Abstract
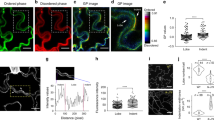
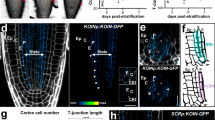
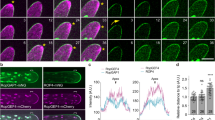
Cell polarity, manifested by the localization of proteins to distinct polar plasma membrane domains, is a key prerequisite of multicellular life. In plants, PIN auxin transporters are prominent polarity markers crucial for a plethora of developmental processes. Cell polarity mechanisms in plants are distinct from other eukaryotes and still largely elusive. In particular, how the cell polarities are propagated and maintained following cell division remains unknown. Plant cytokinesis is orchestrated by the cell plate—a transient centrifugally growing endomembrane compartment ultimately forming the cross wall1. Trafficking of polar membrane proteins is typically redirected to the cell plate, and these will consequently have opposite polarity in at least one of the daughter cells2,3,4,5. Here, we provide mechanistic insights into post-cytokinetic re-establishment of cell polarity as manifested by the apical, polar localization of PIN2. We show that the apical domain is defined in a cell-intrinsic manner and that re-establishment of PIN2 localization to this domain requires de novo protein secretion and endocytosis, but not basal-to-apical transcytosis. Furthermore, we identify a PINOID-related kinase WAG1, which phosphorylates PIN2 in vitro6 and is transcriptionally upregulated specifically in dividing cells, as a crucial regulator of post-cytokinetic PIN2 polarity re-establishment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Change history
14 December 2018
In the version of this Article originally published, Supplementary Video 2 was incorrectly linked to Supplementary Video 1, Supplementary Video 3 was incorrectly linked to Supplementary Video 2, Supplementary Video 4 was incorrectly linked to Supplementary Video 3 and Supplementary Video 5 was incorrectly linked to Supplementary Video 4. Additionally, Supplementary Video 5 was missing from the linked files. The files have now been replaced to rectify this.
References
Smertenko, A. et al. Plant cytokinesis: terminology for structures and processes. Trends Cell Biol. 27, 885–894 (2017).
Geldner, N. et al. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428 (2001).
Mravec, J. et al. Cell plate restricted association of DRP1A and PIN proteins is required for cell polarity establishment in Arabidopsis. Curr. Biol. 21, 1055–1060 (2011).
Men, S. et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 10, 237–244 (2008).
Yoshinari, A. et al. DRP1-dependent endocytosis is essential for polar localization and boron-induced degradation of the borate transporter BOR1 in Arabidopsis thaliana. Plant Cell Physiol. 57, 1985–2000 (2016).
Dhonukshe, P. et al. Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 137, 3245–3255 (2010).
Kania, U. et al. Polar delivery in plants; commonalities and differences to animal epithelial cells. Open Biol. 4, 140017 (2014).
Abas, L. et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8, 249–256 (2006).
Baster, P. et al. SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 32, 260–274 (2013).
Dhonukshe, P. et al. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10, 137–150 (2006).
Gurskaya, N. G. et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461–465 (2006).
Richter, S. et al. Delivery of endocytosed proteins to the cell-division plane requires change of pathway from recycling to secretion. eLife 3, e02131 (2014).
Lauber, M. H. et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J. Cell Biol. 139, 1485–1493 (1997).
Sparks, E. et al. Spatiotemporal signalling in plant development. Nat. Rev. Genet. 14, 631–644 (2013).
Mazur, E. et al. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci. Rep. 6, 33754 (2016).
Prát, T. et al. WRKY23 is a component of the transcriptional network mediating auxin feedback on PIN polarity. PLoS Genet. 14, e1007177 (2018).
Simon, S. et al. Defining the selectivity of processes along the auxin response chain: a study using auxin analogues. New Phytol. 200, 1034–1048 (2013).
Sena, G. et al. Organ regeneration does not require a functional stem cell niche in plants. Nature 457, 1150–1153 (2009).
Kleine-Vehn, J. et al. Cellular and molecular requirements for polar PIN targeting and transcytosis in plants. Mol. Plant 1, 1056–1066 (2008).
Kleine-Vehn, J. et al. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol. Syst. Biol. 7, 540 (2011).
Feraru, E. et al. PIN polarity maintenance by the cell wall in Arabidopsis. Curr. Biol. 21, 338–343 (2011).
Martinière, A. et al. Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl Acad. Sci. USA 109, 12805–12810 (2012).
Kitakura, S. et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 23, 1920–1931 (2011).
Adamowski, M. et al. A functional study of AUXILIN-LIKE1 and 2, two putative clathrin uncoating factors in Arabidopsis. Plant Cell 30, 700–716 (2018).
Friml, J. et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306, 862–865 (2004).
Michniewicz, M. et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056 (2007).
Kleine-Vehn, J. et al. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 21, 3839–3849 (2009).
Zhang, J. et al. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc. Natl Acad. Sci. USA 107, 918–922 (2010).
Huang, F. et al. Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22, 1129–1142 (2010).
Weller, B. et al. Dynamic PIN-FORMED auxin efflux carrier phosphorylation at the plasma membrane controls auxin efflux-dependent growth. Proc. Natl Acad. Sci. USA 114, E887–E896 (2017).
Furutani, M. et al. Polar-localized NPH3-like proteins regulate polarity and endocytosis of PIN-FORMED auxin efflux carriers. Development 138, 2069–2078 (2011).
Salanenka, Y. et al. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc. Natl Acad. Sci. USA 115, 3716–3721 (2018).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Luschnig, C. et al. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187 (1998).
von Wangenheim, D. et al. Live tracking of moving samples in confocal microscopy for vertically grown roots. eLife 6, e26792 (2017).
Jásik, J. et al. PIN2 turnover in Arabidopsis root epidermal cells explored by the photoconvertible protein Dendra2. PLoS ONE 8, e61403 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Grabov, A. et al. Morphometric analysis of root shape. New Phytol. 165, 641–651 (2005).
Acknowledgements
We thank N. Geldner, C. Luschnig, G. Jürgens, R. Offringa and Y. Takano for sharing published material. We would also like to acknowledge M. Adamowski, U. Kania and C. Cuesta for providing entry clones, and the Biomaging Facility at IST Austria for providing excellent imaging service and assistance. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme/ERC grant agreement no. 742985. Additionally, funding was received from the Ministry of Education of the Czech Republic/MŠMT project NPUI - LO1417.
Author information
Authors and Affiliations
Contributions
M.G. designed experiments, performed experiments, analysed data and wrote the manuscript. M.F. designed experiments, analysed data and edited the manuscript. J.F. initiated the project, acquired funding, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–6 and Supplementary Table 1.
Supplementary Video 1
PIN2::PIN2–Dendra dynamics in dividing cells. The experiment was repeated independently three times with similar results.
Supplementary Video 2
KN::PIN2–GFP dynamics in dividing cells. The experiment was repeated independently more than three times with similar results.
Supplementary Video 3
Effect of AXL2 overexpression on KN::PIN2–GFP dynamics. The experiment was repeated independently three times with similar results.
Supplementary Video 4
KN::PIN2–GFP dynamics in pid– wag1– wag2– mutant background. The experiment was repeated independently three times with similar results.
Supplementary Video 5
WAG1::WAG1–GFP dynamics in dividing cells. The experiment was repeated independently three times with similar results.
Rights and permissions
About this article
Cite this article
Glanc, M., Fendrych, M. & Friml, J. Mechanistic framework for cell-intrinsic re-establishment of PIN2 polarity after cell division. Nature Plants 4, 1082–1088 (2018). https://doi.org/10.1038/s41477-018-0318-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0318-3
This article is cited by
-
Antigravitropic PIN polarization maintains non-vertical growth in lateral roots
Nature Plants (2023)
-
Rhizobia induce SYMRK endocytosis in Phaseolus vulgaris root hair cells
Planta (2023)
-
Auxin regulation on crop: from mechanisms to opportunities in soybean breeding
Molecular Breeding (2023)
-
Distinct mechanisms orchestrate the contra-polarity of IRK and KOIN, two LRR-receptor-kinases controlling root cell division
Nature Communications (2022)
-
WAVY GROWTH Arabidopsis E3 ubiquitin ligases affect apical PIN sorting decisions
Nature Communications (2022)