Abstract
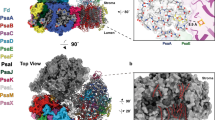
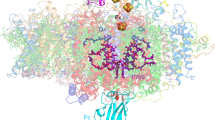
Photosystem I (PSI), a large protein complex located in the thylakoid membrane, mediates the final step in light-driven electron transfer to the stromal electron carrier protein ferredoxin (Fd). Here, we report the first structural description of the PSI–Fd complex from Thermosynechococcus elongatus. The trimeric PSI complex binds three Fds in a non-equivalent manner. While each is recognized by a PSI protomer in a similar orientation, the distances between Fds and the PSI redox centres differ. Fd binding thus entails loss of the exact three-fold symmetry of the PSI’s soluble subunits, inducing structural perturbations which are transferred to the lumen through PsaF. Affinity chromatography and nuclear magnetic resonance analyses of PSI–Fd complexes support the existence of two different Fd-binding states, with one Fd being more tightly bound than the others. We propose a dynamic structural basis for productive complex formation, which supports fast electron transfer between PSI and Fd.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jordan, P. et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411, 909–917 (2001).
Kurisu, G. et al. Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP(+) reductase. Nat. Struct. Biol. 8, 117–121 (2001).
Dai, S. et al. Structural snapshots along the reaction pathway of ferredoxin-thioredoxin reductase. Nature 448, 92–96 (2007).
Kim, J. Y., Nakayama, M., Toyota, H., Kurisu, G. & Hase, T. Structural and mutational studies of an electron transfer complex of maize sulfite reductase and ferredoxin. J. Biochem. 160, 101–109 (2016).
Ruffle, S. V., Mustafa, A. O., Kitmitto, A., Holzenburg, A. & Ford, R. C. The location of the mobile electron carrier ferredoxin in vascular plant photosystem I. J. Biol. Chem. 275, 36250–36255 (2000).
Lelong, C. et al. Characterization of a redox active cross-linked complex between cyanobacterial photosystem I and soluble ferredoxin. EMBO J. 15, 2160–2168 (1996).
Fromme, P., Bottin, H., Krauss, N. & Setif, P. Crystallization and electron paramagnetic resonance characterization of the complex of photosystem I with its natural electron acceptor ferredoxin. Biophys. J. 83, 1760–1773 (2002).
Mutoh, R. et al. X-ray structure and nuclear magnetic resonance analysis of the interaction sites of the Ga-substituted cyanobacterial ferredoxin. Biochemistry 54, 6052–6061 (2015).
Mignée, C., Mutoh, R., Krieger-Liszkay, A., Kurisu, G. & Sétif, P. Gallium ferredoxin as a tool to study the effects of ferredoxin binding to photosystem I without ferredoxin reduction. Photosynth. Res. 134, 251–263 (2017).
Sakakibara, Y. et al. A new structural insight into differential interaction of cyanobacterial and plant ferredoxins with nitrite reductase as revealed by NMR and X-ray crystallographic studies. J. Biochem. 151, 483–492 (2012).
Sétif, P. in Photosystem I: The Light-Driven Plastocyanin:Ferredoxin Oxidoreductase (ed J.H. Golbeck) Ch. 26, 439–454 (Springer, Dordrecht, 2006).
Kovalenko, I. B., Abaturova, A. M., Riznichenko, G. Y. & Rubin, A. B. Computer simulation of interaction of photosystem 1 with plastocyanin and ferredoxin. Biosystems 103, 180–187 (2011).
El-Mohsnawy, E. et al. Structure and function of intact photosystem 1 monomers from the cyanobacterium Thermosynechococcus elongatus. Biochemistry 49, 4740–4751 (2010).
Fischer, N., Sétif, P. & Rochaix, J. D. Site-directed mutagenesis of the PsaC subunit of photosystem I: Fb is the cluster interacting with soluble ferredoxin. J. Biol. Chem. 274, 23333–23340 (1999).
Barth, P., Guillouard, I., Sétif, P. & Lagoutte, B. Essential role of a single arginine of photosystem I in stabilizing the electron transfer complex with ferredoxin. J. Biol. Chem. 275, 7030–7036 (2000).
Bottin, H., Hanley, J. & Lagoutte, B. Role of acidic amino acid residues of PsaD subunit on limiting the affinity of photosystem I for ferredoxin. Biochem. Biophys. Res. Comm. 287, 833–836 (2001).
Hanley, J., Sétif, P., Bottin, H. & Lagoutte, B. Mutagenesis of photosystem I in the region of the ferredoxin cross-linking site: modifications of positively charged amino acids. Biochemistry 35, 8563–8571 (1996).
Chitnis, V. P., Jungs, Y. S., Albee, L., Golbeck, J. H. & Chitnis, P. R. Mutational analysis of photosystem I polypeptides: role of PsaD and the lysyl 106 residue in the reductase activity of the photosystem I. J. Biol. Chem. 271, 11772–11780 (1996).
Liu, H. et al. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 342, 1104–1107 (2013).
Xu, Q., Yu, L., Chitnis, V. P. & Chitnis, P. R. Function and organization of photosystem I in a cyanobacterial mutant strain that lacks PsaF and PsaJ subunits. J. Biol. Chem. 269, 3205–3211 (1994).
Busch, A. & Hippler, M. The structure and function of eukaryotic photosystem I. Biochim. Biophys. Acta 1807, 864–877 (2011).
Jeanjean, R. et al. A photosystem 1 psaFJ-null mutant of the cyanobacterium Synechocystis PCC 6803 expresses the isiAB operon under iron replete conditions. FEBS Lett. 549, 52–56 (2003).
Mazor, Y., Borovikova, A., Caspy, I. & Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nature Plants 3, 17014 (2017).
Amunts, A., Drory, O. & Nelson, N. The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 447, 58–63 (2007).
Qin, X., Suga, M., Kuang, T. & Shen, J. R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348, 989–995 (2015).
Malavath, T., Caspy, I., Netzer-El, S. Y., Klaman, D. & Nelson, N. Structure and function of wild-type and subunit-depleted photosystem I in Synechocystis. Biochim. Biophys. Acta. http://doi.org/cmmt (2018).
Mazor, Y., Nataf, D., Toporik, H. & Nelson, N. Crystal structures of virus-like photosystem I complexes from the mesophilic cyanobacterium Synechocystis PCC 6803. eLife 3, e01496 (2014).
Baker, D. R. et al. Comparative photoactivity and stability of isolated cyanobacterial monomeric and trimeric Photosystem I. J. Phys. Chem. B 118, 2703–2711 (2014).
Klodawska, K. et al. Elevated growth temperature can enhance photosystem I trimer formation and affects xanthophyll biosynthesis in Cyanobacterium Synechocystis sp. PCC6803 cells. Plant Cell Physiol. 56, 558–571 (2015).
Kubota, H. et al. Purification and characterization of photosystem I complex from Synechocystis sp. PCC 6803 by expressing histidine-tagged subunits. Biochim. Biophys. Acta 1797, 98–105 (2010).
Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
The PyMOL Molecular Graphics System, Version 1.3r1 (Schrodinger, LLC, 2010).
Nakanishi, T. et al. Determination of the interface of a large protein complex by transferred cross-saturation measurements. J. Mol. Biol. 318, 245–249 (2002).
Sétif, P. Q. & Bottin, H. Laser flash absorption spectroscopy study of ferredoxin reduction by photosystem I: spectral and kinetic evidence for the existence of several photosystem I-ferredoxin complexes. Biochemistry 34, 9059–9070 (1995).
Akashi, T. et al. Comparison of the electrostatic binding sites on the surface of ferredoxin for two ferredoxin-dependent enzymes, ferredoxin-NADP+ reductase and sulfite reductase. J. Biol. Chem. 274, 29399–29405 (1999).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Info. Model. 51, 2778–2786 (2011).
Acknowledgements
We thank T. Hase for valuable discussions on the Fd-affinity chromatography; T. Kikuchi, P. Liauw, Y. Takagi, E. El-Mohsnawy, N. Muraki and T. Oyama for technical help in the initial stage of this project; E. Yamashita, A. Higashiura and A. Nakagawa at SPring-8, Harima, Japan; and the staff at the Taiwan Light Source, Hsinchu, Taiwan, R.O.C for support during data collection. This work was supported by the Funding Programme for Next Generation World-Leading Researchers (GS016) from the Cabinet Office of Japan (G.K.) and an International Joint Research Promotion Programme, Osaka University (G.K. and M.R.).
Author information
Authors and Affiliations
Contributions
Purification of WTFd was conducted by K.S. Preparation of GaFd was conducted by R.M. Purification of His-tagged PSI was introduced for structural analysis by H.K.-K., M.N. and M.R. Flash-absorption spectroscopy was carried out by P.S. Crystallization and X-ray data collection of PSI–Fd complexes were conducted by H.K-K. The crystal structures of PSI–Fd were solved by H.T., H.K-K. and G.K. NMR analysis was done by R.M. and T.I. G.K. contributed to the design of the experiments and writing the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7 and Supplementary Table 1.
Supplementary GIF 1
Top view of the superimposed models of the free PSI coloured in grey and the Fd-bound PSI trimer in dark green. Each Fd molecule is coloured in yellow, green and cyan.
Supplementary GIF 2
Side view of the same superimposed models of Supplementary GIF 1.
Rights and permissions
About this article
Cite this article
Kubota-Kawai, H., Mutoh, R., Shinmura, K. et al. X-ray structure of an asymmetrical trimeric ferredoxin–photosystem I complex. Nature Plants 4, 218–224 (2018). https://doi.org/10.1038/s41477-018-0130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-018-0130-0
This article is cited by
-
Structure of cyanobacterial photosystem I complexed with ferredoxin at 1.97 Å resolution
Communications Biology (2022)
-
Structure of a tetrameric photosystem I from a glaucophyte alga Cyanophora paradoxa
Nature Communications (2022)
-
Cryo-EM structure of a functional monomeric Photosystem I from Thermosynechococcus elongatus reveals red chlorophyll cluster
Communications Biology (2021)
-
Recent advances in the structural diversity of reaction centers
Photosynthesis Research (2021)
-
Room temperature XFEL crystallography reveals asymmetry in the vicinity of the two phylloquinones in photosystem I
Scientific Reports (2021)