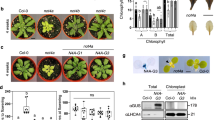
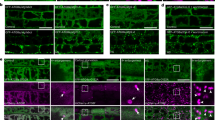
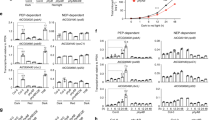
Abstract
Communication between organelles and the nucleus is essential for fitness and survival. Retrograde signals are cues emitted from the organelles to regulate nuclear gene expression. GENOMES UNCOUPLED1 (GUN1), a protein of unknown function, has emerged as a central integrator, participating in multiple retrograde signalling pathways that collectively regulate the nuclear transcriptome. Here, we show that GUN1 regulates chloroplast protein import through interaction with the import-related chaperone cpHSC70-1. We demonstrated that overaccumulation of unimported precursor proteins (preproteins) in the cytosol causes a GUN phenotype in the wild-type background and enhances the GUN phenotype of the gun1 mutant. Furthermore, we identified the cytosolic HSP90 chaperone complex, induced by overaccumulated preproteins, as a central regulator of photosynthetic gene expression that determines the expression of the GUN phenotype. Taken together, our results suggest a model in which protein import capacity, folding stress and the cytosolic HSP90 complex control retrograde communication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Mass spectrometry-based proteomic data have been deposited in the PRIDE partner repository of the ProteomeXchange Consortium with the data set identifiers PXD010730 and PXD013005. All other data are available in the main text or the Supplementary Information.
References
Bradbeer, J. W., Atkinson, Y. E., Borner, T. & Hagemann, R. Cytoplasmic synthesis of plastid polypeptides may be controlled by plastid-synthesized RNA. Nature 279, 816–817 (1979).
Ramel, F. et al. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl Acad. Sci. USA 109, 5535–5540 (2012).
Estavillo, G. M. et al. Evidence for a SAL1–PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23, 3992–4012 (2011).
Xiao, Y. M. et al. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149, 1525–1535 (2012).
Woodson, J. D., Perez-Ruiz, J. M. & Chory, J. Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 21, 897–903 (2011).
Fang, X. et al. Chloroplast-to-nucleus signaling regulates microRNA biogenesis in Arabidopsis. Dev. Cell 48, 371–382.e4 (2018).
Martin, G. et al. Phytochrome and retrograde signalling pathways converge to antagonistically regulate a light-induced transcriptional network. Nat. Commun. 7, 11431 (2016).
Jarvis, P. & Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 14, 787–802 (2013).
Singh, R., Singh, S., Parihar, P., Singh, V. P. & Prasad, S. M. Retrograde signaling between plastid and nucleus: a review. J. Plant Physiol. 181, 55–66 (2015).
Susek, R. E., Ausubel, F. M. & Chory, J. Signal-transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene-expression from chloroplast development. Cell 74, 787–799 (1993).
Koussevitzky, S. et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719 (2007).
Mochizuki, N., Brusslan, J. A., Larkin, R., Nagatani, A. & Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl Acad. Sci. USA 98, 2053–2058 (2001).
Larkin, R. M., Alonso, J. M., Ecker, J. R. & Chory, J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299, 902–906 (2003).
Strand, A., Asami, T., Alonso, J., Ecker, J. R. & Chory, J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421, 79–83 (2003).
von Gromoff, E. D., Alawady, A., Meinecke, L., Grimm, B. & Beck, C. F. Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell 20, 552–567 (2008).
Moulin, M., McCormac, A. C., Terry, M. J. & Smith, A. G. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl Acad. Sci. USA 105, 15178–15183 (2008).
La Rocca, N., Rascio, N., Oster, U. & Rudiger, W. Amitrole treatment of etiolated barley seedlings leads to deregulation of tetrapyrrole synthesis and to reduced expression of Lhc and RbcS genes. Planta 213, 101–108 (2001).
Wu, G. Z. et al. Control of retrograde signaling by rapid turnover of GENOMES UNCOUPLED 1. Plant Physiol. 176, 2472–2495 (2018).
Hernandez-Verdeja, T. & Strand, A. Retrograde signals navigate the path to chloroplast development. Plant Physiol. 176, 967–976 (2018).
Ruckle, M. E., DeMarco, S. M. & Larkin, R. M. Plastid signals remodel light signaling networks and are essential for efficient chloroplast biogenesis in Arabidopsis. Plant Cell 19, 3944–3960 (2007).
Waters, M. T. et al. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21, 1109–1128 (2009).
Kakizaki, T. et al. Coordination of plastid protein import and nuclear gene expression by plastid-to-nucleus retrograde signaling. Plant Physiol. 151, 1339–1353 (2009).
Tadini, L. et al. GUN1 controls accumulation of the plastid ribosomal protein S1 at the protein level and interacts with proteins involved in plastid protein homeostasis. Plant Physiol. 170, 1817–1830 (2016).
Colombo, M., Tadini, L., Peracchio, C., Ferrari, R. & Pesaresi, P. GUN1, a jack-of-all-trades in chloroplast protein homeostasis and signaling. Front. Plant Sci. 7, 1427 (2016).
Llamas, E., Pulido, P. & Rodriguez-Concepcion, M. Interference with plastome gene expression and Clp protease activity in Arabidopsis triggers a chloroplast unfolded protein response to restore protein homeostasis. PLoS Genet. 13, e1007022 (2017).
Sun, X. et al. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2, 477 (2011).
Page, M. T. et al. Seedlings lacking the PTM protein do not show a genomes uncoupled (gun) mutant phenotype. Plant Physiol. 174, 21–26 (2017).
Mochizuki, N., Susek, R. & Chory, J. An intracellular signal transduction pathway between the chloroplast and nucleus is involved in de-etiolation. Plant Physiol. 112, 1465–1469 (1996).
Su, P.-H. & Li, H.-M. Stromal Hsp70 is important for protein translocation into pea and Arabidopsis chloroplasts. Plant Cell 22, 1516–1531 (2010).
Flores-Pérez, U. et al. Functional analysis of the Hsp93/ClpC chaperone at the chloroplast envelope. Plant Physiol. 170, 147–162 (2016).
Shi, L. X. & Theg, S. M. A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss Physcomitrella patens. Plant Cell 22, 205–220 (2010).
Liu, L., McNeilage, R. T., Shi, L. X. & Theg, S. M. ATP requirement for chloroplast protein import is set by the Km for ATP hydrolysis of stromal Hsp70 in Physcomitrella patens. Plant Cell 26, 1246–1255 (2014).
Nielsen, E., Akita, M., Davila-Aponte, J. & Keegstra, K. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 16, 935–946 (1997).
Huang, P.-K., Chan, P.-T., Su, P.-H., Chen, L.-J. & Li, H.-M. Chloroplast Hsp93 directly binds to transit peptides at an early stage of the preprotein import process. Plant Physiol. 170, 857–866 (2016).
Kubis, S. et al. The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell 15, 1859–1871 (2003).
Lee, S. et al. Heat shock protein cognate 70-4 and an E3 ubiquitin ligase, CHIP, mediate plastid-destined precursor degradation through the ubiquitin–26S proteasome system in Arabidopsis. Plant Cell 21, 3984–4001 (2009).
Fellerer, C., Schweiger, R., Schongruber, K., Soll, J. & Schwenkert, S. Cytosolic HSP90 cochaperones HOP and FKBP interact with freshly synthesized chloroplast preproteins of Arabidopsis. Mol. Plant 4, 1133–1145 (2011).
Wrobel, L. et al. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524, 485–488 (2015).
Weidberg, H. & Amon, A. MitoCPR-A surveillance pathway that protects mitochondria in response to protein import stress. Science 360, eaan4146 (2018).
Kovacheva, S., Bedard, J., Wardle, A., Patel, R. & Jarvis, P. Further in vivo studies on the role of the molecular chaperone, Hsp93, in plastid protein import. Plant J. 50, 364–379 (2007).
Zhao, X., Huang, J. & Chory, J. genome uncoupled1 mutants are hypersensitive to norflurazon and lincomycin. Plant Physiol. 178, 960–964 (2018).
Kimura, Y., Yahara, I. & Lindquist, S. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science 268, 1362–1365 (1995).
Cutforth, T. & Rubin, G. M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77, 1027–1036 (1994).
Zhang, X. C., Millet, Y. A., Cheng, Z., Bush, J. & Ausubel, F. M. Jasmonate signalling in Arabidopsis involves SGT1b–HSP70–HSP90 chaperone complexes. Nat. Plants 1, 15049 (2015).
Wang, R. H. et al. HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nat. Commun. 7, 10269 (2016).
Kindgren, P., Noren, L., Lopez Jde, D., Shaikhali, J. & Strand, A. Interplay between heat shock protein 90 and HY5 controls PhANG expression in response to the GUN5 plastid signal. Mol. Plant 5, 901–913 (2012).
Kindgren, P. et al. A novel proteomic approach reveals a role for Mg-protoporphyrin IX in response to oxidative stress. Physiol. Plant 141, 310–320 (2011).
Cardamone, M. D. et al. Mitochondrial retrograde signaling in mammals is mediated by the transcriptional cofactor GPS2 via direct mitochondria-to-nucleus translocation. Mol. Cell 69, 757–772 (2018).
Quiros, P. M., Mottis, A. & Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 17, 213–226 (2016).
Chan, K. X., Phua, S. Y., Crisp, P., McQuinn, R. & Pogson, B. J. Learning the languages of the chloroplast: retrograde signaling and beyond. Annu. Rev. Plant Biol. 67, 25–53 (2016).
Wang, X. & Chen, X. J. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature 524, 481–484 (2015).
Jarvis, P. et al. An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282, 100–103 (1998).
Huang, W., Ling, Q., Bedard, J., Lilley, K. & Jarvis, P. In vivo analyses of the roles of essential Omp85-related proteins in the chloroplast outer envelope membrane. Plant Physiol. 157, 147–159 (2011).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 15, 473–497 (1962).
Azimzadeh, J. et al. Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 20, 2146–2159 (2008).
Grefen, C. et al. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J. 64, 355–365 (2010).
Scharff, L. B. & Koop, H. U. Linear molecules of tobacco ptDNA end at known replication origins and additional loci. Plant Mol. Biol. 62, 611–621 (2006).
Zoschke, R., Watkins, K. P. & Barkan, A. A rapid ribosome profiling method elucidates chloroplast ribosome behavior in vivo. Plant Cell 25, 2265–2275 (2013).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 (2001).
Cahoon, E. B., Shanklin, J. & Ohlrogge, J. B. Expression of a coriander desaturase results in petroselinic acid production in transgenic tobacco. Proc. Natl Acad. Sci. USA 89, 11184–11188 (1992).
Czarnecki, O. et al. An Arabidopsis GluTR binding protein mediates spatial separation of 5-aminolevulinic acid synthesis in chloroplasts. Plant Cell 23, 4476–4491 (2011).
Barkan, A. Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 297, 38–57 (1998).
Aronsson, H. & Jarvis, R. P. Rapid isolation of Arabidopsis chloroplasts and their use for in vitro protein import assays. Methods Mol. Biol. 774, 281–305 (2011).
Mou, Z., He, Y., Dai, Y., Liu, X. & Li, J. Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12, 405–418 (2000).
Walz, C., Juenger, M., Schad, M. & Kehr, J. Evidence for the presence and activity of a complete antioxidant defence system in mature sieve tubes. Plant J. 31, 189–197 (2002).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Patton, D. A. et al. An embryo-defective mutant of arabidopsis disrupted in the final step of biotin synthesis. Plant Physiol. 116, 935–946 (1998).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394 (1989).
Moran, R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 69, 1376–1381 (1982).
Froehlich, J. Studying Arabidopsis envelope protein localization and topology using thermolysin and trypsin proteases. Methods Mol. Biol. 774, 351–367 (2011).
Queitsch, C., Sangster, T. A. & Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 417, 618–624 (2002).
Stebbins, C. E. et al. Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89, 239–250 (1997).
Massey, A. J. et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 66, 535–545 (2010).
Balaburski, G. M. et al. A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol. Cancer Res. 11, 219–229 (2013).
Cho, H. J. et al. A small molecule that binds to an ATPase domain of Hsc70 promotes membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator. J. Am. Chem. Soc. 133, 20267–20276 (2011).
Acknowledgements
We thank the MPI-MP GreenTeam for help with plant transformation. We are grateful to D. Leister (Ludwig-Maximilians-University Munich) for providing the gun1-102 seeds, Å. Strand (Umeå University, Umeå, Sweden) for providing seeds of the HSP90 RNAi lines, A. Clarke (Gothenburg University) for providing antibodies against Clp proteins and M. Gorka from MPI-MP for running the mass spectrometry samples to quantify the TOC–TIC subunits. This research was financed by the Max Planck Society, grants from the Deutsche Forschungsgemeinschaft to R.B. (FOR 804; SFB-TRR 175 A04), R.Z. (ZO 302/4-1; SFB-TRR 175 A04) and B.G. (SFB-TRR 175 C04), and grants from the Biotechnology and Biological Sciences Research Council (BBSRC) to R.P.J. (research grant numbers BB/N006372/1 and BB/R009333/1).
Author information
Authors and Affiliations
Contributions
R.B. and G.-Z.W. conceived and designed the research. G.-Z.W. performed most of the experiments. E.H.M. performed the mass spectrometry-based proteomics. A.R. and B.G. investigated the import of the TPB enzymes. M.S. performed the ribosome profiling experiments, analysed and discussed the results with G.-Z.W. and R.Z. Q.L. and G.-Z.W. conducted the protein import assays and analysed the results with R.P.J. M.A.S. performed the photosynthesis measurements. D.W. mapped peptides to the transit peptide regions of chloroplast proteins. R.B. and G.-Z.W. wrote the manuscript, with input from the co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Plants thanks Chanhong Kim, Thomas Pfannschmidt and Jin-Zheng Wang for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–29 and Supplementary Tables 1–5.
Supplementary Data 1
Data from array-based ribosome profiling experiments.
Supplementary Data 2
Mass spectrometry data from co-IP experiments.
Supplementary Data 3
Mass spectrometry data of proteomic analyses of the wild type, the gun1 and clpc1 single mutants and the gun1 clpc1 double mutant.
Rights and permissions
About this article
Cite this article
Wu, GZ., Meyer, E.H., Richter, A.S. et al. Control of retrograde signalling by protein import and cytosolic folding stress. Nat. Plants 5, 525–538 (2019). https://doi.org/10.1038/s41477-019-0415-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0415-y
This article is cited by
-
Modulation of early gene expression responses to water deprivation stress by the E3 ubiquitin ligase ATL80: implications for retrograde signaling interplay
BMC Plant Biology (2024)
-
In planta expression of human polyQ-expanded huntingtin fragment reveals mechanisms to prevent disease-related protein aggregation
Nature Aging (2023)
-
Regulation of Drought and Salt Tolerance by OsSKL2 and OsASR1 in Rice
Rice (2022)
-
De-etiolation-induced protein 1 (DEIP1) mediates assembly of the cytochrome b6f complex in Arabidopsis
Nature Communications (2022)
-
Transcriptomic and proteomic data provide new insights into cold-treated potato tubers with T- and D-type cytoplasm
Planta (2022)