Abstract
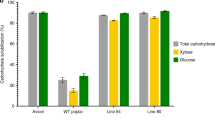
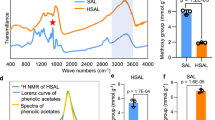
Lignin is the main cause of lignocellulosic biomass recalcitrance to industrial enzymatic hydrolysis. By partially replacing the traditional lignin monomers by alternative ones, lignin extractability can be enhanced. To design a lignin that is easier to degrade under alkaline conditions, curcumin (diferuloylmethane) was produced in the model plant Arabidopsis thaliana via simultaneous expression of the turmeric (Curcuma longa) genes DIKETIDE-CoA SYNTHASE (DCS) and CURCUMIN SYNTHASE 2 (CURS2). The transgenic plants produced a plethora of curcumin- and phenylpentanoid-derived compounds with no negative impact on growth. Catalytic hydrogenolysis gave evidence that both curcumin and phenylpentanoids were incorporated into the lignifying cell wall, thereby significantly increasing saccharification efficiency after alkaline pretreatment of the transgenic lines by 14–24% as compared with the wild type. These results demonstrate that non-native monomers can be synthesized and incorporated into the lignin polymer in plants to enhance their biomass processing efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Boerjan, W., Ralph, J. & Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546 (2003).
Vanholme, R. et al. Metabolic engineering of novel lignin in biomass crops. New Phytol. 196, 978–1000 (2012).
Mottiar, Y., Vanholme, R., Boerjan, W., Ralph, J. & Mansfield, S. D. Designer lignins: harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 37, 190–200 (2016).
Ralph, J. et al. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 3, 29–60 (2004).
Eudes, A. et al. Biosynthesis and incorporation of side-chain-truncated lignin monomers to reduce lignin polymerization and enhance saccharification. Plant Biotechnol. J. 10, 609–620 (2012).
Kim, K. H. et al. Impact of lignin polymer backbone esters on ionic liquid pretreatment of poplar. Biotechnol. Biofuels 10, 101 (2017).
Wilkerson, C. G. et al. Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344, 90–93 (2014).
Zhou, S. et al. Chemical pulping advantages of zip-lignin hybrid poplar. ChemSusChem 10, 3565–3573 (2017).
Bhalla, A. et al. Engineered lignin in poplar biomass facilitates Cu-catalyzed alkaline-oxidative pretreatment. ACS Sustain Chem. Eng. 6, 2932–2941 (2018).
Smith, R. A. et al. Engineering monolignol p-coumarate conjugates into poplar and Arabidopsis lignins. Plant Physiol. 169, 2992–3001 (2015).
Sibout, R. et al. Structural redesigning Arabidopsis lignins into alkali-soluble lignins through the expression of p-coumaroyl-CoA:monolignol transferase PMT. Plant Physiol. 170, 1358–1366 (2016).
Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843 (2014).
Chen, Y.-R. & Tan, T.-H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 17, 173–178 (1998).
Katsuyama, Y., Kita, T., Funa, N. & Horinouchi, S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 284, 11160–11170 (2009).
Hauser, C. R., Swamer, F. W. & Ringler, B. I. Alkaline cleavage of unsymmetrical β-diketones. Ring opening of acylcylohexanones to form ε-acyl caproic acids. J. Am. Chem. Soc. 70, 4023–4026 (1948).
Wang, Y.-J. et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 15, 1867–1876 (1997).
Tomren, M. A., Másson, M., Loftsson, T. & Tønnesen, H. H. Studies on curcumin and curcuminoids: XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int. J. Pharm. 338, 27–34 (2007).
Price, L. C. & Buescher, R. W. Kinetics of alkaline degradation of the food pigments curcumin and curcuminoids. J. Food. Sci. 62, 267–269 (1997).
Pearson, R. G. & Mayerle, E. A. Mechanism of the hydrolytic cleavage of carbon—carbon bonds. I. Alkaline hydrolysis of β-diketones. J. Am. Chem. Soc. 73, 926–930 (1951).
Tønnesen, H. H. & Karlsen, J. Studies on curcumin and curcuminoids. V. Alkaline degradation of curcumin. Z. Lebens. Unters. Forsch. 180, 132–134 (1985).
Tsuji, Y. et al. Introduction of chemically labile substructures into Arabidopsis lignin through the use of LigD, the Cα-dehydrogenase from Sphingobium sp. strain SYK-6. Plant Biotechnol. J. 13, 821–832 (2015).
Criss, D. L., Fisher, T. H. & Schultz, T. P. Alkaline hydrolysis of nonphenolic α-carbonyl β-O-4 lignin dimers substituted on the leaving phenoxide ring: comparison with benzylic hydroxyl analogues. Holzforschung 52, 57–60 (1998).
Mnich, E. et al. Degradation of lignin β‐aryl ether units in Arabidopsis thaliana expressing LigD, LigF and LigG from Sphingomonas paucimobilis SYK‐6. Plant Biotechnol. J. 15, 581–593 (2017).
Imai, A., Yokoyama, T., Matsumoto, Y. & Meshitsukat, G. Significant lability of guaiacylglycerol β-phenacyl ether under alkaline conditions. J. Agric. Food Chem. 55, 9043–9046 (2007).
Freudenberg, K. & Neish, A. C. Constitution and Biosynthesis of Lignin (Springer-Verlag, New York, 1968).
Tobimatsu, Y. et al. Hydroxycinnamate conjugates as potential monolignol replacements: in vitro lignification and cell wall studies with rosmarinic acid. ChemSusChem 5, 676–686 (2012).
Lan, W. et al. Tricin, a flavonoid monomer in monocot lignification. Plant Physiol. 167, 1284–1295 (2015).
Morreel, K. et al. Mass spectrometry-based sequencing of lignin oligomers. Plant Physiol. 153, 1464–1478 (2010).
Morreel, K. et al. Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal. Chem. 82, 8095–8105 (2010).
Tobimatsu, Y. et al. Visualization of plant cell wall lignification using fluorescence‐tagged monolignols. Plant J. 76, 357–366 (2013).
Tobimatsu, Y. et al. A click chemistry strategy for visualization of plant cell wall lignification. Chem. Commun. 50, 12262–12265 (2014).
Singh, R., Tonnesen, H. H., Vogensen, S. B., Loftsson, T. & Masson, M. Studies of curcumin and curcuminoids. XXXVI. The stoichiometry and complexation constants of cyclodextrin complexes as determined by the phase-solubility method and UV-Vis titration. J. Incl. Phenom. Macro. 66, 335–348 (2010).
Katsuyama, Y., Kita, T. & Horinouchi, S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 583, 2799–2803 (2009).
Halpin, C., Cooke, S. E., Barakate, A., Amrani, A. E. & Ryan, M. D. Self‐processing 2A‐polyproteins – a system for co‐ordinate expression of multiple proteins in transgenic plants. Plant J. 17, 453–459 (1999).
Morreel, K. et al. Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol. 136, 3537–3549 (2004).
De Meester, B. et al. Vessel-specific reintroduction of CINNAMOYL-COA REDUCTASE1 (CCR1) in dwarfedccr1 mutants restores vessel and xylary fiber integrity and increases biomass. Plant Physiol. 176, 611–633 (2018).
Vanholme, R. et al. A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell 24, 3506–3529 (2012).
Matsuda, F. et al. AtMetExpress development: a phytochemical atlas of Arabidopsis development. Plant Physiol. 152, 566–578 (2010).
Morreel, K. et al. Systematic structural characterization of metabolites in Arabidopsis via candidate substrate–product pair networks. Plant Cell 26, 929–945 (2014).
Morrison, W. H., Hartley, R. D. & Himmelsbach, D. S. Synthesis of substituted truxillic acids from p-coumaric and ferulic acid: simulation of photodimerization in plant cell walls. J. Agric. Food Chem. 40, 768–771 (1992).
D’Auria, M. & Vantaggi, A. Photochemical dimerization of methoxy substituted cinnamic acid methyl esters. Tetrahedron 48, 2523–2528 (1992).
Hanley, A. B., Russell, W. R. & Chesson, A. Formation of substituted truxillic and truxinic acids in plant cell walls – a rationale. Phytochemistry 33, 957–960 (1993).
Dauwe, R. et al. Molecular phenotyping of lignin-modified tobacco reveals associated changes in cell-wall metabolism, primary metabolism, stress metabolism and photorespiration. Plant J. 52, 263–285 (2007).
Bonawitz, N. D. et al. Disruption of Mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature 509, 376–380 (2014).
Van den Bosch, S. et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 8, 1748–1763 (2015).
Galkin, M. V. & Samec, J. S. M. Selective route to 2-propenyl aryls directly from wood by a tandem organosolv and palladium-catalysed transfer hydrogenolysis. ChemSusChem 7, 2154–2158 (2014).
Galkin, M. V. & Samec, J. S. M. Lignin valorization through catalytic lignocellulose fractionation: a fundamental platform for the future biorefinery. ChemSusChem 9, 1544–1558 (2016).
Renders, T. et al. Synergetic effects of alcohol/water mixing on the catalytic reductive fractionation of poplar wood. ACS Sustain. Chem. Eng. 4, 6894–6904 (2016).
Hendrickson, J. B., Cram, D. J. & Hammond, G. S. Organic Chemistry 3rd edn (McGraw-Hill, Tokyo, 1970).
Rahimi, A., Ulbrich, A., Coon, J. J. & Stahl, S. S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 515, 249–252 (2014).
Van Acker, R. et al. Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in Arabidopsis thaliana. Biotechnol. Biofuels 6, 46 (2013).
Lee, S., Mo, H., Kim, J. I. & Chapple, C. Genetic engineering of Arabidopsis to overproduce disinapoyl esters, potential lignin modification molecules. Biotechnol. Biofuels 10, 40 (2017).
Ralph, J. et al. Identification of the structure and origin of a thioacidolysis marker compound for ferulic acid incorporation into angiosperm lignins (and an indicator for cinnamoyl-CoA reductase deficiency). Plant J. 53, 368–379 (2008).
Sibout, R. & Höfte, H. Plant cell biology: the ABC of monolignol transport. Curr. Biol. 22, R533–R535 (2012).
Karlen, S. D. et al. Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci. Adv. 2, e1600393 (2016).
Leplé, J.-C. et al. Downregulation of cinnamoyl-coenzyme A reductase in poplar: multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure. Plant Cell 19, 3669–3691 (2007).
Van Acker, R. et al. Improved saccharification and ethanol yield from field-grown transgenic poplar deficient in cinnamoyl-CoA reductase. Proc. Natl Acad. Sci. USA 111, 845–850 (2014).
Vanholme, R. et al. Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis. Science 341, 1103–1106 (2013).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Shimada, T. L., Shimada, T. & Hara‐Nishimura, I. A rapid and non‐destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 61, 519–528 (2010).
Sundin, L. et al. Mutation of the inducible ARABIDOPSIS THALIANA CYTOCHROME P450 REDUCTASE2 alters lignin composition and improves saccharification. Plant Physiol. 166, 1956–1971 (2014).
Foster, C. E., Martin, T. M. & Pauly, M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass). Part I: Lignin. J. Vis. Exp 37, e1745 (2010).
Van Acker, R. et al. Different routes for conifer- and sinapaldehyde and higher saccharification upon deficiency in the dehydrogenase CAD1. Plant Physiol. 175, 1018–1039 (2017).
Brendel, O., P.P.M., I. & Stewart, D. A rapid and simple method to isolate pure alpha-cellulose. Phytochem. Anal. 11, 4 (2000).
Van Acker, R., Vanholme, R., Piens, K. & Boerjan, W. Saccharification protocol for small-scale lignocellulosic biomass samples to test processing of cellulose into glucose. Bio-Protocol 6, e1701 (2016).
Acknowledgements
P.O. was funded by the National Commission for Scientific and Technological Research (Chile) for a predoctoral fellowship and by SBO-FISH through the ARBOREF project for a postdoctoral fellowship, B.D.M. and L.d.V. were funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) for predoctoral fellowships, F.F. was funded by the Science Without Borders program from CNPq for a postdoctoral fellowship 206329/2014–8, A.P. was funded by the Research Foundation-Flanders (FWO, grant G0C1914N) and Y.T. was funded by Stanford University’s Global Climate and Energy Program (GCEP). S.V.d.B. was funded by PDM KULeuven and by BIOWOOD (FWO-SBO), J.R. was funded by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494 and DE-SC0018409) and R.V. was funded by the FWO for a postdoctoral fellowship. The authors thank A. Bleys for help in preparing the manuscript, E. Parthoens (VIB BioImaging Core) for assitance with the fluorescence microscopy and D. Loqué for providing the vector containing pCesA4. The work was also funded by a CLEM grant from I. Lieten to the VIB BioImaging Core for the acquisition of a Zeiss LSM780 microscope.
Author information
Authors and Affiliations
Contributions
P.O., B.D.M., J.R., R.V. and W.B. designed the experiments. P.O., B.D.M., F.F., L.d.V., G.G., R.D.R., Y.T., Y.L. and S.V.d.B. performed the experiments. P.O., B.D.M., G.G., A.P., Y.T., B.S., J.R. and R.V. collected and analysed data. P.O., B.D.M., R.V. and W.B. wrote the article with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–11 and Supplementary Tables 1 and 2.
Supplementary Dataset 1
Supplementary Dataset 1.
Rights and permissions
About this article
Cite this article
Oyarce, P., De Meester, B., Fonseca, F. et al. Introducing curcumin biosynthesis in Arabidopsis enhances lignocellulosic biomass processing. Nature Plants 5, 225–237 (2019). https://doi.org/10.1038/s41477-018-0350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0350-3
This article is cited by
-
Tailoring renewable materials via plant biotechnology
Biotechnology for Biofuels (2021)
-
Rice cell suspension culture as a model for producing high-value recombinant proteins and plant specialized metabolites
Plant Cell, Tissue and Organ Culture (PCTOC) (2021)
-
Identification of enzymatic genes with the potential to reduce biomass recalcitrance through lignin manipulation in Arabidopsis
Biotechnology for Biofuels (2020)
-
Pretreatment of sweet sorghum straw and its enzymatic digestion: insight into the structural changes and visualization of hydrolysis process
Biotechnology for Biofuels (2019)
-
The unexpected malleability of lignin
Nature Plants (2019)