Abstract
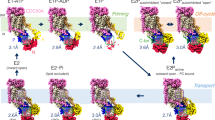
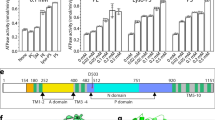
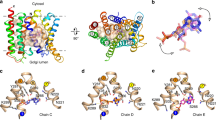
The triose-phosphate/phosphate translocator (TPT) catalyses the strict 1:1 exchange of triose-phosphate, 3-phosphoglycerate and inorganic phosphate across the chloroplast envelope, and plays crucial roles in photosynthesis. Despite rigorous study for more than 40 years, the molecular mechanism of TPT is poorly understood because of the lack of structural information. Here we report crystal structures of TPT bound to two different substrates, 3-phosphoglycerate and inorganic phosphate, in occluded conformations. The structures reveal that TPT adopts a 10-transmembrane drug/metabolite transporter fold. Both substrates are bound within the same central pocket, where conserved lysine, arginine and tyrosine residues recognize the shared phosphate group. A structural comparison with the outward-open conformation of the bacterial drug/metabolite transporter suggests a rocker-switch motion of helix bundles, and molecular dynamics simulations support a model in which this rocker-switch motion is tightly coupled to the substrate binding, to ensure strict 1:1 exchange. These results reveal the unique mechanism of sugar phosphate/phosphate exchange by TPT.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bassham, J. A. Photosynthetic carbon metabolism. Proc. Natl Acad. Sci. USA 68, 2877–2882 (1971).
Stocking, C. R. & Larson, S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem. Biophys. Res. Commun. 37, 278–282 (1969).
Heldt, H. W. & Rapley, L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 10, 143–148 (1970).
Flügge, U. I. et al. The triose phosphate-3-phosphoglycerate-phosphate translocator from spinach chloroplasts: nucleotide sequence of a full-length cDNA clone and import of the in vitro synthesized precursor protein into chloroplasts. EMBO J. 8, 39–46 (1989).
Weber, A. P. & Linka, N. Connecting the plastid: transporters of the plastid envelope and their role in linking plastidial with cytosolic metabolism. Annu. Rev. Plant Biol. 62, 53–77 (2011).
Flügge, U. I. et al. The major chloroplast envelope polypeptide is the phosphate translocator and not the protein import receptor. Nature 353, 364–367 (1991).
Knappe, S., Flügge, U. I. & Fischer, K. Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol. 131, 1178–1190 (2003).
Weber, A. P., Linka, M. & Bhattacharya, D. Single, ancient origin of a plastid metabolite translocator family in Plantae from an endomembrane-derived ancestor. Eukaryot. Cell 5, 609–612 (2006).
Fischer, K. et al. A new class of plastidic phosphate translocators: a putative link between primary and secondary metabolism by the phosphoenolpyruvate/phosphate antiporter. Plant Cell 9, 453–462 (1997).
Kammerer, B. et al. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate/phosphate antiporter. Plant Cell 10, 105–117 (1998).
Eicks, M., Maurino, V., Knappe, S., Flügge, U. I. & Fischer, K. The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol. 128, 512–522 (2002).
Weber, A. P. & Bräutigam, A. The role of membrane transport in metabolic engineering of plant primary metabolism. Curr. Opin. Biotechnol. 24, 256–262 (2013).
Wang, Y., Long, S. P. & Zhu, X. G. Elements required for an efficient NADP-malic enzyme type C4 photosynthesis. Plant Physiol. 164, 2231–2246 (2014).
Zhang, L. et al. Overriding the co-limiting import of carbon and energy into tuber amyloplasts increases the starch content and yield of transgenic potato plants. Plant Biotechnol. J. 6, 453–464 (2008).
Cho, M. H., Jang, A., Bhoo, S. H., Jeon, J. S. & Hahn, T. R. Manipulation of triose phosphate/phosphate translocator and cytosolic fructose-1,6-bisphosphatase, the key components in photosynthetic sucrose synthesis, enhances the source capacity of transgenic Arabidopsis plants. Photosynth. Res. 111, 261–268 (2012).
Mullin, K. A. et al. Membrane transporters in the relict plastid of malaria parasites. Proc. Natl Acad. Sci. USA 103, 9572–9577 (2006).
Lim, L. & McFadden, G. I. The evolution, metabolism and functions of the apicoplast. Philos. Trans. R. Soc. Lond. B. Biol. Sci 365, 749–763 (2010).
Brooks, C. F. et al. The toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival.Cell Host Microbe 7, 62–73 (2010).
Banerjee, T., Jaijyan, D. K., Surolia, N., Singh, A. P. & Surolia, A. Apicoplast triose phosphate transporter (TPT) gene knockout is lethal for Plasmodium. Mol. Biochem. Parasitol. 186, 44–50 (2012).
Lim, L., Linka, M., Mullin, K. A., Weber, A. P. & McFadden, G. I. The carbon and energy sources of the non-photosynthetic plastid in the malaria parasite. FEBS Lett. 584, 549–554 (2010).
Facchinelli, F. & Weber, A. P. The metabolite transporters of the plastid envelope: an update. Front. Plant Sci. 2, 50 (2011).
Hadley, B. et al. Structure and function of nucleotide sugar transporters: Current progress. Comput. Struct. Biotechnol. J. 10, 23–32 (2014).
Orellana, A., Moraga, C., Araya, M. & Moreno, A. Overview of nucleotide sugar transporter gene family functions across multiple species. J. Mol. Biol. 428, 3150–3165 (2016).
Ishida, N. & Kawakita, M. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch. 447, 768–775 (2004).
Reyes, F. & Orellana, A. Golgi transporters: opening the gate to cell wall polysaccharide biosynthesis. Curr. Opin. Plant Biol. 11, 244–251 (2008).
Heldt, H. W. Three decades in transport business: studies of metabolite transport in chloroplasts - a personal perspective. Photosynth. Res. 73, 265–272 (2002).
Linka, M., Jamai, A. & Weber, A. P. Functional characterization of the plastidic phosphate translocator gene family from the thermo-acidophilic red alga Galdieria sulphuraria reveals specific adaptations of primary carbon partitioning in green plants and red algae. Plant Physiol. 148, 1487–1496 (2008).
Landau, E. M. & Rosenbusch, J. P. Lipidic cubic phases: a novel concept for the crystallization of membrane proteins. Proc. Natl Acad. Sci. USA 93, 14532–14535 (1996).
Jack, D. L., Yang, N. M. & Saier, M. H. Jr. The drug/metabolite transporter superfamily. Eur. J. Biochem 268, 3620–3639 (2001).
Tsuchiya, H. et al. Structural basis for amino acid export by DMT superfamily transporter YddG. Nature 534, 417–420 (2016).
Weber, A. P., Schwacke, R. & Flügge, U. I. Solute transporters of the plastid envelope membrane. Annu. Rev. Plant Biol. 56, 133–164 (2005).
Flügge, U. I. Hydrodynamic properties of the Triton X-100-solubilized chloroplast phosphate translocator. BBA-Biomembranes 815, 299–305 (1985).
Gross, A., Bruckner, G., Heldt, H. W. & Flügge, U. I. Comparison of the kinetic properties, inhibition and labelling of the phosphate translocators from maize and spinach mesophyll chloroplasts. Planta 180, 262–271 (1990).
Nozawa, A. et al. A Cell-free translation and proteoliposome reconstitution system for functional analysis of plant solute transporters. Plant Cell Physiol. 48, 1815–1820 (2007).
Drew, D. & Boudker, O. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85, 543–572 (2016).
Hayashi, Y., Matsui, H. & Takagi, T. Membrane protein molecular weight determined by low-angle laser light-scattering photometry coupled with high-performance gel chromatography. Methods Enzymol. 172, 514–528 (1989).
Caffrey, M. Crystallizing membrane proteins for structure determination: use of lipidic mesophases. Annu. Rev. Biophys. 38, 29–51 (2009).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Foadi, J. et al. Clustering procedures for the optimal selection of data sets from multiple crystals in macromolecular crystallography. Acta Crystallogr. D 69, 1617–1632 (2013).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Javanainen, M. Universal method for embedding proteins into complex lipid bilayers for molecular dynamics simulations. J. Chem. Theory Comput. 10, 2577–2582 (2014).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Nose, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Polymorphic transitions in single-crystals – a new molecular-dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Darden, T., York, D. & Pedersen, L. Particle Mesh Ewald – an N.Log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Acknowledgements
We thank H. Nishimasu for comments on the manuscript; A. Kurabayashi, K. Ogomori, W. Shihoya and R. Taniguchi for technical assistance; and the beam-line scientists at SPring-8 BL32XU for assistance in data collection. The diffraction experiments were performed at SPring-8 BL32XU (proposals 2015B0119, 2015B2057 and 2016B2527). Computations of MD simulations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics. This work was supported by grants from the Platform for Drug Discovery, Informatics and Structural Life Science by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), JSPS KAKENHI (Grant Nos. 24227004, 25291011), the FIRST program, JST PRESTO; and a Grant-in-Aid for JSPS Fellows (Grant No. 16J07405).
Author information
Authors and Affiliations
Contributions
Y.L. designed the research, expressed, purified and crystallized GsGPT, determined the structures and performed biochemical assays. Y.L., K.Y. and K.H. collected and processed diffraction data. T.N. and K.K. assisted with the structure determination. M.T. performed the molecular dynamics simulations. A.M. prepared G. sulphuraria cDNA. S.N. and K.T. performed the SEC-MALLS experiment. Y.L., T.N., R.I. and O.N. wrote the manuscript with help from all authors. O.N. directed and supervised all of the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Figures 1–11, Supplementary Tables 1 and 2
Reporting Checklist
Reporting Checklist file
Supplementary Data 1
Multiple sequence alignment between human NSTs, Arabidopsis NST/TPT members and GsGPT
Supplementary Video 1
Conformational change of GsGPT
Supplementary Video 2
Molecular dynamics simulation of GsGPT without Pi
Rights and permissions
About this article
Cite this article
Lee, Y., Nishizawa, T., Takemoto, M. et al. Structure of the triose-phosphate/phosphate translocator reveals the basis of substrate specificity. Nature Plants 3, 825–832 (2017). https://doi.org/10.1038/s41477-017-0022-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-017-0022-8
This article is cited by
-
Structural basis of metabolite transport by the chloroplast outer envelope channel OEP21
Nature Structural & Molecular Biology (2023)
-
pH-dependence of the Plasmodium falciparum chloroquine resistance transporter is linked to the transport cycle
Nature Communications (2023)
-
Structure and mechanism of oxalate transporter OxlT in an oxalate-degrading bacterium in the gut microbiota
Nature Communications (2023)
-
Structural and evolutionary analyses of the Plasmodium falciparum chloroquine resistance transporter
Scientific Reports (2020)
-
Substrate specificity of plastid phosphate transporters in a non-photosynthetic diatom and its implication in evolution of red alga-derived complex plastids
Scientific Reports (2020)