Abstract
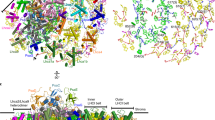
The photosynthesis machinery in chloroplast thylakoid membranes is comprised of multiple protein complexes and supercomplexes1,2. Here, we show a novel supramolecular organization of photosystem I (PSI) in the moss Physcomitrella patens by single-particle cryo-electron microscopy. The moss-specific light-harvesting complex (LHC) protein Lhcb9 is involved in this PSI supercomplex, which has been shown to have a molecular density similar to that of the green alga Chlamydomonas reinhardtii3. Our results show that the structural organization is unexpectedly different—two rows of the LHCI belt exist as in C. reinhardtii4, but the outer one is shifted toward the PsaK side. Furthermore, one trimeric LHC protein and one monomeric LHC protein position alongside PsaL/K, filling the gap between these subunits and the outer LHCI belt. We provide evidence showing that Lhcb9 is a key factor, acting as a linkage between the PSI core and the outer LHCI belt to form the unique supramolecular organization of the PSI supercomplex in P. patens.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The 3D cryo-EM density map of Pp-PSI-L has been deposited in the Electron Microscopy Data Bank under accession code EMD-9107, and with the Protein Data Bank (PDB) under accession code 6MEM. The data are available from the corresponding authors (M.I. and K.K.N.) upon request.
References
Dekker, J. P. & Boekema, E. J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706, 12–39 (2005).
Nelson, N. & Yocum, C. F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 57, 521–565 (2006).
Iwai, M. et al. Light-harvesting complex Lhcb9 confers a green alga-type photosystem I supercomplex to the moss Physcomitrella patens. Nat. Plants 1, 14008 (2015).
Drop, B. et al. Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J. Biol. Chem. 286, 44878–44887 (2011).
Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 (1999).
Wobbe, L., Bassi, R. & Kruse, O. Multi-level light capture control in plants and green algae. Trends Plant Sci. 21, 55–68 (2016).
Nelson, N. & Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 84, 659–683 (2015).
Büchel, C. Evolution and function of light harvesting proteins. J. Plant Physiol. 172, 62–75 (2015).
Neilson, J. A. & Durnford, D. G. Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes. Photosynth. Res. 106, 57–71 (2010).
Jensen, P. E. et al. Structure, function and regulation of plant photosystem I. Biochim. Biophys. Acta 1767, 335–352 (2007).
Qin, X., Suga, M., Kuang, T. & Shen, J. R. Structural basis for energy transfer pathways in the plant PSI–LHCI supercomplex. Science 348, 989–995 (2015).
Mazor, Y., Borovikova, A., Caspy, I. & Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 3, 17014 (2017).
Wientjes, E., Oostergetel, G. T., Jansson, S., Boekema, E. J. & Croce, R. The role of Lhca complexes in the supramolecular organization of higher plant photosystem I. J. Biol. Chem. 284, 7803–7810 (2009).
Germano, M. et al. Supramolecular organization of photosystem I and light-harvesting complex I in Chlamydomonas reinhardtii. FEBS Lett. 525, 121–125 (2002).
Kargul, J., Nield, J. & Barber, J. Three-dimensional reconstruction of a light-harvesting complex I-photosystem I (LHCI-PSI) supercomplex from the green alga Chlamydomonas reinhardtii. Insights into light harvesting for PSI. J. Biol. Chem. 278, 16135–16141 (2003).
Rensing, S. A. et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319, 64–69 (2008).
Alboresi, A., Caffarri, S., Nogue, F., Bassi, R. & Morosinotto, T. In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: identification of subunits which evolved upon land adaptation. PLoS ONE 3, e2033 (2008).
Iwai, M. & Yokono, M. Light-harvesting antenna complexes in the moss Physcomitrella patens: implications for the evolutionary transition from green algae to land plants. Curr. Opin. Plant Biol. 37, 94–101 (2017).
Alboresi, A., Gerotto, C., Cazzaniga, S., Bassi, R. & Morosinotto, T. A red-shifted antenna protein associated with photosystem II in Physcomitrella patens. J. Biol. Chem. 286, 28978–28987 (2011).
Busch, A. et al. Composition and structure of photosystem I in the moss Physcomitrella patens. J. Exp. Bot. 64, 2689–2699 (2013).
Drop, B., Yadav, K. N. S., Boekema, E. J. & Croce, R. Consequences of state transitions on the structural and functional organization of photosystem I in the green alga Chlamydomonas reinhardtii. Plant J. 78, 181–191 (2014).
Kouřil, R. et al. Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44, 10935–10940 (2005).
Olsson, T., Thelander, M. & Ronne, H. A novel type of chloroplast stromal hexokinase is the major glucose-phosphorylating enzyme in the moss Physcomitrella patens. J. Biol. Chem. 278, 44439–44447 (2003).
Thelander, M., Olsson, T. & Ronne, H. Effect of the energy supply on filamentous growth and development in Physcomitrella patens. J. Exp. Bot. 56, 653–662 (2005).
Schumaker, K. S. & Dietrich, M. A. Programmed changes in form during moss development. Plant Cell 9, 1099–1107 (1997).
Sugiyama, T. et al. Involvement of PpDof1 transcriptional repressor in the nutrient condition-dependent growth control of protonemal filaments in Physcomitrella patens. J. Exp. Bot. 63, 3185–3197 (2012).
Pinnola, A. et al. A LHCB9-dependent photosystem I megacomplex induced under low light in Physcomitrella patens. Nat. Plants https://doi.org/10.1038/s41477-018-0270-2 (2018).
Gorman, D. S. & Levine, R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 54, 1665–1669 (1965).
Nishiyama, T., Hiwatashi, Y., Sakakibara, I., Kato, M. & Hasebe, M. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 7, 9–17 (2000).
Iwai, M., Takahashi, Y. & Minagawa, J. Molecular remodeling of photosystem II during state transitions in Chlamydomonas reinhardtii. Plant Cell 20, 2177–2189 (2008).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394 (1989).
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
The QB3/Chemistry Mass Spectrometry Facility at the University of California, Berkeley receives support from the National Institutes of Health (grant 1S10OD020062-01). This work was supported by the US Department of Energy, Office of Science, through the Photosynthetic Systems programme in the Office of Basic Energy Sciences. E.N. and K.K.N. are investigators of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
M.I. designed the research and performed the sample preparation and protein analysis. P.G. performed electron microscopy analysis and image processing. M.I. and P.G. wrote the paper. A.T.I. performed mass spectrometry analysis. E.N. and K.K.N. provided resources and supervision. All authors analysed the data, discussed the results and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–8 and Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Iwai, M., Grob, P., Iavarone, A.T. et al. A unique supramolecular organization of photosystem I in the moss Physcomitrella patens. Nature Plants 4, 904–909 (2018). https://doi.org/10.1038/s41477-018-0271-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0271-1
This article is cited by
-
Uncovering the photosystem I assembly pathway in land plants
Nature Plants (2024)
-
Structural insights into a unique PSI–LHCI–LHCII–Lhcb9 supercomplex from moss Physcomitrium patens
Nature Plants (2023)
-
Structural insights into the assembly and energy transfer of the Lhcb9-dependent photosystem I from moss Physcomitrium patens
Nature Plants (2023)
-
The structure of the Physcomitrium patens photosystem I reveals a unique Lhca2 paralogue replacing Lhca4
Nature Plants (2022)
-
The revolution evolution
Nature Plants (2021)