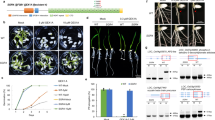
Abstract
Recent findings suggest that alternative splicing has a critical role in controlling the responses of plants to temperature variations. However, alternative splicing factors in plants are largely uncharacterized. Here we establish the putative splice regulator, PORCUPINE (PCP), as temperature-specific regulator of development in Arabidopsis thaliana. Our findings point to the misregulation of WUSCHEL and CLAVATA3 as the possible cause for the meristem defects affecting the pcp-1 loss-of-function mutants at low temperatures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Suzuki, D. T. Science 170, 695–706 (1970).
O’Rourke, S. M. et al. PLoS ONE 6, e16644 (2011).
Eki, T. et al. J. Biol. Chem. 265, 26–33 (1990).
Hartwell, L. H., Culotti, J., & Reid, B. Proc. Natl Acad. Sci. USA 66, 352–359 (1970).
Moir, D., Stewart, S. E., Osmond, B. C. & Botstein, D. Genetics 100, 547–563 (1982).
Pickett, F. B., Champagne, M. M. & Meeks-Wagner, D. R. Development 122, 3799–3807 (1996).
Yasutani, I., Ozawa, S., Nishida, T., Sugiyama, M. & Komamine, A. Plant Physiol. 105, 815–822 (1994).
Verhage, L. et al. PLoS ONE 12, e0172950 (2017).
Streitner, C. et al. Plant Signal. Behav. 8, e24638 (2013).
Capovilla, G., Pajoro, A., Immink, R. G. H. & Schmid, M. Curr. Opin. Plant Biol. 27, 97–103 (2015).
Wang, B.-B. & Brendel, V. Genome Biol. 5, R102 (2004).
Ito, J. et al. J. Proteome Res. 10, 1571–1582 (2011).
Cao, J. et al. J. Biomol. Struct. Dyn. 28, 535–544 (2011).
Kalyna, M., Lopato, S., Voronin, V. & Barta, A. Nucleic Acids Res. 34, 4395–4405 (2006).
Kalyna, M. et al. Nucleic Acids Res. 40, 2454–2469 (2012).
Han, P. & Zhu, Y.-X. Plant Signal. Behav. 4, 52–54 (2009).
Black, D. L., Chabot, B. & Steitz, J. A. Cell 42, 737–750 (1985).
Rosbash, M. & Séraphin, B. Trends Biochem. Sci. 16, 187–190 (1991).
Somssich, M., Je, B. I., Simon, R. & Jackson, D. Development 143, 3238–3248 (2016).
Capovilla, G., Symeonidi, E., Wu, R. & Schmid, M. J. Exp. Bot. 68, 5117–5127 (2017).
de Francisco Amorim, M. et al. Preprint at bioRxiv. https://doi.org/10.1101/150805 (2017).
Kopylova, E., Noé, L. & Touzet, H. Bioinformatics 28, 3211–3217 (2012).
Bolger, A. M., Lohse, M. & Usadel, B. Bioinformatics 30, 2114–2120 (2014).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Nat. Biotechnol. 34, 525–527 (2016).
Berardini, T. Z. et al. Genesis 53, 474–485 (2015).
Gentleman, R. C. et al. Genome Biol. 5, R80 (2004).
Soneson, C., Love, M. I. & Robinson, M. D. F1000Res. 4, 1521 (2015).
Love, M. I., Huber, W. & Anders, S. Genome Biol. 15, 550 (2014).
Anders, S., Reyes, A. & Huber, W. Genome Res. 22, 2008–2017 (2012).
Sundell, D. et al. New Phytol. 208, 1149–1156 (2015).
Supek, F., Bošnjak, M., Škunca, N., & Šmuc, T. PLoS ONE 6, e21800 (2011).
Palatnik, J. F. et al. Nature 425, 257–263 (2003).
Dinneny, J. R., Yadegari, R., Fischer, R. L., Yanofsky, M. F. & Weigel, D. Development 131, 1101–1110 (2004).
Roeder, A. H. K., Ferrándiz, C. & Yanofsky, M. F. Curr. Biol. 13, 1630–1635 (2003).
Cheng, M.-M., Mitra, N. J., Huang, X., Torr, P. H. S. & Hu, S.-M. IEEE Trans. Pattern Anal. Mach. Intell. 37, 569–582 (2015).
Acknowledgements
We thank J. Berger for assistance with scanning electron microscopy, N. Davidson for help with the initial RNA-seq analyses, A. Mangilet for sharing U1-70K plasmids and E. Scacchi for discussion. Supported by the DFG through the Sonderforschungsbereich 1101 (Collaborative Research Centre 1101), project SFB1101/1-C04 and the Knut and Alice Wallenberg Foundation (KAW 2016.0025) to M.S.
Author information
Authors and Affiliations
Contributions
G.C. and M.S. designed the experiments. G.C. performed the RNA-seq experiments, identified and phenotyped the pcp mutants and performed RNA in situ hybridization experiments. S.C. performed the expression analysis of the RNA-seq data obtained from Col-0 grown at 16 °C, 23 °C and 27 °C. N.D. performed the alternative splicing RNA-seq analyses with help from I.S. I.B. analysed the growth rate in pcp-1 seedlings. M.d.F.A. and S.L. generated the U1-70K construct used in this study. E.S. contributed to genotyping of the transgenic lines generated by G.C. G.C. and M.S. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7, Supplementary Tables 1–5 and legends for Supplementary Files 1–4.
Supplementary File 1
List of DE genes in Col-0 at 16°C and 27°C.
Supplementary File 2
Differential expression analyses results for pcp-1 and WT grown under different temperatures.
Supplementary File 3
Lists of DE genes between Col-0 and pcp-1.
Supplementary File 4
Curated list of meristem genes.
Rights and permissions
About this article
Cite this article
Capovilla, G., Delhomme, N., Collani, S. et al. PORCUPINE regulates development in response to temperature through alternative splicing. Nature Plants 4, 534–539 (2018). https://doi.org/10.1038/s41477-018-0176-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0176-z
This article is cited by
-
Importance of pre-mRNA splicing and its study tools in plants
Advanced Biotechnology (2024)
-
Integrated analysis of transcriptome and small RNAome reveals regulatory network of rapid and long-term response to heat stress in Rhododendron moulmainense
Planta (2024)
-
Pervasive under-dominance in gene expression underlying emergent growth trajectories in Arabidopsis thaliana hybrids
Genome Biology (2023)
-
The Arabidopsis spliceosomal protein SmEb modulates ABA responses by maintaining proper alternative splicing of HAB1
Stress Biology (2021)
-
Alternative splicing is a Sorghum bicolor defense response to fungal infection
Planta (2020)