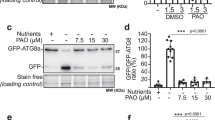
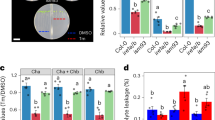
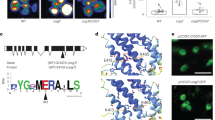
Abstract
Protein homeostasis is essential for cellular functions and longevity, and the loss of proteostasis is one of the hallmarks of senescence. Autophagy is an evolutionarily conserved cellular degradation pathway that is critical for the maintenance of proteostasis. Paradoxically, autophagy deficiency leads to accelerated protein loss by unknown mechanisms. We discover that the ABNORMAL SHOOT3 (ABS3) subfamily of multidrug and toxic compound extrusion transporters promote senescence under natural and carbon-deprivation conditions in Arabidopsis thaliana. The senescence-promoting ABS3 pathway functions in parallel with the longevity-promoting autophagy to balance plant senescence and survival. Surprisingly, ABS3 subfamily multidrug and toxic compound extrusion proteins interact with AUTOPHAGY-RELATED PROTEIN 8 (ATG8) at the late endosome to promote senescence and protein degradation without canonical cleavage and lipidation of ATG8. This non-autophagic ATG8–ABS3 interaction paradigm is probably conserved among dicots and monocots. Our findings uncover a previously unknown non-autophagic function of ATG8 and an unrecognized senescence regulatory pathway controlled by ATG8–ABS3-mediated proteostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Riera, C. E., Merkwirth, C., De Magalhaes Filho, C. D. & Dillin, A. Signaling networks determining life span. Annu. Rev. Biochem. 85, 35–64 (2016).
Li, F. & Vierstra, R. D. Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 17, 526–537 (2012).
Liu, Y. & Bassham, D. C. Autophagy: pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 63, 215–237 (2012).
Xiong, Y. et al. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496, 181–186 (2013).
Sheen, J. Master regulators in plant glucose signaling networks. J. Plant Biol. 57, 67–79 (2014).
Xiong, Y. & Sheen, J. Novel links in the plant TOR kinase signaling network. Curr. Opin. Plant Biol. 28, 83–91 (2015).
Baena-González, E., Rolland, F., Thevelein, J. M. & Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942 (2007).
Lim, P. O., Kim, H. J. & Nam, H. G. Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136 (2007).
Thomas, H. Senescence, ageing and death of the whole plant. New Phytol. 197, 696–711 (2013).
Woo, H. R., Kim, H. J., Nam, H. G. & Lim, P. O. Plant leaf senescence and death—regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 126, 4823–4833 (2013).
Kim, J., Woo, H. R. & Nam, H. G. Toward systems understanding of leaf senescence: an integrated multi-omics perspective on leaf senescence research. Mol. Plant 9, 813–825 (2016).
Guo, Y. & Gan, S.-S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 35, 644–655 (2012).
Liebsch, D. & Keech, O. Dark-induced leaf senescence: new insights into a complex light-dependent regulatory pathway. New Phytol. 212, 563–570 (2016).
Kaur, J. & Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 16, 461–472 (2015).
Xie, Z. & Klionsky, D. J. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 (2007).
Michaeli, S., Galili, G., Genschik, P., Fernie, A. R. & Avin-Wittenberg, T. Autophagy in plants—what's new on the menu?. Trends Plant Sci. 21, 134–144 (2016).
Soto-Burgos, J., Zhuang, X., Jiang, L. & Bassham, D. C. Dynamics of autophagosome formation. Plant Physiol. 176, 219–229 (2018).
Morita, Y., Kataoka, A., Shiota, S., Mizushima, T. & Tsuchiya, T. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J. Bacteriol. 182, 6694–6697 (2000).
Ogawa, T. et al. Stimulating S-adenosyl-l-methionine synthesis extends lifespan via activation of AMPK. Proc. Natl Acad. Sci. USA 113, 11913–11918 (2016).
Otsuka, M. et al. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl Acad. Sci. USA 102, 17923–17928 (2005).
Li, L., He, Z., Pandey, G. K., Tsuchiya, T. & Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 277, 5360–5368 (2002).
Diener, A. C., Gaxiola, R. A. & Fink, G. R. Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13, 1625–1638 (2001).
Rogers, E. E. & Guerinot, M. L. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14, 1787–1799 (2002).
Magalhaes, J. V. et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 39, 1156–1161 (2007).
Marinova, K. et al. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19, 2023–2038 (2007).
Serrano, M. et al. Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol. 162, 1815–1821 (2013).
Li, R. et al. ADP1 affects plant architecture by regulating local auxin biosynthesis. PLoS Genet. 10, e1003954 (2014).
Zhang, H. et al. A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 7, 1522–1532 (2014).
Tian, W. et al. A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 6, 6057 (2015).
Wang, R. et al. A subgroup of MATE transporter genes regulates hypocotyl cell elongation in Arabidopsis. J. Exp. Bot. 66, 6327–6343 (2015).
Zhang, H. et al. Two tonoplast mate proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E2036–E2045 (2017).
Dobritzsch, M. et al. MATE transporter-dependent export of hydroxycinnamic acid amides. Plant Cell 28, 583–596 (2016).
Kim, J. H. et al. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057 (2009).
Sato, Y., Morita, R., Nishimura, M., Yamaguchi, H. & Kusaba, M. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc. Natl Acad. Sci. USA 104, 14169–14174 (2007).
Hanaoka, H. et al. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 129, 1181–1193 (2002).
Doelling, J. H., Walker, J. M., Friedman, E. M., Thompson, A. R. & Vierstra, R. D. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 277, 33105–33114 (2002).
Thompson, A. R., Doelling, J. H., Suttangkakul, A. & Vierstra, R. D. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 138, 2097–2110 (2005).
Phillips, A. R., Suttangkakul, A. & Vierstra, R. D. The ATG12-conjugating enzyme ATG10 is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics 178, 1339–1353 (2008).
Svenning, S., Lamark, T., Krause, K. & Johansen, T. Plant NBR1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters NBR1 and p62/SQSTM1. Autophagy 7, 993–1010 (2011).
Cui, Y. et al. Biogenesis of plant prevacuolar multivesicular bodies. Mol. Plant 9, 774–786 (2016).
Lee, G.-J., Sohn, E. J., Lee, M. H. & Hwang, I. The Arabidopsis rab5 homologs rha1 and ara7 localize to the prevacuolar compartment. Plant Cell Physiol. 45, 1211–1220 (2004).
Bindels, D. S. et al. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14, 53–56 (2017).
Waadt, R. et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 56, 505–516 (2008).
Bashline, L. & Gu, Y. Using the split-ubiquitin yeast two-hybrid system to test protein–protein interactions of transmembrane proteins. Methods Mol. Biol. 1242, 143–158 (2015).
Tanaka, Y. et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature 496, 247–251 (2013).
Miyauchi, H. et al. Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat. Commun. 8, 1633 (2017).
Birgisdottir, Å. B., Lamark, T. & Johansen, T. The LIR motif—crucial for selective autophagy. J. Cell Sci. 126, 3237–3247 (2013).
Jacomin, A.-C., Samavedam, S., Charles, H. & Nezis, I. P. iLIR@viral: a web resource for LIR motif-containing proteins in viruses. Autophagy 13, 1782–1789 (2017).
Subramani, S. & Malhotra, V. Non-autophagic roles of autophagy-related proteins. EMBO Rep. 14, 143–151 (2013).
Schaaf, M. B. E., Keulers, T. G., Vooijs, M. A. & Rouschop, K. M. A. LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J. 30, 3961–3978 (2016).
Kriegenburg, F., Ungermann, C. & Reggiori, F. Coordination of autophagosome–lysosome fusion by ATG8 family members. Curr. Biol. 28, R512–R518 (2018).
Wild, P., McEwan, D. G. & Dikic, I. The LC3 interactome at a glance. J. Cell Sci. 127, 3–9 (2014).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Lai, Z., Wang, F., Zheng, Z., Fan, B. & Chen, Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 66, 953–968 (2011).
Geldner, N. et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59, 169–178 (2009).
Yu, F., Park, S. & Rodermel, S. R. The Arabidopsis FtsH metalloprotease gene family: interchangeability of subunits in chloroplast oligomeric complexes. Plant J. 37, 864–876 (2004).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Lichtenthaler, H. K. [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987).
Schägger, H. & von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987).
Zhuang, X. et al. A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell 25, 4596–4615 (2013).
Yoo, S.-D., Cho, Y.-H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Avila, J. R., Lee, J. S. & Torii, K. U. Co-immunoprecipitation of membrane-bound receptors. Arabidopsis Book 13, e0180 (2015).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31570267 to F.Y., 31770205 to X.L. and 31741010 to Y.Q.) and Northwest A&F University (2452016001 to F.Y.). Y.W., L.S. and J.S. were supported by US National Institute of Health grant R01GM06493. We thank the Teaching and Research Core Facility at the College of Life Sciences, NWAFU for support in this work. We thank members of the Sheen Laboratory and K. Mao of Massachusetts General Hospital and Harvard Medical School, USA for stimulating discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
X.L., J.Sheen and F.Y. conceived the study and designed the experiments. M.J., H.X, R.W., Y.C., N.X., J.Z., J.Shao and Y.Q. performed the experiments. M.J., X.L., Y.W., L.S. and L.A. analysed the data. M.J., X.L., J.Sheen and F.Y. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Jia, M., Liu, X., Xue, H. et al. Noncanonical ATG8–ABS3 interaction controls senescence in plants. Nature Plants 5, 212–224 (2019). https://doi.org/10.1038/s41477-018-0348-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0348-x
This article is cited by
-
Enigmas of senescence: a reappraisal on the hormonal crosstalk and the molecular mechanisms
Theoretical and Experimental Plant Physiology (2024)
-
Phospholipid:diacylglycerol acyltransferase1-overexpression stimulates lipid turnover, oil production and fitness in cold-grown plants
BMC Plant Biology (2023)
-
Transmembrane potential, an indicator in situ reporting cellular senescence and stress response in plant tissues
Plant Methods (2023)
-
The ABA–AtNAP–SAG113 PP2C module regulates leaf senescence by dephoshorylating SAG114 SnRK3.25 in Arabidopsis
Molecular Horticulture (2023)
-
A deeply conserved protease, acylamino acid-releasing enzyme (AARE), acts in ageing in Physcomitrella and Arabidopsis
Communications Biology (2023)