Abstract
Environmental temperature affects physiological functions, representing a barrier for the range expansions of ectothermic species. To understand the link between thermal physiology and biogeography, a key question is whether among-species thermal sensitivity of metabolic rates is mechanistically constrained or buffered through physiological remodeling over evolutionary time. The former conception, the Universal Temperature Dependence hypothesis, predicts similar among- and within-species thermal sensitivity. The latter conception, the Metabolic Cold Adaptation hypothesis, predicts lower among-species thermal sensitivity than within-species sensitivity. Previous studies that tested these hypotheses for fishes overwhelmingly investigated teleosts with elasmobranchs understudied. Here, we show that among-species thermal sensitivity of resting metabolic rates is lower than within-species sensitivity in teleosts but not in elasmobranchs. Further, species richness declines with latitude more rapidly in elasmobranchs than in teleosts. Metabolic Cold Adaptation exhibited by teleosts might underpin their high diversity at high latitudes, whereas the inflexible thermal sensitivity approximated by Universal Temperature Dependence of elasmobranchs might explain their low diversity at high latitudes.
Similar content being viewed by others
Introduction
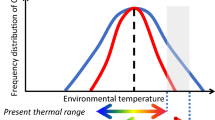
Environmental temperature is a major barrier for the range expansion of species. For marine ectotherms, range expansion into subpolar and polar regions presents particularly severe challenges, because their physiological functions are impacted by permanently cold waters and the impacts can be lethal to many species1. Among many important physiological functions, energy metabolism and its thermal sensitivity are of direct relevance to life history and ecology2 and have been extensively studied. Metabolic rates of individual animals, normally measured as oxygen consumption rates of animals in captivity, decline with decreasing temperature. However, ectothermic species have some capacity to reduce the thermal sensitivity of their metabolic rates if they are given sufficient time to remodel their physiology under new thermal regimes (a process called acclimation) (Fig. 1a). Previous experiments showed that Q10 [the factorial increase (or decrease) in physiological rates associated with a 10 °C increase (or decrease) in temperature] of metabolic rates of acclimated animals is lower than that of animals exposed to an acute temperature change, especially in aquatic taxa3. A key question in an evolutionary context is whether thermal sensitivity of metabolic rates across species can also be reduced by the remodeling of physiology associated with genetic changes in phenotypes (Fig. 1b).
a Experimentally induced acute cooling causes a rapid decline in physiological rate of an individual, but the rate can recover to some extents following sufficient time. This process is called acclimation and represents reversible phenotypic changes. b Across species, physiological rate tends to decline with decreasing environmental temperature. As such, range expansion into cold waters associated with speciation will cause a decline in physiological rate. However, the effects of temperature may be reduced compared to that predicted from the temperature dependence of individual animals, given that physiological remodeling occurs over evolutionary time. This unreversible temperature compensation could be called adaptation if it occurs.
There are two different conceptions. The Universal Temperature Dependence (UTD) hypothesis states that metabolic rates scales with temperature in a similar fashion within and among species because of an inflexible thermal sensitivity of biochemical reactions4. The other conception is that thermal sensitivity of metabolic rates across species is more complex and can be adjusted via physiological remodeling and genetic changes over evolutionary time5. A form of this latter conception is the Metabolic Cold Adaptation (MCA) hypothesis, which states that the metabolic rate of cold-adapted species is higher at their low temperatures than would be predicted from the observed thermal sensitivity of temperate or tropical species6,7. If UTD is correct, the interspecific Q10 calculated across species from a range of climate zones would be similar to intraspecific Q10 of acclimated, individual species. If MCA is correct, interspecific Q10 measured across polar-to-tropical species would be lower than intraspecific Q10.
Fishes represent an ideal model to explore thermal sensitivity of metabolic rates and its possible link to biogeography due to their extraordinary high diversity across different climate zones. Diverse fishes are present even in polar waters, and polar fishes are often more active than the warm-water fishes placed in cold waters8. Driven by these curious observations, many previous studies tested MCA using various methods and datasets, providing mixed support6,7,8,9,10. Crucially, however, these previous tests, and indeed most studies that examined the interspecific relationships of metabolic rates in fishes11,12 (including tests of UTD4,13), were based overwhelmingly on data for teleosts, with a few elasmobranch species included if any. Bony fishes (Osteichthyes, including teleosts) and cartilaginous fishes (Chondrichthyes, including elasmobranchs) diverged from common ancestors of jawed vertebrates (gnathostomes) about 450 Myr ago14. With the long evolutionary separation, teleosts and elasmobranchs have many distinct biological traits, including energy metabolism15, but share many environmental and ecological constraints. Curiously, although both groups are distributed globally in today’s oceans, elasmobranchs are relatively rare in the Southern Ocean16. Accordingly, comparing thermal sensitivity of metabolic rates and biogeography between teleosts and elasmobranchs could provide clearer evidence for UTD or MCA and insights into the roles of physiological remodeling regarding energy metabolism in the range expansion of species.
In this study, we compile the published reports of resting metabolic rates (RMRs) of thermally acclimated elasmobranchs as widely as possible (i.e., for different species and for different temperatures and body masses within a species). By combining this dataset with previously compiled datasets on teleost RMRs11,12, we explore among- and within-species patterns of thermal sensitivity in the two major clades of fishes. Further, we analyze large datasets on marine fish diversity17,18 to test whether teleosts and elasmobranchs have expanded toward the poles to similar extents. We hypothesize that the thermal sensitivity of metabolic rates mirrors latitudinal gradient of species richness. That is, a group exhibiting a clearer signature of MCA is predicted to have a higher species diversity at high latitudes than a group to which UTD is better fitted.
Results
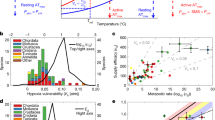
RMRs of elasmobranchs composed of 377 estimates from 34 species (mean body mass 0.10–55.6 kg, temperature 4–31 °C) were compiled (Supplementary Data. 1). A dataset on teleost RMRs previously compiled12 was filtered using the same criteria as we used for elasmobranchs (Methods), leaving 100 species (mean body mass 0.0005 − 3.0 kg, temperature −1.5 − 30 °C) (Supplementary Data. 2). Elasmobranchs had larger body mass than teleosts and the mass ranges overlapped only partially. A phylogenetic generalized least squares (PGLS) model with log10(RMR) as the response variable and log10(mass) and temperature as the predictor variables showed that the allometric slopes (with 95% confidence interval) were similar between teleosts [0.95 (0.88 − 1.02)] and elasmobranchs [0.89 (0.75 − 1.01)] (Fig. 2a). By contrast, temperature dependance was different (Fig. 2b, c). Interspecific Q10, reflecting the slope of regression lines in Fig. 2b, c, was lower in teleosts [1.43 (1.14 − 1.76)] than elasmobranchs [2.37 (1.81 − 3.30)] with no overlaps of 95% confidence intervals. This result primarily stemmed from higher teleost RMRs at low temperatures, rather than lower teleost RMRs at high temperature, compared to elasmobranch RMRs (inset in Fig. 2c). Phylogenetic signals, quantified by d values that can range from 0 to 119, were 0.28 and 0.13 for teleosts and elasmobranchs, respectively. These d values indicate some tendency for closely related species to have similar RMRs for a given body mass and temperature. Model selection analyses using the combined dataset of the two clades (teleost and elasmobranch) showed that the best model for explaining log10(RMR) has log10(mass), temperature, clade, and the interaction between temperature and clade as predictor variables (Table 1). Thus, the effect of temperature on RMRs across species was clearly different between teleosts and elasmobranchs, whereas that of body mass was not.
a Allometric relationships of RMRs standardized to 20 °C using the interspecific Q10 (1.43 for teleosts and 2.37 for elasmobranchs) estimated with the phylogenetic generalized least squares (PGLS) method. Thick lines are the estimates of RMRs for a temperature of 20 °C based on PGLS [teleost, log10(RMR) = 0.95*log10(mass) + 1.87; elasmobranch, log10(RMR) = 0.89*log10(mass) + 1.86]. b, c Temperature dependance of RMRs standardized to a 1-kg body mass using the allometric slope (0.95 for teleosts and 0.89 for elasmobranchs) estimated with PGLS. Thick lines are the estimates of RMRs for a 1-kg body mass based on PGLS [teleost, log10(RMR) = 0.0154*temp + 1.56; elasmobranch, log10(RMR) = 0.0375*temp + 1.11]. c Inset shows a comparison between teleosts and elasmobranchs. Source data are provided as a Source Data file.
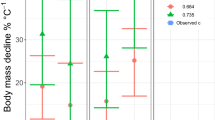
Interspecific Q10 based on the PGLS model (Model 1) was compared to intraspecific Q10. In teleosts, interspecific Q10 was lower than intraspecific Q10 [mean 2.41 (2.10 − 2.71)] (Table S1) estimated for 31 species using a different published dataset11, with no overlaps of 95% confidence interval (Fig. 3a). In elasmobranchs, by contrast, interspecific Q10 was similar to intraspecific Q10 [mean 2.29 (1.96−2.61)] (Table S1) estimated for 10 species using our dataset (Fig. 3b). These results did not change (i) when the variation of lifestyles (i.e., pelagic, benthopelagic, or demersal) among species (Table S2) was added as a categorical predictor variable (Model 2), or (ii) when multiple measures from single species of elasmobranchs, rather than the average of each species, were included in a phylogenetic mixed model (Model 3) (Fig. 3a, b). Moreover, stricter rules for data inclusion were applied and only data for thermally acclimated individuals for ≥2 weeks prior to measurements were analyzed. Despite decreased sample sizes (from 100 to 50 species in teleosts and from 34 to 19 species in elasmobranchs), the subset still showed a large difference in interspecific Q10 between teleosts [1.41 (1.07−1.74)] and elasmobranchs [2.17 (1.42−3.31)] based on PGLS. Comparing our results with previous estimate of interspecific Q10 of RMRs for major vertebrate groups11, interspecific Q10 of elasmobranchs was not exceptionally high. Rather, interspecific Q10 of teleosts was exceptionally low (Fig. 3c).
a, b Interspecific Q10 (with 95% confidence interval) estimated from the coefficient of the temperature term of three phylogenetic regression models (Models 1–3) compared to intraspecific Q10 (shown for each species and as the mean and 95% confidence interval) for a teleosts and b elasmobranchs. Models 1–3 are a phylogenetic generalized least squares (PGLS) model, a PGLS model with species’ lifestyle as the additional predictor variable, and a phylogenetic mixed model, respectively (Methods). Intraspecific Q10 of teleosts is positively skewed, and an outlier (5.4) is not shown. Sample size for teleosts a: 100 species for all models of interspecific Q10, 31 species for intraspecific Q10. Sample size for elasmobranchs b: 34 species for Models 1 and 2 and 377 estimates for Model 3 of interspecific Q10, 10 species for intraspecific Q10. c A comparison of interspecific Q10 of resting metabolic rates across major vertebrate groups. Data for teleosts and elasmobranchs are from this study and data for other groups are from White et al.11.
Based on AquaMaps17, species richness declines at high latitude in both teleosts and elasmobranchs. However, the richness of elasmobranchs at high latitudes is disproportionally low, especially in the southern hemisphere (Fig. 4a, b). Our investigation of FishBase18 showed that teleosts are approximately 10 times more speciose than elasmobranchs for a given latitude lower than 60°. The teleost/elasmobranch ratio of species count rose at higher latitudes, especially in the southern hemisphere, reaching 68 at 75°S (Fig. 4c, d). Species count averaged across the northern and southern hemisphere for a given absolute latitude showed that it is rather stable at low and middle latitudes but declines with latitude at higher latitudes (Fig. 4e). The broken-line regression analyses20 indicated that the breakpoint latitude is 25.6 and 34.5° for teleosts and elasmobranchs, respectively. Beyond the breakpoint latitudes, the log10 of species count declined with latitude more rapidly for elasmobranchs [0.0394 degree−1 (0.0379 − 0.0408)] than teleost [0.0289 degree−1 (0.0283 − 0.0295)], with no overlaps of 95% confidence interval.
a, b Diversity maps created by AquaMaps for a teleosts and b elasmobranchs, with areas with relatively high and low diversity shown in red and blue, respectively. Elasmobranch diversity is disproportionally low at high latitudes, especially in the Southern Ocean. c Species count in a log scale plotted against latitude for teleosts (light blue) and elasmobranchs (pink) based on FishBase. d Teleost/elasmobranch species count ratio (in a log scale) plotted against latitudes, showing that it rises at high latitudes, especially in the southern hemisphere. e Species count (in a log scale) plotted against absolute latitude for teleosts and elasmobranchs, fitted with the broken-line regression model. At latitudes above the breakpoints (vertical bars), species count declines with latitude more rapidly in elasmobranchs than teleosts. Source data for c–e are provided as a Source Data file.
Discussion
Contrasting thermal sensitivity of metabolic rates
We found that the among-species influence of temperature on RMRs is strikingly different between teleosts and elasmobranchs. By contrast, the among-species influence of body mass is similar and individual species from both clades have similar intraspecific Q10 (Figs. 2 and 3). Our estimate of interspecific Q10 of elasmobranch RMRs (2.37) is consistent with the previous study that analyzed a smaller dataset (2.23)21. The interspecific Q10 of teleost RMRs (1.43) is somewhat different from the previous study that shared data with this study but had less strict rules for data inclusion (1.62)12 and an older study that used a different dataset and statistical method (1.83)7. Due to this variability, a range of 1.4 − 1.8 can be considered the best estimate for interspecific Q10 of teleost RMRs. In all such case, teleosts have lower interspecific Q10 than elasmobranchs, although no previous studies compiled data for both clades and compared them with a consistent methodology. Lower interspecific Q10 of teleosts arises primarily from elevated RMRs of cold-water species, despite large variability for RMR at a given temperature in this clade (Fig. 2b, c). Notably, interspecific Q10 is lower than intraspecific Q10 in teleosts (as shown before7), whereas interspecific and intraspecific Q10 of RMRs are similar in elasmobranchs. This finding is robust, as shown by the three different phylogenetic regression models we tested (Fig. 3a, b). Interspecific Q10 of teleost RMRs is remarkably low even among major vertebrate groups (Fig. 3c). Taken together, our results present strong support for MCA in teleosts but not in elasmobranchs. RMRs of elasmobranchs are better explained by UTD that assumes an inflexible thermal sensitivity among and within species.
Metabolic rates are an integrative proxy for a variety of fitness-related processes and traits, including enzyme reactions, locomotion, growth, and reproduction22. Besides RMRs we analyzed in this study, other metrics of metabolic rates, such as maximum metabolic rates and aerobic scope (i.e., the difference between maximum and resting metabolic rates), are ecologically relevant23; however, these metrics are positively associated with one another across species at least in teleosts12. Therefore, our finding of reduced thermal sensitivity of RMRs across teleost species suggests that teleosts in cold waters may have better competitive ability than predicted for that temperature at the cost of higher energy expenditure. By contrast, elasmobranch species in cold waters do not appear to exhibit such evolutionary changes as teleosts. This observation is exemplified by Greenland sharks (the species with the lowest water temperature in our elasmobranch dataset) that exhibit unusually slow swim speed24, low metabolic rate21, and slow growth rate25.
We acknowledge that our elasmobranch dataset is still limited compared to teleosts, for which large amounts of data on metabolic rates have long been collected and complied. The data on elasmobranchs should be expanded in future studies with regard to the number of species, phylogeny, lifestyle, temperature coverage, and the types of metabolic rate metrics. Moreover, to better characterize the difference in thermal sensitivity of physiological rates between teleosts and elasmobranchs, other important temperature-dependent traits (e.g., enzyme activity, locomotor performance) of elasmobranchs should be examined in future studies, as previously demonstrated for teleosts9.
Link between metabolic rates and biogeography
Our findings of contrasting thermal sensitivity of RMRs between teleosts and elasmobranchs apparently mirror the contrasting biogeographic patterns of the two groups. Species richness are highest at low and middle latitudes in both teleosts and elasmobranchs, as previously reported26,27. However, we found that the rate of declines in species richness with increasing latitudes differs, leading to increased teleost/elasmobranch richness ratio at high latitudes (Fig. 4). This pattern is especially evident in the Southern Ocean, where diverse teleosts are present whereas elasmobranchs are rare16. According to FishBase, 247 species (1.5%) of marine teleosts (total, 16,611 species), comprised of 38 families across 12 orders, occur in polar waters. By contrast, only 8 species (0.7%) of marine elasmobranchs (total, 1,198 species), comprised of two genera of skates (Bathyraja and Amblyraja spp.), are categorized as polar species. Thus, with regard to both the number of extant species and taxonomic diversity, elasmobranchs are less successful in expanding their ranges into cold waters than teleosts. This difference cannot be explained by the development of anti-freeze proteins, which prevent ice formation and growth and are found in the plasma or epidermis (e.g., skin) of multiple linages of polar teleosts28. Unlike teleosts, elasmobranchs maintain osmotic equilibrium with the surrounding seawater primarily by high concentrations of urea29 and have no risk of freezing even at sub-zero water temperature. Therefore, we propose that MCA exhibited by teleosts underpins their high diversity at high latitudes, whereas the inflexible thermal sensitivity of RMRs approximated by UTD of elasmobranchs is associated with their low diversity at high latitudes.
It is important to note that any ecological benefits associated with MCA (i.e., elevated RMRs in cold waters) come with challenges such as elevated energy requirements. In the environment where resources (e.g., food, oxygen) are scarce, less active lifestyles with reduced RMRs might be favored. For example, some teleosts (e.g., carp) with low RMRs are highly tolerant to hypoxia12, allowing them to expand their range into the environment where oxygen concentrations occasionally drop30. Tolerance to hypoxia is unlikely an important factor affecting the survivals of marine fishes at high latitudes due to increased dissolved oxygen concentrations in colder waters. However, tolerance to food shortage associated with low RMRs can undoubtedly be a selective advantage for marine fishes at high latitudes, where seasonal fluctuations in food availability is pronounced. As such, while it is possible that the capacity for MCA in teleosts (but not elasmobranchs) facilitated their range expansion into cold waters, causality may be the reverse. Highly successful diversification and range expansions of teleosts may have caused high variability in RMRs for a given temperature (Fig. 2b), which can be interpreted as the evidence of MCA. High variability in teleost RMRs reflects a broad range of ecological niches occupied by this group. Notably, some teleosts (e.g., salmons) have highly active lifestyles in cold waters, despite their ectothermic physiology like many other species. As an approximation, we refer to waters of <15 °C as “cold waters”, because 15 °C lies roughly halfway between typical average sea surface temperature of ~30 °C in the tropics and ~0 °C in polar waters. Teleosts and elasmobranchs have diverged RMRs below 15 °C (Fig. 2b, c). It is our view that, in general, cold-water elasmobranchs are either sluggish (e.g., Greenland sharks24) or active with regional endothermic physiology (e.g., salmon sharks31). There seem to be no “salmon-like” elasmobranchs, being highly active cold-water ectotherms—a view that can partly explain low elasmobranch diversity at high latitudes. Thus, while stopping short of claiming that one pattern causes the other, we propose that contrasting thermal sensitivities of RMRs between teleosts and elasmobranchs are intrinsically linked to their biogeographic differences.
An unresolved issue is why teleost/elasmobranch richness ratio is much higher at high latitudes in the southern hemisphere (the Southern Ocean) than northern hemisphere (the Arctic Ocean) (Fig. 4). Compared to the Arctic Ocean, the Southern Ocean has an older history of the present thermal regime and higher degree of isolation from adjacent seas32. These differences may have caused increased difference in species richness between teleosts (that have capacity for MCA) and elasmobranchs (that do not) in the southern hemisphere compared to the northern hemisphere. Detailed analyses separating northern and southern fauna with taxonomically and geographically expanded datasets on metabolic rates could address this issue in future studies.
Possible physiological mechanism
An intriguing question arising from our findings is why teleosts have capacity for MCA but elasmobranchs do not. We refer to a previous review article15 for the possible physiological mechanism, which will be summarized below.
Teleosts and elasmobranchs are distinct in their pathways of energy metabolism. Adipose tissues are stored as oxidative fuels in teleost muscles but are absent in elasmobranch muscles, where ketone bodies and amino acids are main oxidative fuels15. This unusual energy metabolism of elasmobranchs is likely related to their osmoregulatory strategy. Unlike teleosts, elasmobranchs are iso-osmotic to the surrounding seawater primarily by maintaining high concentration of urea29. They use amino acids for both osmoregulation (as essential nitrogen donor for urea synthesis) and energy metabolism (as oxidative fuels and ketogenic precursors). Importantly, fatty acids in adipose tissues are preferred oxidative fuels in cold-water teleost species32, which often have increased mitochondria density in muscles33. Therefore, the inability of elasmobranchs to utilize fatty acids as energy source may limit their RMRs in cold water (as previously suggested15), potentially leading to the lack of capacity for MCA.
We acknowledge that our discussion is rather speculative. To advance our understanding, the energy metabolism of cold-water elasmobranchs needs to be studied at lower levels of biological organization (the molecular, organellar, cellular, and tissue levels) than the whole-organism level, as previous studies exclusively targeted cold-water teleosts32,33.
In conclusion, we show that the thermal sensitivity of metabolic rates is different between teleosts and elasmobranchs and propose that the difference may underpin their contrasting diversity at high latitudes. In teleosts, among-species thermal sensitivity is lower than within-species thermal sensitivity, consistent with the MCA hypothesis that might explain high diversity of this group at high latitudes. In elasmobranchs, by contrast, among- and within-species thermal sensitivity is similar, consistent with the UTD hypothesis that may be associated with poor diversity of this group at high latitudes. Fishes have been a central study model to explore whether thermal sensitivity of organisms’ metabolic rates are constrained mechanistically by biochemical kinetics4, or if it represents a complex interplay between physics and evolutionary adaptations to particular temperature and lifestyle5. Findings from this study provide improved precision of our understanding of thermal sensitivities across organisms. It is teleosts – not fishes in general9 – that exhibit a clear signature of evolutionary adaptation and depart from the traditional view that interspecific Q10 of RMR in vertebrates is approximately 2–3 (Fig. 3C). Understanding the underlying mechanisms of the divergence between teleosts and other vertebrates could help reveal why metabolic rate scales with temperature in the first place and predict how different species will react to changing climates.
Methods
Metabolic rate data compilation
Data on the resting metabolic rates (RMRs) of elasmobranchs, measured as the oxygen consumption rates of fasted, thermally acclimated animals during resting (i.e., not actively swimming) periods, were compiled with body mass and water temperature from the literature (Supplementary Data. 1). RMR is an approximation of standard (or basal) metabolic rate in fishes, although the effects of some body movements are often included. In most of the compiled studies, individuals were acclimated to the experimental temperature for ≥1 week and fasted for ≥2 days prior to measurements; however, these conditions were not met in some cases, such as experiments on large species in remote areas21. Thermal acclimations of RMR can require days to weeks, depending on the extent and direction of the temperature change34. To examine the robustness of our results, the studies that acclimated individual fish for ≥2 weeks were noted and analyzed separately. When RMRs were measured at different controlled temperatures, we ensured that (i) target temperature was achieved slowly (1 °C day−1 or less in most cases) and (ii) test temperatures fall within the natural range of the species. When the effects of acute temperature changes on RMRs were reported, we only extracted the estimates for the initial, control conditions. Following a review article34, we did not accept RMRs measured for chemically sedated animals, or surgically operated animals (e.g., canulation) that likely incur increased stress. In addition to direct measurements, we accepted the RMRs of continuous swimmers estimated by extrapolating the relationship between metabolic rate and swim speed (or other activity measures such as body acceleration) to zero activity level. The four species for which the extrapolation method was used (i.e., blacknose sharks, blacktip sharks, bull sharks, and scalloped hammerhead sharks) had relatively high RMRs for a given body mass and temperature, likely reflecting their active lifestyles. Limited data available for the species with regional endothermy (e.g., shortfin mako sharks) were excluded in this study. With large body mass and elevated metabolic rates, these species would have disproportionally large effects on allometric relationships, precluding a fair comparison of RMRs between teleosts and elasmobranchs. While some literature only reported the mean RMR of multiple individuals, other literature reported more detailed information (RMR of individual animals and for different temperature treatments) in various forms. We extracted as detailed information as possible by digitalizing the figures or referring to the supplemental materials and data repository of the literature, when applicable. As such, each datapoint in Supplementary Data. 1 represents either an individual or the mean of multiple individuals. Scientific names of species were matched to those in FishBase18.
For teleosts, a published dataset of RMRs composed of 112 species12, measured within the natural temperature range of the species, was used to examine among-species patterns. We referred to the original literature cited by the dataset and excluded species that do not meet the selection criteria we used for elasmobranchs, leaving 100 species (Supplementary Data. 2). In some instances where reported values did not match our calculation based on the original sources, we used the values we calculated. As for elasmobranchs, the studies with thermal acclimation durations of ≥2 weeks were noted and analyzed separately. We also modified scientific names of species following “The Fish Tree of Life”26, a large dataset of bony fish phylogeny that is mostly consistent with the information in FishBase18. Because Killen et al.’s dataset12 only included species-averaged data, we used a different dataset11 to examine intraspecific Q10 of teleost RMRs.
Metabolic rate analyses
RMR data for elasmobranchs were averaged for each species. Log10 values, rather than raw values, were averaged for RMR and body mass, because these are the input of subsequent analyses. Possible effects of local adaptations among populations of a species35 were considered to be beyond the scope of this study. The phylogenetic generalized least squares (PGLS) method was used to examine the effect of body mass and temperature on RMR across species with the effect of phylogeny accounted for. Phylogenetic trees were created for the compiled species of teleosts and elasmobranchs with the published relationships among species26,36,37 and an arbitrary branch length38 (Figs. S1 and S2). PGLS analyses were performed with log10(RMR) as the response variable and log10(mass) and temperature as the predictor variables under the Ornstein-Uhlenbeck evolutionary process model using the Regressionv2 program19 of the software Matlab (MathWorks). Interspecific Q10 was calculated as 1010*a, where a is the coefficient (or slope) for temperature in the PGLS analyses. The 95% confidence interval of each estimate was computed by the bootstrap method19. Phylogenetic signals were quantified as d value (ranging from 0 to 1) using restricted maximum likelihood19. d = 0 indicates that non-phylogenetic (i.e., the ordinary least squares) model best fit the data, whereas d = 1 indicates that the statistical model with the provided branch lengths best fit the data.
To test whether the effects of body mass and temperature on RMRs across species are different between teleosts and elasmobranchs, RMR data for the two groups were combined, and their phylogenetic trees were connected at the roots. Model selection analyses were performed using PGLS with clade (teleost or elasmobranch) as a categorical predictor variable. Five representative models, including those having interaction between clade and other predictor variables, were compared based on Akaike Information Criterion (AIC) (Table 1).
Intraspecific Q10 was estimated for 31 species of teleosts (using White et al.’s dataset11) and 10 species of elasmobranchs (using our dataset; Supplementary Data. 1), for which RMR data are available over a ≥ 7 °C temperature difference (Table S1). The ordinary least squares regressions were performed with log10(RMR) as the response variable and log10(mass) and temperature as the predictor variables. As for interspecific Q10, intraspecific Q10 was calculated as 1010*a, where a is the coefficient (or slope) for temperature.
In addition to the main PGLS analysis (Model 1), two different models (Models 2 and 3) were tested to examine the robustness in our estimates of interspecific Q10. Model 2 also used the PGLS method but had the lifestyle of each species as the additional predictor variable [log10(RMR) ~ log10(mass) + temp + lifestyle]. We tested this model because metabolic rates are often affected by lifestyles (i.e., species with active lifestyles tend to have elevated metabolic rates)12. For each species of our datasets, the categorical information on lifestyle (i.e., pelagic, benthopelagic, or demersal) was extracted from FishBase18 with the software R and the package rfishbase39 (Table S2). For simplicity, the category of “reef associated” was merged into “benthopelagic”. Model 3 was a phylogenetic mixed model [log10(RMR) ~ log10(mass) + temp] with phylogeny included as a random factor and was fitted to all datapoints for elasmobranchs (rather than the data averaged for each species). This analysis was performed with the software R and the package MCMCglmm40. Model 3 used much larger datasets than Models 1 and 2 for elasmobranchs, making 95% confidence interval narrower (Fig. 3b). The same model was applied for the teleost dataset, which is the data averaged for each species.
Biogeography analyses
Global maps for the species diversity of teleosts and elasmobranchs were created using AquaMaps17 (Fig. 4a, b). The group categories of “bony fish” and “sharks and rays” in AquaMaps were considered nearly the same as teleosts and elasmobranchs, respectively. Latitudinal gradients of species richness were analyzed based on FishBase18 with the software R and the package rfishbase39. For all existing taxonomic orders of teleosts (following “The Fish Tree of Life”26) and elasmobranchs, lists of species were extracted with latitudinal range, habitat types (freshwater, brackish water, and saltwater), and climate zones (e.g., tropical, temperate, boreal, polar). The species that do not occur in saltwater were excluded, leaving 16,611 and 1198 species of marine teleosts and elasmobranchs, respectively. The number of species that occurs for each degree of latitude was calculated both for teleosts and elasmobranchs (Fig. 4c, d). Further, species count was averaged across the northern and southern hemisphere for a given absolute latitude up to 78° (Fig. 4e). The relationships between log10(species count) and absolute latitude were examined by the broken-line regression models20 with the software R.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Metabolic rate data used in this study are available in Supplementary Data 1 and 2 and the supplementary material of ref. 11. Data used in biogeography analyses are available at open databases (AquaMaps and FishBase). Source data are provided with this paper.
Code availability
R codes used in this study are available at Github repository (https://github.com/yuuki412/fish_metabolic_rate_and_biogeography).
Change history
25 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41467-023-38166-5
References
Pörtner, H. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146 (2001).
Clarke, A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 18, 573–581 (2003).
Seebacher, F., White, C. R. & Franklin, C. E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66 (2015).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Clarke, A. Is there a universal temperature dependence of metabolism? Funct. Ecol. 18, 252–256 (2004).
Holeton, G. F. Metabolic cold adaptation of polar fish: fact or artefact? Physiol. Zool. 47, 137–152 (1974).
Clarke, A. & Johnston, N. M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 68, 893–905 (1999).
Scholander, P. F., Flagg, W., Walters, V. & Irving, L. Climatic adaptation in arctic and tropical poikilotherms. Physiol. Zool. 26, 67–92 (1953).
White, C. R., Alton, L. A. & Frappell, P. B. Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc. R. Soc. B 279, 1740–1747 (2012).
Steffensen, J. F. Metabolic cold adaptation of polar fish based on measurements of aerobic oxygen consumption: fact or artefact? Artefact! Comp. Biochem. Physiol. A 132, 789–795 (2002).
White, C. R., Phillips, N. F. & Seymour, R. S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2, 125–127 (2006).
Killen, S. S. et al. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 187, 592–606 (2016).
White, C. R., Frappell, P. B. & Chown, S. L. An information-theoretic approach to evaluating the size and temperature dependence of metabolic rate. Proc. R. Soc. B 279, 3616–3621 (2012).
Venkatesh, B. et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174–179 (2014).
Speers-Roesch, B. & Treberg, J. R. The unusual energy metabolism of elasmobranch fishes. Comp. Biochem. Physiol. A 155, 417–434 (2010).
Verde, C. et al. Structure, function and molecular adaptations of haemoglobins of the polar cartilaginous fish Bathyraja eatonii and Raja hyperborea. Biochem. J. 389, 297–306 (2005).
Kaschner, K., et al. AquaMaps: Predicted range maps for aquatic species. Retrieved from https://www.aquamaps.org. (2019).
Froese, R. & Pauly, D. FishBase. World Wide Web electronic publication. www.fishbase.org. (2022).
Lavin, S. R., Karasov, W. H., Ives, A. R., Middleton, K. M. & Garland, T. Jr. Morphometrics of the avian small intestine compared with that of nonflying mammals: A phylogenetic approach. Physiol. Biochem. Zool. 81, 526–550 (2008).
Muggeo, V. M. Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25 (2008).
Ste-Marie, E., Watanabe, Y. Y., Semmens, J. M., Marcoux, M. & Hussey, N. E. A first look at the metabolic rate of Greenland sharks (Somniosus microcephalus) in the Canadian Arctic. Sci. Rep. 10, 19297 (2020).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Payne, N. L. et al. Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Funct. Ecol. 30, 903–912 (2016).
Watanabe, Y. Y., Lydersen, C., Fisk, A. T. & Kovacs, K. M. The slowest fish: Swim speed and tail-beat frequency of Greenland sharks. J. Exp. Mar. Biol. Ecol. 426, 5–11 (2012).
Nielsen, J. et al. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 353, 702–704 (2016).
Rabosky, D. L. et al. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395 (2018).
Lucifora, L. O., García, V. B. & Worm, B. Global diversity hotspots and conservation priorities for sharks. PLoS ONE 6, e19356 (2011).
Fletcher, G. L., Hew, C. L. & Davies, P. L. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 63, 359–390 (2001).
Pang, P. K., Griffith, R. W. & Atz, J. W. Osmoregulation in elasmobranchs. Am. Zool. 17, 365–377 (1977).
Nilsson, G. E. & Renshaw, G. M. C. Hypoxic survival strategies in two fishes: extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shark. J. Exp. Biol. 207, 3131–3139 (2004).
Watanabe, Y. Y., Goldman, K. J., Caselle, J. E., Chapman, D. D. & Papastamatiou, Y. P. Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proc. Natl Acad. Sci. USA 112, 6104–6109 (2015).
Pörtner, H.-O., Lucassen, M. & Storch, D. Metabolic biochemistry: its role in thermal tolerance and in the capacities of physiological and ecological function. In Fish Physiology: The Physiology of Polar Fishes (eds A. P. Farrell & J. F. Steffensen) 79–154 (Academic Press, 2005).
Guderley, H. Metabolic responses to low temperature in fish muscle. Biol. Rev. 79, 409–427 (2004).
Chabot, D., Steffensen, J. & Farrell, A. The determination of standard metabolic rate in fishes. J. Fish. Biol. 88, 81–121 (2016).
Eliason, E. J. et al. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112 (2011).
Aschliman, N. C. et al. Body plan convergence in the evolution of skates and rays (Chondrichthyes: Batoidea). Mol. Phylogen. Evol. 63, 28–42 (2012).
Velez-Zuazo, X. & Agnarsson, I. Shark tales: A molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes). Mol. Phylogen. Evol. 58, 207–217 (2011).
Grafen, A. The phylogenetic regression. Philos. Trans. Roy. Soc. B 326, 119–157 (1989).
Boettiger, C., Lang, D. T. & Wainwright, P. rfishbase: exploring, manipulating and visualizing FishBase data from R. J. Fish. Biol. 81, 2030–2039 (2012).
Hadfield, J. D. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22 (2010).
Acknowledgements
We thank C. White for comments on the draft. N.L. Payne was supported by a Science Foundation Ireland Starting Investigator grant (18/SIRG/5549).
Author information
Authors and Affiliations
Contributions
Y.Y.W. conceived idea and compiled and analyzed data with discussion with N.L.P. Y.Y.W. lead the writing with substantial inputs from N.L.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ben Speers-Roesch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, Y.Y., Payne, N.L. Thermal sensitivity of metabolic rate mirrors biogeographic differences between teleosts and elasmobranchs. Nat Commun 14, 2054 (2023). https://doi.org/10.1038/s41467-023-37637-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-37637-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.