A. & Rinnan, R. Acclimation of biogenic volatile organic compound emission from subarctic heath under long-term moderate warming. J. Geophys. Res. Biogeosci. 123, 95–105 (2018).
Ameye, M. et al. Green leaf volatile production by plants: a meta-analysis. New Phytol. 220, 666–683 (2018).
Rowen, E. & Kaplan, I. Eco-evolutionary factors drive induced plant volatiles: a meta-analysis. New Phytol. 210, 284–294 (2016).
Bergström, R., Hallquist, M., Simpson, D., Wildt, J. & Mentel, T. F. Biotic stress: a significant contributor to organic aerosol in Europe? Atmos. Chem. Phys. 14, 13643–13660 (2014).
Deutsch, C. A. et al. Increase in crop losses to insect pests in a warming climate. Science 361, 916–919 (2018).
Post, E. et al. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358 (2009).
Lesk, C., Coffel, E., D’Amato, A. W., Dodds, K. & Horton, R. Threats to North American forests from southern pine beetle with warming winters. Nat. Clim. Change 7, 713–717 (2017).
Barrio, I. C., Bueno, C. G. & Hik, D. S. Warming th e tundra: reciprocal responses of invertebrate herbivores and plants. Oikos 125, 20–28 (2016).
Jepsen, J. U. et al. Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Glob. Change Biol. 17, 2071–2083 (2011).
Sistla, S. A. et al. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 497, 615–618 (2013).
Hollesen, J. et al. Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century. Glob. Change Biol. 21, 2410–2423 (2015).
Schuman, M. C., Heinzel, N., Gaquerel, E., Svatos, A. & Baldwin, I. T. Polymorphism in jasmonate signaling partially accounts for the variety of volatiles produced by Nicotiana attenuata plants in a native population. New Phytol. 183, 1134–1148 (2009).
Barrio, I. C. et al. Background invertebrate herbivory on dwarf birch (Betula glandulosa– nana complex) increases with temperature and precipitation across the tundra biome. Polar Biol. 40, 2265–2278 (2017).
Kozlov, M. V., Lanta, V., Zverev, V. & Zvereva, E. L. Global patterns in background losses of woody plant foliage to insects. Glob. Ecol. Biogeogr. 24, 1126–1135 (2015).
Halitschke, R., Stenberg, J. A., Kessler, D., Kessler, A. & Baldwin, I. T. Shared signals—‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 11, 24–34 (2008).
Mentel, T. F. et al. Secondary aerosol formation from stress-induced biogenic emissions and possible climate feedbacks. Atmos. Chem. Phys. 13, 8755–8770 (2013).
Paasonen, P. et al. Warming-induced increase in aerosol number concentration likely to moderate climate change. Nat. Geosci. 6, 438–442 (2013).
Abisko Scientific Research Station. Temperature and precipitation data 1913–2015 (Polarforskningssekretariatet Press, 2016).
Tholl, D. et al. Practical approaches to plant volatile analysis. Plant J. 45, 540–560 (2006).
Johnsen, L. G., Skou, P. B., Khakimov, B. & Bro, R. Gas chromatography–mass spectrometry data processing made easy. J. Chromatogr. A 1503, 57–64 (2017).
Guenther, A. B., Zimmerman, P. R., Harley, P. C., Monson, R. K. & Fall, R. Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. J. Geophys. Res. Atmos. 98, 12609–12617 (1993).
Schuman, M. C., Allmann, S. & Baldwin, I. T. Plant defense phenotypes determine the consequences of volatile emission for individuals and neighbors. eLife 4, e04490 (2015).
Graus, M., Müller, M. & Hansel, A. High resolution PTR–TOF: quantification and formula confirmation of VOC in real time. J. Am. Soc. Mass Spectrom. 21, 1037–1044 (2010).
Holzinger, R. PTRwid: a new widget tool for processing PTR–TOF–MS data. Atmos. Meas. Tech. 8, 3903–3922 (2015).
Kozlov, M. V., Filippov, B. Y., Zubrij, N. A. & Zverev, V. Abrupt changes in invertebrate herbivory on woody plants at the forest–tundra ecotone. Polar Biol. 38, 967–974 (2015).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2009).
Oksanen, J. F. et al. Vegan: Community ecology package v.2.0-5 (CRAN, 2012); http://CRAN.R-project.org/package=vegan
Brückner, A. & Heethoff, M. A chemo-ecologists’ practical guide to compositional data analysis. Chemoecology 27, 33–46 (2017).
Acknowledgements
We thank M. Jylkkä for providing the image of E. autumnata and C.L. Davie-Martin for language editing. We gratefully acknowledge financial support from the Danish Council for Independent Research/Natural Sciences, the Danish National Research Foundation (grant No. CENPERM DNRF100), the Marie Sklodowska-Curie grant (No. 751684) and the European Research Council (grant No. 771012) under the European Union’s Horizon 2020 research and innovation programme. The Abisko Scientific Research Station (Sweden) is thanked for housing and logistics support.
Author information
Authors and Affiliations
Contributions
T.L. and R.R. designed the experiment. A.M. established the experimental site. T.L. and T.H. developed the methodology for the volatile emission time course experiments. T.L. collected, analysed and interpreted the data and wrote the manuscript. All authors commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal Peer Review Information: Nature Plants thanks Robert Hollister and the other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figs. 1–10.
Supplementary Tables 1–6
Supplementary Table 1: VOC emissions of Betula nana under different treatments. Supplementary Table 2: Summary of mixed-effects models testing for main warming and mimicked herbivory effects and their interactions. Supplementary Table 3: Summary of Kruskal–Wallis tests for warming and herbivory effects. Supplementary Table 4: Timelines of real time measurements of VOC emissions. Supplementary Table 5: List of ion masses and molecular formulae measured with the PTR–ToF–MS. Supplementary Table 6: Summary of mixed-effects models testing for warming, litter addition, herbivory effects and their interactions.
Rights and permissions
About this article
Cite this article
Li, T., Holst, T., Michelsen, A. et al. Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nat. Plants 5, 568–574 (2019). https://doi.org/10.1038/s41477-019-0439-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0439-3
This article is cited by
-
Impact of Volatile Mediated Indirect Defense Response of Plant and Herbivore Refuge in Tritrophic Cascade
Differential Equations and Dynamical Systems (2024)
-
BVOC Emissions From a Subarctic Ecosystem, as Controlled by Insect Herbivore Pressure and Temperature
Ecosystems (2022)
-
The fate of carbon in a mature forest under carbon dioxide enrichment
Nature (2020)