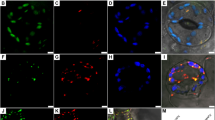
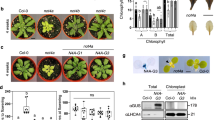
Abstract
Mitochondria and chloroplasts (plastids) both harbour extranuclear DNA that originates from the ancestral endosymbiotic bacteria. These organelle DNAs (orgDNAs) encode limited genetic information but are highly abundant, with multiple copies in vegetative tissues, such as mature leaves. Abundant orgDNA constitutes a substantial pool of organic phosphate along with RNA in chloroplasts, which could potentially contribute to phosphate recycling when it is degraded and relocated. However, whether orgDNA is degraded nucleolytically in leaves remains unclear. In this study, we revealed the prevailing mechanism in which organelle exonuclease DPD1 degrades abundant orgDNA during leaf senescence. The DPD1 degradation system is conserved in seed plants and, more remarkably, we found that it was correlated with the efficient use of phosphate when plants were exposed to nutrient-deficient conditions. The loss of DPD1 compromised both the relocation of phosphorus to upper tissues and the response to phosphate starvation, resulting in reduced plant fitness. Our findings highlighted that DNA is also an internal phosphate-rich reservoir retained in organelles since their endosymbiotic origin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Accession numbers of the genes used in this study are listed in Supplementary Table 9. Precise P values calculated by statistical tests in this study are listed in Supplementary Table 10. The raw data used to construct graphs in this study are presented as Supplementary Dataset. The raw transcriptomic data are deposited in the DDBJ with the accession number DRA007138, under the BioProject with the accession number PRJDB7233. All transcriptomic data used in Fig. 5 and Supplementary Figs. 10 and 11 are available in Supplementary Tables 1–8.
References
Dyall, S. D., Brown, M. T. & Johnson, P. J. Ancient invasions: from endosymbionts to organelles. Science 304, 253–257 (2004).
Gray, M. W. Evolution of organellar genomes. Curr. Opin. Genet. Dev. 9, 678–687 (1999).
Sugiura, M. History of chloroplast genomics. Photosynth. Res. 76, 371–377 (2003).
Wallace, D. C. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 76, 781–821 (2007).
Sato, S., Nakamura, Y., Kaneko, T., Asamizu, E. & Tabata, S. Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res. 6, 283–290 (1999).
Jarvis, P. & Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 14, 787–802 (2013).
Sakamoto, W., Miyagishima, S. Y. & Jarvis, P. Chloroplast biogenesis: control of plastid development, protein import, division and inheritance. Arabidopsis Book 6, e0110 (2008).
Gualberto, J. M. & Newton, K. J. Plant mitochondrial genomes: dynamics and mechanisms of mutation. Annu. Rev. Plant Biol. 68, 225–252 (2017).
Marechal, A. & Brisson, N. Recombination and the maintenance of plant organelle genome stability. New Phytol. 186, 299–317 (2010).
Oldenburg, D. J. & Bendich, A. J. DNA maintenance in plastids and mitochondria of plants. Front. Plant Sci. 6, 883 (2015).
Rauwolf, U., Golczyk, H., Greiner, S. & Herrmann, R. G. Variable amounts of DNA related to the size of chloroplasts III. Biochemical determinations of DNA amounts per organelle. Mol. Genet. Genomics 283, 35–47 (2010).
Fujie, M., Kuroiwa, H., Kawano, S., Mutoh, S. & Kuroiwa, T. Behavior of organelles and their nucleoids in the shoot apical meristem during leaf development in Arabidopsis thaliana L. Planta 194, 395–405 (1994).
Zoschke, R., Liere, K. & Borner, T. From seedling to mature plant: Arabidopsis plastidial genome copy number, RNA accumulation and transcription are differentially regulated during leaf development. Plant J. 50, 710–722 (2007).
Dean, C. & Leech, R. M. Genome expression during normal leaf development: I. Cellular and chloroplast numbers and DNA, RNA, and protein levels in tissues of different ages within a seven-day-old wheat leaf. Plant Physiol. 69, 904–910 (1982).
Veneklaas, E. J. et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 195, 306–320 (2012).
Golczyk, H. et al. Chloroplast DNA in mature and senescing leaves: a reappraisal. Plant Cell 26, 847–854 (2014).
Oldenburg, D. J., Rowan, B. A., Kumar, R. A. & Bendich, A. J. On the fate of plastid DNA molecules during leaf development: response to the Golczyk et al. commentary. Plant Cell 26, 855–861 (2014).
Sato, M. & Sato, K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim. Biophys. Acta 1833, 1979–1984 (2013).
Kuroiwa, T. Review of cytological studies on cellular and molecular mechanisms of uniparental (maternal or paternal) inheritance of plastid and mitochondrial genomes induced by active digestion of organelle nuclei (nucleoids). J. Plant Res. 123, 207–230 (2010).
Zhou, Q. et al. Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science 353, 394–399 (2016).
Nishimura, Y. et al. An mt + gamete-specific nuclease that targets mt − chloroplasts during sexual reproduction in C. reinhardtii. Genes Dev. 16, 1116–1128 (2002).
Matsushima, R. et al. A conserved, Mg2+-dependent exonuclease degrades organelle DNA during Arabidopsis pollen development. Plant Cell 23, 1608–1624 (2011).
Sakamoto, W. & Takami, T. Nucleases in higher plants and their possible involvement in DNA degradation during leaf senescence. J. Exp. Bot. 65, 3835–3843 (2014).
Portis, A. R. Jr & Heldt, H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim. Biophys. Acta 449, 434–436 (1976).
Parent, J. S., Lepage, E. & Brisson, N. Divergent roles for the two PolI-like organelle DNA polymerases of Arabidopsis. Plant Physiol. 156, 254–262 (2011).
Moriyama, T. & Sato, N. Enzymes involved in organellar DNA replication in photosynthetic eukaryotes. Front. Plant Sci. 5, 480 (2014).
Wang, D. Y. et al. The levels of male gametic mitochondrial DNA are highly regulated in angiosperms with regard to mitochondrial inheritance. Plant Cell 22, 2402–2416 (2010).
Preuten, T. et al. Fewer genes than organelles: extremely low and variable gene copy numbers in mitochondria of somatic plant cells. Plant J. 64, 948–959 (2010).
Arimura, S. I. Fission and fusion of plant mitochondria, and genome maintenance. Plant Physiol. 176, 152–161 (2018).
Conn, S. J. et al. Protocol: optimising hydroponic growth systems for nutritional and physiological analysis of Arabidopsis thaliana and other plants. Plant Methods 9, 4 (2013).
Rubio, V. et al. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 15, 2122–2133 (2001).
Castrillo, G. et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 543, 513–518 (2017).
Krapp, A. et al. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 157, 1255–1282 (2011).
Peng, M., Bi, Y. M., Zhu, T. & Rothstein, S. J. Genome-wide analysis of Arabidopsis responsive transcriptome to nitrogen limitation and its regulation by the ubiquitin ligase gene NLA. Plant Mol. Biol. 65, 775–797 (2007).
Keskitalo, J., Bergquist, G., Gardestrom, P. & Jansson, S. A cellular timetable of autumn senescence. Plant Physiol. 139, 1635–1648 (2005).
Kurita, Y. et al. Establishment of a shortened annual cycle system; a tool for the analysis of annual re-translocation of phosphorus in the deciduous woody plant (Populus alba L.). J. Plant Res. 127, 545–551 (2014).
Kurita, Y. et al. Inositol hexakis phosphate is the seasonal phosphorus reservoir in the deciduous woody plant Populus alba L. Plant Cell Physiol. 58, 1477–1485 (2017).
Fulgosi, H. et al. Degradation of chloroplast DNA during natural senescence of maple leaves. Tree Physiol. 32, 346–354 (2012).
Sodmergen, Kawano, S., Tano, S. & Kuroiwa, T. Preferential digestion of chloroplast nuclei (nucleoids) during senescence of the coleoptile of Oryza sativa. Protoplasma 152, 65–68 (1989).
Inada, N., Sakai, A., Kuroiwa, H. & Kuroiwa, T. Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles. Planta 206, 585–597 (1998).
Sakamoto, W. & Takami, T. Chloroplast DNA dynamics: copy number, quality control and degradation. Plant Cell Physiol. 59, 1120–1127 (2018).
Zhang, Q., Liu, Y. & Sodmergen. Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol. 44, 941–951 (2003).
Mogensen, H. L. The hows and whys of cytoplasmic inheritance in seed plants. Am. J. Bot. 83, 383–404 (1996).
Corriveau, J. L. & Coleman, A. W. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 75, 1443–1458 (1988).
Tang, L. Y., Matsushima, R. & Sakamoto, W. Mutations defective in ribonucleotide reductase activity interfere with pollen plastid DNA degradation mediated by DPD1 exonuclease. Plant J. 70, 637–649 (2012).
Lim, P. O., Kim, H. J. & Nam, H. G. Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136 (2007).
Gregersen, P. L., Culetic, A., Boschian, L. & Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 82, 603–622 (2013).
Krupinska, K. in The Structure and Function of Plastids (eds Wise, R. R. & Hoober, J. K.) 433–449 (Springer, Dordrecht, 2006).
Makino, A. & Osmond, B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 96, 355–362 (1991).
Smith, D. W., Fontenot, E. B., Zhahraeifard, S. & DiTusa, S. F. Molecular components that drive phosphorus-remobilization during leaf senescence. Annu. Plant Rev. 48, 159–186 (2015).
Stigter, K. A. & Plaxton, W. C. Molecular mechanisms of phosphorus metabolism and transport during leaf senescence. Plants (Basel) 4, 773–798 (2015).
Robinson, W. D., Carson, I., Ying, S., Ellis, K. & Plaxton, W. C. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 196, 1024–1029 (2012).
Bariola, P. A., MacIntosh, G. C. & Green, P. J. Regulation of S-like ribonuclease levels in Arabidopsis. Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol. 119, 331–342 (1999).
Perez-Amador, M. A. et al. Identification of BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. Plant Physiol. 122, 169–180 (2000).
Matallana-Ramirez, L. P. et al. NAC transcription factor ORE1 and senescence-induced BIFUNCTIONAL NUCLEASE1 (BFN1) constitute a regulatory cascade in Arabidopsis. Mol. Plant 6, 1432–1452 (2013).
Liere, K. & Borner, T. in Plastid Development in Leaves During Growth and Senescence. Advances in Photosynthesis and Respiration Vol. 36 (eds Biswal, B. et al.) 215–237 (Springer, Dordrecht, 2013).
Chiou, T. J. & Lin, S. I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 62, 185–206 (2011).
Versaw, W. K. & Garcia, L. R. Intracellular transport and compartmentation of phosphate in plants. Curr. Opin. Plant Biol. 39, 25–30 (2017).
Liu, T. Y., Lin, W. Y., Huang, T. K. & Chiou, T. J. MicroRNA-mediated surveillance of phosphate transporters on the move. Trends Plant Sci. 19, 647–655 (2014).
Ishida, H., Izumi, M., Wada, S. & Makino, A. Roles of autophagy in chloroplast recycling. Biochim. Biophys. Acta 1837, 512–521 (2014).
Bendich, A. J. Circular chloroplast chromosomes: the grand illusion. Plant Cell 16, 1661–1666 (2004).
Sears, B. B. & VanWinkle-Swift, K. The salvage/turnover/repair (STOR) model for uniparental inheritance in Chlamydomonas: DNA as a source of sustenance. J. Hered. 85, 366–376 (1994).
Yang, Y. G., Lindahl, T. & Barnes, D. E. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131, 873–886 (2007).
Acknowledgements
We thank R. Hijiya (Institute of Plant Science and Resources, Okayama University, Kurashiki, Japan) for technical support, H. Kanegae (Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan) for assisting mtDNA sequence alignment in Populus species and K. Baba (Research Institute for Sustainable Humanosphere, Kyoto University, Kyoto, Japan) for supporting poplar leaf sampling. This work was supported by KAKENHI grants from JSPS (16H06554 and 17H03699 to W.S.) and from the Oohara Foundation (to W.S.).
Author information
Authors and Affiliations
Contributions
W.S. designed the project. T.T. performed all qPCR and qRT–PCR measurements for various environments, in addition to photosynthetic activity measurements and RNA-seq analysis. N.O. performed the nuclease assays. Y.K., S.I., M.O. and T.M. prepared the poplar samples and conducted primary work related to poplar. T.T., M.K. and W.S. analysed the data. W.S. wrote the manuscript with consultation among all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–15 and Supplementary Table 9.
Supplementary Tables 1–8
Supplementary Table 1: Differential expression analysis of Col under P deprivation by edgeR. Supplementary Table 2: Differential expression analysis of dpd1-1 under P deprivation by edgeR. Supplementary Table 3: Expression profile of literature-curated phosphate starvation marker genes. Supplementary Table 4: Expression profile of PHR1 regulon genes. Supplementary Table 5: Differential expression analysis of Col under N deprivation by edgeR. Supplementary Table 6: Differential expression analysis of dpd1-1 under N deprivation by edgeR. Supplementary Table 7: Comparative analysis between Krapp et al. (ref. 32) and this study on upregulated gene profile under long term nitrogen starvation. Supplementary Table 8: Comparative analysis between Peng et al. (ref. 34) and this study on upregulated gene profile under long term nitrogen starvation.
Supplementary Supplementary Table 10
Precise P values in this study.
Supplementary Dataset
Raw data used to construct bar graphs.
Rights and permissions
About this article
Cite this article
Takami, T., Ohnishi, N., Kurita, Y. et al. Organelle DNA degradation contributes to the efficient use of phosphate in seed plants. Nature Plants 4, 1044–1055 (2018). https://doi.org/10.1038/s41477-018-0291-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0291-x
This article is cited by
-
An update on Glycerophosphodiester Phosphodiesterases; From Bacteria to Human
The Protein Journal (2024)
-
The ABA–AtNAP–SAG113 PP2C module regulates leaf senescence by dephoshorylating SAG114 SnRK3.25 in Arabidopsis
Molecular Horticulture (2023)
-
Control of plastid inheritance by environmental and genetic factors
Nature Plants (2023)
-
Comparative physiology of canopy tree leaves in evergreen and deciduous forests in lowland Thailand
Scientific Data (2023)
-
Metabolic footprints in phosphate-starved plants
Physiology and Molecular Biology of Plants (2023)