Abstract
Recent data have shown that liver fibrosis can regress even at later stages of cirrhosis and shifting the immune response from pro-inflammatory towards a resolutive profile is considered as a promising option. The immune regulatory networks that govern the shift of the inflammatory phenotype and thus potential reversal of liver fibrosis are lesser known. Here we show that in precision-cut human liver slices obtained from patients with end-stage fibrosis and in mouse models, inhibiting Mucosal-Associated Invariant T (MAIT) cells using pharmacological or antibody-driven approaches, limits fibrosis progression and even regresses fibrosis, following chronic toxic- or non-alcoholic steatohepatitis (NASH)-induced liver injury. Mechanistic studies, combining RNA sequencing, in vivo functional studies (performed in male mice) and co-culture experiments indicate that disruption of the MAIT cell-monocyte/macrophage interaction results in resolution of fibrosis both by increasing the frequency of restorative Ly6Clo at the expenses of pro-fibrogenic Ly6Chi monocyte-derived macrophages and promoting an autophagic phenotype in both subsets. Thus, our data show that MAIT cell activation and the consequential phenotype shift of liver macrophages are important pathogenic features of liver fibrosis and could be targeted by anti-fibrogenic therapy.
Similar content being viewed by others
Introduction
Chronic liver injury exposes to fibrosis and its end-stage cirrhosis is associated with life-threatening complications. Although cirrhosis has long been considered irreversible, data from patients with chronic liver diseases and murine models have clearly established that fibrosis can revert, even at later stages, following the elimination of the underlying cause1,2. However, in many cases this goal cannot be achieved, highlighting the urgent unmet need for therapies halting progression, and/or promoting regression of established fibrosis/cirrhosis. Removal or elimination of the causative agent promotes inhibition of the inflammatory and profibrogenic response, together with deactivation and/or disappearance of fibrogenic cells, leading to the resolution of liver fibrosis3,4,5,6,7. Among immune cells, different subsets of liver macrophages play a dual role in the control of fibrosis progression and regression8,9. In particular, data from human samples and experimental mouse models have shown that during liver injury, recruited monocyte-derived macrophages (MoMac) with high expression of Ly6C secrete pro-inflammatory and profibrogenic cytokines which perpetuate the fibrogenic response8,9,10. In contrast, studies in mouse models have shown that during fibrosis regression, a restorative MoMac population with a Ly6Clo phenotype promotes scar degradation8,9. Therefore, controlling the profibrogenic vs restorative macrophage balance is considered an attractive strategy to promote fibrosis regression.
Mucosal-Associated Invariant T (MAIT) cells are non-conventional innate-like T cells at the interface between innate and adaptive immunity11. They are restricted by the non-polymorphic MHC-related class 1 (MR1) molecule and express the evolutionary conserved αβ-T cell receptor (TCR), consisting of an invariant alpha chain Vα7.2-Jα33 in humans (Vα19-Jα33 in mice) and a restricted set of TCRβ chains11,12. MAIT cells recognize bacterial ligands presented by MR1, that comprise agonist metabolites derived from the synthesis of riboflavin (vitamin B2) biosynthesis pathway, as well as ligands derived from folate metabolism that behave as antagonists11,13,14,15. In particular, 6-Formylpterin (6-FP) and acetyl-6-formylpterin (Ac-6-FP), the resulting products of folic acid photodegradation, can bind to MR1, increasing its stability and its surface expression, but inhibit MAIT cell activation by competing with the activating ligands16. The regulatory role of MAIT cells in the pathogenesis of chronic liver diseases has recently emerged11. In particular, we and others have shown that MAIT cells are decreased in the blood and in the liver of patients with fibrosis or end-stage cirrhosis17,18. However, they accumulate in the fibrotic septa and display an activated phenotype associated with profibrogenic properties, via direct effects on hepatic myofibroblasts, and may promote fibrosis progression, by fostering a local inflammatory reaction18. These findings identified MAIT cells as a potential target for the prevention of liver fibrosis progression. In the present study, we combine studies in human precision-cut liver slices (PCLS) and mouse models to investigate whether a MAIT cell inhibition-based strategy may impact liver fibrosis progression and regression. We show that inhibiting MAIT cell activation halts fibrosis progression and promotes fibrosis regression via a change in the profibrogenic vs resolutive macrophage signature and an increase in Ly6Clo restorative MoMac frequency.
Results
MAIT cell inhibition reduces inflammatory and fibrogenic genes in human PCLS
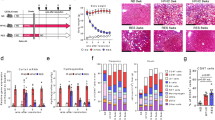
We evaluated the consequences of MAIT cell inhibition on liver fibrogenesis in human liver samples, using ex vivo PCLS from patients with fibrosis and end-stage cirrhosis from various etiologies (viral, metabolic and alcoholic, Table 1). PCLS were exposed for 48 h to the non-agonist synthetic folate derivative Acetyl-6-formylpterin (Ac-6-FP), which dampens MR1 stimulation and inhibits MAIT cell activation16. There was no impact of Ac-6-FP on the viability of PCLS (Fig. 1a). As previously observed in surgical liver samples from patients with cirrhosis18, MAIT cells, identified by their Vα7.2 positivity by immunostaining, were found in proximity to fibrogenic smooth muscle alpha-actin (α-SMA) positive myofibroblasts in PCLS from patients with fibrosis or cirrhosis (Fig. 1b). Exposure of PCLS to Ac-6-FP did not affect the number of Vα7.2+ cells but strongly reduced their activation, as reflected by the decreased number of CD69 + Vα7.2+ cells assessed by immunostaining (Fig. 1c). The MAIT cell antagonist significantly decreased the expression of CCL2 chemokine and its receptor CCR2 but did not significantly modify TNFA (p = 0.09) and IL1B expressions (Fig. 1d, Table 2). Interestingly, Ac-6-FP caused a significant reduction in the expression of the fibrogenic genes COL1A1 and A2, ACTA2, and there was a trend to decrease that of the fibrogenic cytokine TGFB (p = 0.07) (Fig. 1d). These data were further confirmed by a decrease in the number of α-SMA+ cells in response to Ac-6-FP (Fig. 1e). These findings indicate that inhibiting MAIT cell activation decreases inflammatory and fibrogenic gene expression in human liver samples.
Human PCLS were incubated with 10 µM Ac-6-FP or its vehicle for 48 h. a PCLS viability was assayed using PrestoBlue™ Cell Viability assay by fluorescence. b MAIT cell localization was evaluated by immunofluorescent co-staining of Vα7.2 and α-SMA. A representative image of two experiments is shown. c Representative images and quantification of Vα7.2+ cell number per PCLS and %CD69 + Vα7.2+ activated cells. Results are expressed as % of CD69+Vα7.2+/total Vα7.2+ cells. Each point is the mean value per PCLS. d Expression of inflammatory and fibrogenic genes in PCLS normalized to housekeeping gene PPIA. e Representative images of α-SMA immunostaining on liver tissue sections from those cirrhotic patients and respective quantifications of positive areas. a, d, e n = 7 patients (see Table 1 Group 1) except for CCR2, TNFA, and IL1B where expression was not detected for one patient. c Experiments were performed on four patients (see Table 1 Group 2) using two-tailed paired t test (**p = 0.006). d, e *p < 0.05 by two-tailed Wilcoxon matched-pairs signed rank test. Bars show the mean. Scale bar is 10 µm for b, c and 100 µm for e. Source data are provided as a Source Data file.
MAIT cell inhibition blocks liver fibrosis progression and promotes regression
We next evaluated whether blocking MAIT cells in a therapeutic setting (i.e., administration of Ac-6-FP at the stage of fibrosis, while continuing the injury) may prevent fibrosis progression. To that aim, male mice were administered CCl4 for 4 weeks, with daily injection of Ac-6-FP starting from 2.5 weeks, at a time point where mild fibrosis is already observed19 (Fig. 2a). We found that blocking MAIT cell activation limits fibrosis progression, as shown by reduced Sirius red staining and decreased number of α-SMA+ cells in mice administered Ac-6-FP as compared to vehicle-injected animals (Fig. 2b). These data demonstrate the efficacy of therapeutic intervention targeting MAIT cells for liver fibrosis.
a Timeline of the CCl4-induced fibrosis progression protocol in C57BL/6 J mice. b Representative images and quantification of Sirius red (SR; ***p = 0.0002) and α-SMA-positive areas (***p = 0.0006) in liver tissue sections (n = 8 mice/group). c Timeline of the CDAA-HFD-induced fibrosis regression protocol in C57BL/6 J mice. Representative images and quantification of Sirius red areas in liver tissue sections at day 1 (n = 5 mice/group) and day 8 (n = 8 mice/group). *p = 0.02; **p = 0.0016. d Timeline of the CCl4-induced fibrosis regression protocol in C57BL/6 J and MR1−/− mice. Representative images and quantification of Sirius red areas in liver tissue sections from C57BL/6 J (n = 8 mice/group) and MR1−/− mice (n = 5 mice/group) at days 1 and 4. **p = 0.002. e Flow cytometry analysis of CD4−CD8−GFP+ MAIT cell frequency in CCl4-exposed B6-MAITCAST mice injected with Ac-6-FP or vehicle (pooled data from three experiments n = 22 mice in vehicle group and 25 mice in Ac-6-FP group at day 1; n = 13 mice/group at day 2; n = 19 mice for vehicle and 21 mice for Ac-6-FP at day 4). Oil-injected mice served as control (pooled data from 7 experiments n = 36 mice). f Intracellular staining of CD4−CD8−GFP+ MAIT cells for TNFα and IL17 after 4 h intrahepatic leukocyte stimulation with PMA/ionomycin and Brefeldin A. Pooled data from 3 experiments for TNFα at day 1 (n = 16 mice for oil, n = 14 mice for vehicle and n = 18 mice for Ac-6-FP; *p = 0.04, **p = 0.003), and from 2 experiments for IL17 (n = 9 for oil, n = 11 for vehicle and n = 12 for Ac-6-FP; *p = 0.03, **p = 0.004). g Representative images and quantification of Sirius red areas in liver tissue sections from B6-MAITCAST congenic mice daily injected either with Ac-6-FP or vehicle (n = 7 mice/group; ***p = 0.0006), anti-MR1 or isotype (n = 5 mice/group at day 1, n = 8 at day 4; ***p = 0.0002). a, c, d Timelines of injections were created with BioRender® software. Representative images were taken at ×10 magnification. Scale bar is 100 µm. Data are mean ± S.D. Statistical analysis was performed by two-tailed Mann–Whitney test. SR sirius red, CDAA-HFD choline-deficient l-amino-acid defined high-fat diet, PMA phorbol 12-meristate 13-acetate. Source data are provided as a Source Data file.
We also evaluated whether blocking MAIT cell activation impacts fibrosis regression in mice. We used two mouse models of fibrosis reversibility, which consist either in the switch to normal diet from Choline-Deficient, L-Amino-Acid defined High Fat Diet (CDAA-HFD), a model of non-alcoholic steatohepatitis (NASH)-induced fibrosis, or the cessation of CCl4 administration in mice with established CCl4-induced fibrosis8,20,21. In the first model, male C57BL/6 J mice were fed with CDAA-HFD for 8 weeks, and switched to a normal diet together with injection with Ac-6-FP for 1 day, or daily for 8 days after switching (Fig. 2c). Switching to a normal diet for 8 days led to steatosis reversal in vehicle-exposed mice, as shown by the decrease in the steatosis area, that was not affected by administration of Ac-6-FP (Fig. S1a). In contrast, switching to a normal diet did not affect the extent of collagen accumulation and fibrogenic cell density in vehicle-injected mice as compared to day 1, but Ac-6-FP promoted fibrosis regression, as shown by the reduction of Sirius red (Fig. 2c) and α-SMA (Fig. S1b) staining areas, compared to vehicle at day 8. In the second model, male C57BL/6 J mice were chronically i.p administered CCl4 for 4 weeks, and injected Ac-6-FP for 1 day, or daily for 4 days after the last CCl4 injection (Fig. 2d). As previously described20, accumulation of collagen and the density of α-SMA-expressing fibrogenic cells remained elevated 4 days after the last CCl4 injection, in C57BL/6 J mice injected with vehicle. In contrast, mice daily administered Ac-6-FP showed fibrosis regression, as reflected by reduced Sirius red (Fig. 2d) and α-SMA staining areas (Fig. S1c). Importantly, Ac-6-FP did not modify Sirius red staining when administered to male MR1−/− mice, which are deficient in MAIT cells (Fig. 2d). These data, obtained in two different settings, i.e., a NASH and a toxic model of liver fibrosis, demonstrate that inhibiting MAIT cell activation promotes fibrosis regression.
The efficacy and specificity of Ac-6-FP were further evaluated in B6-MAITCAST mice22. These mice are enriched in MAIT cells and constitute a relevant tool for functional evaluation of MAIT cells, since they have been bred with a transgenic RORγtGFP reporter strain, and express GFP in RORγT-expressing cells, including MAIT cells22. The frequency of liver MAIT cells, identified as CD45+ CD19−CD11b−TCRβ+ CD4−CD8−RORγtGFP+ (Fig. S2) was not modified upon exposure to CCl4 and was not affected by Ac-6-FP (Fig. 2e). Similar findings were obtained when identifying MAIT cells with MR1 tetramers loaded with 5-OP-RU (CD45+ CD19-CD11b-TCRβ+ CD4−CD8-MR1tet+, Fig. S3a). The compound did not alter the frequency of total TCRβ+, CD4+, CD8+, CD4−CD8−, NK1.1+, γδT, or NKT cells, either in B6-MAITCAST mice or C57BL/6 J mice (Fig. S3b, c). We previously observed that in patients with end-stage liver disease, MAIT cells display an activated phenotype18. In keeping, in mice chronically exposed to CCl4, liver MAIT cells also exhibited an activated phenotype characterized by an increased frequency of TNFα+ and IL17 + MAIT cells, as compared to that of oil-injected animals (Fig. 2f). The MR1-blocking ligand Ac-6-FP decreased MAIT cell activation, since it abolished the increase in TNFα+ or IL17+ liver MAIT cell frequency in CCl4-injected mice (Fig. 2f). As observed in C57BL/6 J mice, the blocking ligand also promoted fibrosis regression in B6-MAITCAST mice after 4 days (Fig. 2g).
Beneficial impact of a MR1-blocking strategy was further confirmed with a monoclonal neutralizing antibody to MR1. Injection of the MR1 antibody to mice with established fibrosis for 4 days after the last CCl4 injection decreased MAIT cell activation, as reflected by reduced frequency of CD69+ MAIT cells (Fig. S3d) while it did not affect MAIT cell frequency (Fig. S3e). Mice injected with the MR1 antibody showed decreased Sirius red staining compared to mice injected with isotype (Fig. 2g). Taken together, these data demonstrate through two different, pharmacological and antibody-driven, approaches the beneficial impact of MAIT cell inhibition on fibrosis regression.
Inhibiting MAIT cells alters the frequency and phenotype of MoMac
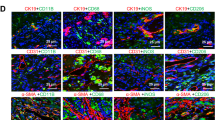
MoMac play a dual role in fibrosis progression and regression, with CCR2-dependent recruitment of Ly6Chi inflammatory monocytes to the liver during fibrosis progression, and a shift to Ly6Clo restorative monocyte-derived macrophages (MoMac) during resolution8,21,23. We, therefore, evaluated their contribution to the acceleration of fibrosis regression induced by MAIT cell inhibition. Time course flow cytometry analysis of monocyte/macrophage populations during fibrosis regression showed that, among the total CD45+, Kupffer cells (F4/80+TIM4+) were the main macrophage population in the liver of control oil-injected mice (Fig. 3a). However, the proportion of Kupffer cells was strongly decreased by CCl4 at day 1 and up to 4 days after cessation of the injury. In contrast, and as previously reported8 the proportion of profibrogenic Ly6Chi MoMac population strongly increased one day after cessation of injury but decreased thereafter; the restorative Ly6Clo MoMac population emerged one day after cessation of the injury and was sustained for up to 4 days (Fig. 3a, Fig. S4a). Interestingly, flow cytometry analysis showed that Kupffer cells were the only population expressing MR1 among the monocyte/macrophages in control oil-injected mice, with marginal expression in Ly6Clo and Ly6Chi MoMac. In CCl4-injected animals, where Kupffer cells are lost, the Ly6Clo and to a lesser extent Ly6Chi MoMac populations convert into MR1-expressing cells, as evidenced by up-regulation of MR1 expression at the cell surface (Fig. 3b).
a–d Mice were injected CCl4 or oil for 4 weeks along the protocol described in Fig. 2d and frequencies of resident Kupffer cells (KC) and MoMac analyzed after cessation of injury. a Frequency of intrahepatic KC, Ly6Clo and Ly6Chi MoMac in C57BL/6 J. Each point is the mean of five mice except for oil where n = 13 mice. b Frequencies of MR1+ cells among KC, Ly6Clo and Ly6Chi MoMac at day 1 after CCl4 cessation (n = 7 mice/group) vs oil (n = 6 mice/group). **p = 0.001. c Frequencies of Ly6Chi, Ly6Clo (pooled data from two experiments, n = 11 mice/group), and CCR2+ (n = 6 mice/group) MoMac from C57BL/6 J mice at day 1 after the last CCl4 injection. *p = 0.01, **p = 0.002. d Frequencies of Ly6Chi and Ly6Clo from MR1−/− mice exposed to Ac-6-FP or vehicle, at day 1 after CCl4 cessation (n = 5 mice/group) compared to oil (n = 6). **p = 0.009. e, f Representative images of immunofluorescent staining of CD206 and CCR2 and quantification of the CCR2/CD206 mean intensity ratio in BMDM alone or co-cultured with MAIT cells for 48 h in the presence of e Ac-6-FP or vehicle (n = 5 experiments except for BMDM + Ac-6-FP where n = 2; each point represent the mean per experiment; *p = 0.02 for BMDM + MAIT vs BMDM alone, and *p = 0.04 for BMDM + MAIT + Ac-6-FP vs BMDM + MAIT + vehicle), or f antibodies to TNFα and/or IL17 or control isotype (typical representative of two experiments; each point is one field; n = 12 fields/group except for BMDM + MAIT + isotype where n = 15 fields). *p = 0.02; **p = 0.002; ****p < 0.0001. Scale bar is 10 µm. g Timeline of CCl4, Ac-6-FP and clodronate or PBS liposome administration in C57BL/6 J mice created with BioRender® software. h Clodronate vs PBS liposome fold change of Ly6Chi and Ly6Clo MoMac frequencies at day 1, 2, and 4 after CCl4 cessation. Each point represents the mean value of 5 mice for day 1 and day 2, and 4 mice for day 4. i Representative images and quantification of Sirius red areas (typical representative of n = 4 experiments) of liver tissue sections from mice treated with Ac-6-FP or vehicle (n = 5 mice/group except for vehicle clodronate group where n = 4 mice). Sample were collected 4 days after the last CCl4 injection. Scale bar is 100 µm. *p = 0.05. Data are mean ± S.D. Statistical analysis was performed by (b–d, i) two-tailed Mann–Whitney test or (e, f) Kruskal–Wallis followed by Dunn’s multiple comparisons post-test. KC Kupffer cells, BMDM bone marrow-derived macrophages, clodro clodronate-encapsulated liposomes, PBS phosphate-buffered saline-encapsulated liposomes. Source data are provided as a Source Data file.
Flow cytometry analysis of hepatic leukocytes showed that administration of Ac-6-FP resulted in a decrease in the frequency of the Ly6Chi and CCR2+ MoMac, and a concordant increase in the Ly6Clo MoMac (Fig. 3c, Fig. S4a–c). Strikingly, the frequency of Ly6Chi and Ly6Clo MoMac was not affected by Ac-6-FP in MAIT cell-deficient MR1−/− mice (Fig. 3d). Taken together, these data demonstrate that blocking MAIT cell activation results in a decrease in the recruitment of Ly6Chi /CCR2+ MoMac and the emergence of a Ly6Clo MoMac phenotype.
We next investigated whether MAIT cells have a direct impact on macrophage phenotype in co-culture experiments. Naive bone marrow-derived macrophages (BMDM) were co-cultured either alone, or with anti-CD3/CD28-activated MAIT cells exposed to Ac-6-FP or vehicle (FACS gating strategy of MAIT cells Fig. S5). In the presence of activated MAIT cells, BMDM showed an increase in the CCR2/CD206 ratio, reflecting a shift toward a pro-inflammatory macrophage phenotype, while no increase was observed in the presence of Ac-6-FP (Fig. 3e); Ac-6-FP had no direct effect when added alone to BMDM (Fig. 3e). In addition to MR1 requirement, neutralizing antibodies to TNFα also blunted the increase in the CCR2/CD206 ratio, whereas blocking IL17 had no effect (Fig. 3f).
We next examined the consequences of i.v clodronate administration on Ac-6-FP-induced fibrosis regression. Liposome-encapsulated clodronate or Phosphate Buffer Saline (PBS) counterparts were administered to mice with established fibrosis following 4 weeks of CCl4 administration, and Ac-6-FP was daily administered for 1, 2, or 4 days following cessation of CCl4 exposure (Fig. 3g). Compared to the liposome-encapsulated PBS control group, clodronate treatment depleted half of intrahepatic Ly6Clo MoMac at day 1 and 2, which was restored at day 4. In contrast, clodronate did not deplete Ly6Chi at day 1, in keeping with their low phagocytic capacity8, and a compensatory increase was observed from day 2 (Fig. 3h). MAIT cell inhibition by Ac-6-FP did not affect the extent of MoMac depletion (Fig. S6). Finally, whereas Ac-6-FP promoted fibrosis regression in mice exposed to control liposomes, this effect was totally abolished upon depletion with liposome-encapsulated clodronate (Fig. 3i).
Altogether, these data highlight that acceleration of fibrosis regression by Ac-6-FP is supported by a MAIT-monocyte/macrophage dialog, that results in a shift from a pro-inflammatory Ly6Chi toward a restorative Ly6Clo MoMac phenotype.
Inhibiting MAIT cell activation alters the MoMac signature
In order to determine whether Ac-6-FP-induced inactivation of MAIT cells also impacts on MoMac signature, we performed RNA sequencing analysis on sorted Ly6Chi and Ly6Clo MoMac isolated from the liver of CCl4-injected mice exposed to Ac-6-FP or vehicle. Impact of Ac-6-FP on both populations was evaluated by comparing genes differentially regulated between Ac-6-FP in Ly6Chi and Ly6Clo MoMac. A total of 2687 genes were differentially regulated by Ac-6-FP in Ly6Chi vs Ly6Clo MoMac with 1428 genes upregulated and 1259 downregulated (p ≤ 0.05 and fold change ≥ 1.5) (Fig. 4a). Among the 89 deregulated KEGG pathways, the first 25 could be classified into “apoptosis/cell cycle”, “metabolism”, “autophagy” and “inflammation” (Fig. 4b).
a Heatmap of the 2687 genes with a significant (p ≤ 0.05, paired t test) difference in the ratio of Ly6Chi Ac-6-FP/vehicle and Ly6Clo Ac-6-FP/vehicle and a fold change ≥ 1.5. Clustering used Euclidean distance and Ward.D2 agglomeration method. b Top KEGG pathways from the enrichment analysis based on the 2687 genes with a significant difference in the ratio of Ly6Chi Ac-6-FP/vehicle vs Ly6Clo Ac-6-FP/vehicle. KEGG pathways are ordered by −log10(FDR). c Ac-6-FP/mean vehicle ratios in Ly6Chi (red) and Ly6Clo (blue) for selected genes from Apoptosis (mmu04210) and d Autophagy (mmu04140) KEGG pathways. e Geometric Mean of LC3II-positive cells in total macrophages (F4/80 + CD11b + ; *p = 0.03) and Ly6Chi and Ly6Clo (*p = 0.05) macrophages sorted from C57BL/6 J mice either injected Ac-6-FP or its vehicle. Statistical analyses were performed using two-tailed Mann–Whitney test. f Ac-6-FP/mean vehicle ratios in Ly6Chi (red) and Ly6Clo (blue) for selected genes from Glycerophospholipid metabolism (mmu00564). c–f Experiments were performed on n = 5 mice/group. Data are presented as mean values ± S.E.M. Up upregulated genes, Down downregulated genes. Source data are provided as a Source Data file.
Apoptosis was far the most significant pathway deregulated by Ac-6-FP in Ly6Chi vs Ly6Clo MoMac. Indeed, Ac-6-FP mainly upregulated apoptotic genes in Ly6Chi MoMac such as members of the caspase family (Casp 2, 3, and 7), but only marginally in Ly6Clo liver MoMac. In contrast, Ac-6-FP enhanced the expression of some anti-apoptotic genes in resolutive Ly6Clo macrophages (Fig. 4c), including Akt2, Pdpk1 or Birc3. Interestingly, the autophagy pathway was also upregulated by Ac-6-FP, with the induction of a set of non-overlapping autophagic genes in Ly6Chi vs Ly6Clo MoMac (Fig. 4d). In particular, genes of the ATG family, such as Atg4a, Atg5, Atg10, and Atg14, as well as Wipi1 or Ctsl were upregulated by Ac-6-FP in Ly6Chi, whereas Pik3c3, Tsc1 or Prkaa1 were induced in Ly6Clo MoMac. Functional FACS analysis of the autophagy marker LC3II further showed an increase in the frequency of both LC3II + Ly6Chi and Ly6Clo MoMac isolated from the liver of CCl4-injected mice exposed to Ac-6-FP (Fig. 4e). Ac-6-FP also deregulated several metabolic pathways, among which one of the most significant pathways was the glycerophospholipid metabolic pathway, converging to phosphatidic acid and phosphatidylcholine synthesis, with 12 genes out of 18 mainly upregulated in Ly6Chi MoMac (Fig. 4f), including Taz, Gpat4, Agpat2, Agpat9, Dgka, and Dgkz, Lpcat1, and Pemt. Interestingly, certain lipids including phosphatidic acid are required in the autophagy process during autophagosome formation and fusion with lysosomes24. Intriguingly, the impact of Ac-6-FP on the inflammatory signature of macrophages was minor (Fig. S7). Indeed, deregulated pathways (“Malaria”, “Hepatitis B”, “Leishmaniasis”, “NOD-like receptor signaling pathway”, “TNF signaling pathway” and “Measles”) were all related to inflammatory signaling but mainly included genes of the KEGG “apoptosis” or “autophagy” pathways (Atg5, Bcl2, Birc3, Akt2… Fig. 4c, d). In addition, some genes were not restricted to inflammatory signaling but also related to survival/apoptosis and autophagy, such as Mapk13, Mapk 14, Tbk1, Nfkbia, and Nfkbib. Finally, variations in both pro (Myd88, Aim2, Il18, Il1b, Ptgs2) and anti-inflammatory/fibrogenic genes (Ifng, Ticam1, Tbk1and Mmp9) were observed in one or both Ly6Chi and Ly6Clo MoMac (Supplementary data 1).
Taken together, these data demonstrate that inhibition of MAIT cell activation by Ac-6-FP shifts Ly6Chi MoMac phenotype toward a profile characterized by enhanced apoptotic and autophagic features and induction of the glycerophospholipid metabolic pathway. The MAIT cell inhibitory ligand also promotes a shift toward resolutive Ly6Clo MoMac with enhanced survival and autophagic features.
Discussion
Despite major advances in the understanding of the mechanisms underlying the resolution of liver fibrosis and its end-stage cirrhosis, pharmacological therapies are still lacking in humans. Because specific immune cell subsets drive fibrosis progression and regression, recent studies have underscored that anti-inflammatory approaches may serve as the basis for promoting fibrosis resolution. Combining human data in ex vivo precision-cut liver slices with mouse models and cell culture experiments, the present study demonstrates that inhibiting MAIT cell activation by MR1 inhibitory ligands constitutes an interesting strategy to limit liver fibrosis progression and promote fibrosis resolution. Mechanistic studies show that fibrosis resolution initiated by inhibition of MAIT cell activation triggers a change in the profibrogenic vs resolutive macrophage signature and a decrease in their frequency, in favor of Ly6Clo resolutive MoMac phenotype.
The control of inflammatory signals originating from innate, innate-like, and adaptive immunity is considered an interesting option for promoting fibrosis regression3,4,6. As such, the capacity of activated MAIT cells to produce profibrogenic cytokines such as IL17 and TNFα makes these immune cells an attractive target for liver fibrosis resolution. As we previously observed in human liver samples18, mouse models of chronic liver injury demonstrated that liver MAIT cells were activated at the onset of fibrosis and adopted an IL17/TNFα phenotype, therefore validating their use to test the potential of MAIT cell inhibition-based antifibrogenic strategy. Identification of interventional treatments modulating MAIT cell frequency and/or phenotype is a major challenge. As of now, proposed therapies to block MAIT cell activation favor the blockade of MR1 presentation by MR1 inhibitory ligands such as Ac-6-FP or MR1-blocking antibodies, rather than directly targeting MAIT cells, given their important role in the homeostasis of mucosal tissues against invading pathogens25. These MR1 inhibitory ligands have shown their efficacy in preclinical models, with antitumoral effects26, beneficial effects on bacterial lung infection27 and improved insulin sensitivity and glucose tolerance in obese mice28. Using this strategy, we demonstrate that administration of Ac-6-FP following initiation of the CCl4-induced injury slows down fibrosis progression, demonstrating the efficacy of targeting MAIT cells in a therapeutic setting. Importantly, we also unravel that acceleration of liver fibrosis regression is promoted by blocking MR1 presentation by either Ac-6-FP or an MR1 antibody. We could extend our findings to a mouse model of NASH-induced fibrosis, in which Ac-6-FP also accelerated fibrosis regression when switching to a normal diet. Interestingly, the antifibrogenic efficacy of Ac-6-FP was also demonstrated in pilot studies using human precision-cut liver slices, which preserve the complex liver architecture and retain cell-cell and cell-matrix contacts. Indeed, exposure of PCLS from patients with fibrosis or end-stage cirrhosis to Ac-6-FP resulted in a decrease in MAIT cell activation and reduced the expression of fibrogenic genes, including COL1A1 and COL1A2, ACTA2, and TGFB1, together with the number of fibrogenic cells. Interestingly, Ac-6-FP also caused a reduction in the expression of the pro-inflammatory/fibrogenic chemokine CCL2 and of its receptor CCR2, a receptor expressed both by hepatic stellate cells and pro-inflammatory macrophages29. The nature of the bacterial-derived MAIT ligand presented by MR1 remains to be characterized but is most likely a gut-derived bacterial component that flows to the liver13,14, as a consequence of the characteristic increase in gut permeability, intestinal bacteria overgrowth and dysbiosis observed in patients and animal models during chronic liver injury30. Taken together, our data in mice and human samples reveal MAIT cells as one major actor that promotes fibrosis progression and limits resolution during chronic liver injury and validate the use of a MAIT cell-based inhibitory antifibrogenic approach.
Another major finding relates to the demonstration that an MR1-blocking strategy impacts on the MAIT macrophage crosstalk to drive fibrosis regression. Previous studies in mice have undoubtedly established the crucial role of monocytes and macrophages in fibrosis resolution, which is orchestrated by restorative Ly6Clo MoMac at the expense of scar-associated Ly6Chi MoMac8,10,21,23. Therefore, approaches directly impacting monocyte/macrophage recruitment, macrophage activation, and/or macrophage functions and polarization are the most investigated3,9. Our data demonstrate that indirect targeting of macrophage phenotype through inhibition of MAIT cell activation may represent a promising alternative to promote fibrosis regression. We and others have shown that bidirectional crosstalk exists between MAIT cells and macrophages, since activated MAIT cells can switch macrophages toward a pro-inflammatory phenotype while pro-inflammatory macrophages can promote activation of MAIT cells, at least in part via TCR-MR1-dependent interactions18,28,31. Importantly, our findings further argue for a direct MAIT macrophage dialog and specifically demonstrate that activated MAIT cells increase the CCR2/CD206 macrophage ratio, an effect that is blunted by disrupting MAIT cell activation upon macrophage presentation of Ac-6-FP. We also demonstrate that inhibiting MAIT cell activation by Ac-6-FP affects both the frequency and the phenotype of Ly6Chi vs Ly6Clo macrophages. Indeed, there was a decrease in the frequency of Ly6Chi/CCR2+ MoMac and a concomitant increase in that of Ly6Clo MoMac, when CCl4-exposed mice were injected Ac-6-FP after cessation of the injury. Interestingly, although Kupffer cells are the main population expressing MR1 in physiological conditions, their depletion in the fibrotic liver gave rise to Ly6Chi and Ly6Clo MoMac populations that upregulated MR1 at their cell surface. Finally, the use of clodronate liposomes in mice with established fibrosis depleted, at least in part, Ly6Clo MoMac while it marginally affected Ly6Chi MoMac. Interestingly, clodronate liposomes abolished the beneficial effect of Ac-6-FP on fibrosis regression, suggesting that restorative Ly6Clo MoMac is required for Ac-6-FP beneficial effects. Taken together, these data also further reinforce that modulating the MAIT cell/macrophage dialog is required and sufficient for promoting fibrosis regression, independently of interactions with other liver cells such as hepatic stellate cells or hepatocytes.
RNA sequencing and functional studies also showed that Ac-6-FP profoundly modified the Ly6Chi vs Ly6Clo signature and caused deregulation of pathways related to cell cycle/apoptosis, metabolism, autophagy, and to a minor extent inflammation. In particular, and in keeping with the decrease in Ly6Chi MoMac frequency, apoptosis was the most significant deregulated KEGG pathway affected by Ac-6-FP, with apoptotic genes mainly upregulated in Ly6Chi and survival genes in Ly6Clo counterparts. One of the proposed mechanisms underlying the shift from fibrogenic to resolutive macrophages is through a phenotypic switch8. Our data also suggest that enhancing apoptosis of Ly6Chi MoMac, while sustaining Ly6Clo could constitute an additional mechanism. In keeping, we have previously demonstrated that inducing apoptosis of pro-inflammatory monocyte/macrophages protects against chronic liver injury32.
Another anti-inflammatory and antifibrogenic pathway targeted by Ac-6-FP is autophagy, with the increase of a set of non-overlapping but essential autophagic genes both in Ly6Chi and Ly6Clo subtypes. The MR1-blocking ligand upregulated genes that contribute to different steps of the autophagic process, including autophagosome formation (Pi3kc3, Atg14, Atg10) and maturation (Wipi1, Atg5/Atg16 and Atg16l2). It should also be underlined that some of these autophagic genes, such as Pik3c3, Atg5, or Atg16 also drive LC3-associated phagocytosis, a non-canonical form of autophagy with anti-inflammatory properties in macrophages33. These data were also confirmed in functional studies, which showed an increase in the frequency of LC3II-positive cells in both subtypes of macrophages. They are in line with the antifibrogenic properties of canonical and non-canonical autophagy in macrophages19,33,34.
Finally, Ac-6-FP also regulated genes related to lipid metabolism, mainly affecting lipid metabolic pathway in Ly6Chi MoMac. Changes in lipid metabolism are increasingly recognized as a driving force for macrophage reprogramming, although data regarding scar-associated macrophages in the context of liver fibrosis are scarce35. RNA sequencing analysis revealed that Ac-6-FP mainly induced a large set of genes in Ly6Chi MoMac related to glycerophospholipid metabolism, that converged to phosphatidic acid formation from glycerol-3 phosphate/LPA (Gpd2, Gpcpd1, Gpat4, Agpact2, and 9), diacylglycerol formation and degradation (Dgka and z) or lysophosphatidylcholine/phosphatidylcholine (Lpcat1). Interestingly, enhanced expression of genes affiliated with glycerophospholipid metabolic pathway may also provide a link between lipid metabolism and autophagy. Indeed, besides PI(3)P, recognized as a master regulator of autophagosome biogenesis and fusion, glycerophospholipids such as phosphatidic acid have also been reported to stimulate autophagosome biogenesis24, or reduce the inhibitory protein mTORC2 activity36. In addition, we also found that Ac-6-FP-exposed Ly6Chi MoMac showed increased Taz gene expression, coding for tafazzin, which promotes maturation of cardiolipin, and allows initiation of mitophagy through interaction with LC337.
In conclusion, our data demonstrate that inhibiting MAIT cells promotes scar resolution via reprogramming of macrophage profile. They open interesting perspectives for therapies promoting liver fibrosis regression, based on the use of MAIT cell inhibitory ligand-directed approaches.
Methods
Our research complies with all relevant ethical regulations. Human study protocol was approved by the Institutional Review Board of Paris North Hospitals, Paris Cité University, AP-HP (N°CER-2021-88), and animal study protocol was approved by the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation (APAFIS #30284-2021030912208867).
Human liver samples
Liver samples were obtained from patients from both genders with advanced fibrosis (F2-F4 Metavir score, n = 11) undergoing liver resection or liver transplantation at the digestive surgical department of Beaujon Hospital (Clichy, France). Patients’ clinical data are presented in Table 1. Patients’ age is between 33 and 73. All patients gave written consent to participate in the study. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Paris North Hospitals, Paris Cité University, AP-HP (N°CER-2021-88). Fresh liver specimens were examined by a pathologist and samples were collected at distance from the tumor (when present) and surgical margins.
Human ex vivo PCLS
Fresh liver specimens were harvested and immediately kept on ice in a sterile University of Wisconsin solution (Belzer UW® Cold Storage Solution, Bridge to Life, BTLBUW-1000). Liver cores were generated from liver specimens using 8 mm diameter biopsy punches, embedded into 5% low-gelling-temperature agarose (Sigma-Aldrich) and mounted in a tissue slicer (automated vibrating blade microtome, Leica Biosystems VT1200 S) filled with Hanks Balanced Salt Solution supplemented with 25 mM of d-Glucose (Sigma-Aldrich), 100 μg/mL streptomycin, 1 μg/mL amphotericin B (GibcoTM). Precision-cut liver slices (PCLS, 8 mm diameter, 250 µm thickness) were generated using the following slicing parameters: speed 0.5 mm/s, thickness 250 µm, amplitude 3 mm. Human PCLS were transferred on 8 µm PET tissue culture inserts (ThinCertTM, Greiner bio-one) in six-well plates and pre-incubated in William’s E Medium (GibcoTM) supplemented with 100 IU/mL penicillin and 100 μg/mL streptomycin, 1 μg/mL amphotericin B (GibcoTM), and 25 mM d-Glucose (Sigma-Aldrich). After 1 h pre-incubation, fresh culture medium containing 10 µM Ac-6-FP or vehicle (0.01%DMSO) was added and PCLS were cultivated at 37 °C, 5% CO2, in normoxic conditions, under continuous orbital agitation (70 rpm). Culture medium was daily renewed. The viability of PCLS was assessed using PrestoBlue™ Cell Viability Reagent (Invitrogen, A13262) according to the manufacturer’s instructions (Fig. 1a, Table 1 Group 1) and was not affected by Ac-6-FP. After 48 h of culture, PCLS were washed in cold PBS and immediately snap frozen in liquid nitrogen and stored at −80 °C until processing for RNA extraction or fixed in 10% formalin for 24 h for immunohistochemistry experiments, as described below.
Immunofluorescent staining of PCLS
Frozen sections (3µm-thick) generated from PCLS (n = 4, Table 1 Group 2) were fixed in 4% paraformaldehyde and further blocked in 1%BSA-PBS and 0.2% Triton X100 for 1 h at room temperature (RT). Slides were incubated with rabbit polyclonal anti-human CD69 antibody (1/50, #ab175391, Abcam) mouse anti-human Vα7.2 (3C10, 1/50, #351702, BioLegend) or mouse anti-human α-SMA (1A4, 1/1000, #A5228, Sigma-Aldrich) for 2 h at RT, followed by secondary antibody, either goat anti-rabbit Alexa488 (1/500, #A11034, Thermofisher Scientific), goat anti-mouse Alexa555 (1/200, #A28180, Thermofisher Scientific), or goat anti-mouse Alexa488 (1/1000, #A11001, Thermofisher Scientific) for 1 h at RT. PCLS were mounted by ProLong Gold Antifade Mounting medium with DAPI (Table 3). Representative images were acquired using a confocal microscope (Zeiss LSM 780), equipped with a ×63 oil immersion objective. For quantification, 20 to 28 fields were randomly selected, and the number of activated MAIT cells (CD69+Vα7.2+) and total MAIT cells (total Vα7.2+) was quantified. Results were expressed as % of CD69+Vα7.2+/total Vα7.2+ cells. No staining signal was observed when omitting the primary antibody.
RNA extraction and quantitative polymerase chain reaction blot
RNA from PCLS (n = 7, Table 1 Group 1) was extracted in TRIzol™ reagent (Life Technologies) according to manufacturers’ instructions. cDNA synthesis was performed with QuantiTect Reverse Transcription Kit (Qiagen, 205313). Quantitative polymerase chain reaction (qPCR) was performed on Real-Time PCR system LightCycler® 96 Instrument (Roche) using TaqMan® probes for ACTA2, CCL2, COL1A1, COL1A2, IL1B, TGFB1, TNFA, CCR2 and PPIA (#4331182, Applied Biosystems). Probes sequences are detailed in Table 2. PCR was performed with the following parameters: 1 cycle at 95 °C for 10 min followed by 45 cycles at 95 °C for 15 s, 60 °C for 30 s. Gene expression was normalized to PPIA housekeeping gene. Relative expression was calculated using the (2−ΔΔCt) method.
Immunohistochemistry analysis of PCLS
Αlpha-smooth muscle actin (α-SMA) immunostaining was performed using formalin-fixed paraffin-embedded PCLS (n = 7 Table 1 Group 1) with an automated immunohistochemical stainer (Ventana Benchmark®) according to the manufacturer’s instructions. Briefly, 3 µm thickness tissue sections were dewaxed, rehydrated, and incubated with antibody targeting α-SMA (mouse monoclonal antibody, 1A4, 1/600, #M0851, DAKO, Table 3). Immunostained slides were digitized (Scanscope AT turbo®, Leica), and α-SMA staining was quantified using a dedicated algorithm (quantification of positive area, Indica labs) on the whole surface of each liver slice.
Animals
B6-MAITCAST and MR1−/− mice were obtained as previously reported22. Both strains have been backcrossed on a C57BL/6 J background for more than 10 generations. They were bred separately and housed in different cages in specific pathogen-free (SPF) animal facilities and fed ad libitum. Dark/light cycles were 12 h/12 h, with temperature of 21 °C and humidity of 50%. C57BL/6 J mice were purchased from Janvier Labs (C57BL/6 JRj). Cervical dislocation was used for mice euthanasia. Males were used for animal studies since it has been extensively reported that they are more susceptible to developing liver fibrosis than females (https://doi.org/10.1152/ajpendo.00427.2019). Experiments were performed in accordance with protocols approved by the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation and the ethic committee APAFIS n°121 Paris Nord (APAFIS #30284-2021030912208867).
Mouse models of liver fibrosis progression and regression
Liver fibrosis was induced in male C57BL/6J, B6-MAITCAST, or MR1−/− mice (10 to 12-week-old) by i.p injections of 0.6 ml/kg body weight (BW) carbon tetrachloride (CCl4, Sigma-Aldrich #87030, 1/10 dilution in mineral oil, Sigma-Aldrich #M-5310), twice a week for 4 weeks. Control animals received mineral oil (referred as to “oil” in the text) at the same frequency. To evaluate the impact of MAIT cell inhibition on fibrosis progression mice were administered CCl4 for 4 weeks with daily injection of Ac-6-FP (0.25 mg/kg body weight (b.w), #SMB01333, Sigma-Aldrich,) starting from 2.5 weeks (Fig. 2a).
To evaluate the impact of MAIT cell inhibition on fibrosis regression, two models were used. First, mice were injected intraperitoneally 2 h prior to the last CCl4 injection with MAIT cell inhibitors anti-MR1 (12.5 mg/kg b.w, 26.5, #361110, BioLegend), or Ac-6-FP, or their respective anti-IgG2a kappa isotype control (12.5 mg/kg b.w, #400281, BioLegend) or vehicle (1% DMSO in ×1 PBS) and daily until the sacrifice by cervical dislocation at day 1, day 2 or day 4 following the last CCl4 injection (Fig. 2d).
A second model of NASH-induced liver fibrosis consisted in 8 weeks choline-deficient l-amino acid defined High Fat Diet (CDAA-HFD) feeding (#A06071302i, Brogaarden, 60 kcal% fat, and 0.1% methionine, irradiated). Fibrosis regression was studied at 1 and 8 days after switching to normal diet, with daily injection of Ac-6-FP or its vehicle (Fig. 2c).
Timelines of injections shown in Fig. 2a, c, d were created with BioRender® software.
Histological analysis
Sirius Red staining was performed on 4-μm-thick formalin-fixed paraffin-embedded tissue sections at the Pathology Department of Bichat Hospital, Paris. Sirius red-stained areas from ten fields (×10 magnification) of each mouse were quantified with the software ImageJ as previously described18.
Immunohistochemistry analysis of mice liver
Immunohistochemical detection of α-SMA was performed as previously described18 (Table 4), on paraffin-embedded mice liver tissue sections (4 μm-thick) using the MOM immunodetection kit (Vector, #PK2002) and a mouse monoclonal anti-α-SMA antibody (1A4, 1/1000, #A2547, Sigma). α-SMA-positive area from ten fields (×10 magnification) from 5–8 mice/group was quantified with ImageJ software. No staining was observed when the primary antibody was omitted.
Macrophage depletion by clodronate liposomes
To deplete macrophages, C57BL/6 J mice with established fibrosis were given one i.v injection of liposome-encapsulated clodronate (Clodronate Liposomes, 0.1 ml/10 g, B#C23J0518, Liposoma). Liposome-encapsulated PBS was used as control (B#P24J0518, Liposoma). Ac-6-FP was then administered daily until the sacrifice at day 1, day 2, or day 4 following the last CCl4 injection. Timeline of injections shown in Fig. 3g were created with BioRender® software.
Intrahepatic leukocyte isolation
Following perfusion of 1× PBS through the portal vein, livers were placed into DMEM Dulbecco’s Modified Eagle Medium (DMEM, ThermoFisher, Table 4) and digested using the Liver Dissociation kit (#130-105-807, Miltenyi), according to the manufacturer’s instructions. Digests were passed through 100 µm cell strainer and washed in 30 ml DMEM and centrifuged 300 × g for 10 min. Pellets were resuspended in a 33% Percoll (GE Healthcare) diluted with RPMI complete medium (RPMI 1640 from ThermoFisher containing 10% FBS) at room temperature and centrifuged 690 × g for 20 min, with minimum break and accelerator. The pellet, containing non-parenchymal cells, was washed with RPMI complete medium. Red blood cells were lysed using 5 ml RBC Lysis buffer (420301, BioLegend) for 3 min and washed with RPMI complete medium. The pelleted cells were resuspended in 2 ml FACS buffer (1× PBS supplemented with 2%FBS and 2 mM EDTA) and transferred to FACS tubes for staining.
Ly6Chi and Ly6Clo macrophage sorting
C57BL/6 J mice with established fibrosis (0.6 ml/kg b.w CCl4 twice a week for 4 weeks), were i.p injected with Ac-6-FP (0.25 mg/kg b.w) or vehicle (1% DMSO in 1× PBS) 2 h prior to the last injection and daily during 2 days following the last CCl4 injection. Ly6G-CD11c-CD3-CD11b + F4/80 + Ly6Chi or Ly6Clo macrophages were sorted according to their Ly6C expression level using BD Biosciences FACSAria III sorter (Fig. S4a).
Flow cytometric analysis
Cell suspensions prepared from non-parenchymal liver were stained at 4 °C in FACS buffer (1× PBS containing 2%FBS and 2 mM EDTA). Surface staining was performed with the following antibodies (Table 5) fluorochrome-conjugated anti-mouse: TCRβ (H57-597, 1/200, #109241), CD3 (17A2, 1/50, #100236), CD4 (RM4-5BL, 1/100, #100526), CD8 (53-6.7, 1/100, #100742), CD69 (H1.2F3, 1/100, #104509), F4/80 (BM8, 1/100, #123147), Ly6C (HK1.4, 1/200, #128041), CD19 (6D5, 1/200, #115553), CD11c (N418, 1/100, #117316), CCR2 (SA203G11, 1/100, #150613), NK1.1 (PK136, 1/50, #108727), and Tim-4 (RMT-54, 1/200, #130009) mAbs obtained from BioLegend, France; CD45 (30-F11, 1/200, #560510), CD11b (M1/70, 1/50, #560456), Ly6G (IA8, 1/200, #560602), and TCRγδ (GL3, 1/500, #563532) mAbs obtained from BD Biosciences. Dead cells were excluded from the analysis using the fixable viability dye eFluor 506 (1/200, #65-0866-14, eBiosciences).
Liver MAIT cells were identified in B6-MAITCAST by multicolor flow cytometry from CD45+CD19−CD11b−TCRβ+ as GFP+ CD4−CD8− T cells. Similar findings were obtained when MAIT cells were gated with APC-conjugated mouse MR1 tetramers loaded with the active ligand 5-OP-RU (NIH, USA); APC-conjugated mouse MR1 tetramers loaded with the non-activating ligand Ac-6-FP were used as negative control “control tet” (Fig. S3). Data acquisition was performed using a BD Biosciences Fortessa X20 cytometer and analyzed using FlowJo software (Tree Star, 10.7.1 version).
Intracellular staining
For detection of cytokine production in MAIT cells, intrahepatic leukocytes were isolated, and cells were stimulated for 4 h at 37 °C with 25 ng/ml PMA and 1 μg/ml ionomycin (Sigma-Aldrich), in the presence of 10 μg/ml Brefeldin A (BioLegend) in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco). After surface staining, cells were fixed and permeabilized with Cytofix/Cytoperm kit (#554714, BD Biosciences), according to manufacturers’ instructions, washed and labeled for 30 min at 4 °C with anti-TNFα (MP6-TX22, 1/50, #506324), anti-IL17 (TC11-18H10, 1/50, #506904), from BioLegend. Detection of LC3II in Ly6Chi and Ly6Clo macrophages was performed by flow cytometry with the Guava Autophagy LC3 antibody-based detection kit (FCCH100171, Luminex, Table 4), according to the manufacturer’s instructions.
RNA isolation and RNA-Seq analysis
Following Ly6Chi and Ly6Clo macrophage sorting, cells were directly frozen at −80 °C and RNA extracted extemporaneously using RNeasy Micro kit (74004, QIAgen). RNAs were qualified and quantified with AGILENT Tapestation 2200 on High Sensitivity RNA chip. RNA library preparation was realized following manufacturer’s recommendations (NEBNext Single Cell/Low Input RNA Library Prep from New england Biolabs). cDNA and final libraries were controlled with AGILENT Tapestation 2200 on High Sensitivity DNA chip. Final equimolar pooled library preparations were sequenced on Nextseq 500 ILLUMINA with HighOutPut cartridge (2x400Millions of 75 bases reads). Corresponding to 2x20Millions of reads per sample after demultiplexing.
RNA-Seq data analysis was performed by GenoSplice technology (www.genosplice.com). Sequencing, data quality, reads repartition (e.g., for potential ribosomal contamination), and inner distance estimation was performed using FastQC, Picard-Tools, Samtools, and RSeQC. Reads were mapped using STARv2.4.038 on the mm10 Mouse genome assembly. Gene expression regulation study was performed as already described39,40,41,42. Briefly, for each gene present in the Mouse FAST DB v2018_1 annotations, reads aligning on constitutive regions (that are not prone to alternative splicing) were counted. Based on these read counts, normalization was performed using DESeq243. Genes were considered as expressed if their FPKM value was greater than 95% of the background FPKM value based on intergenic regions defined from the Mouse FAST DB v2018_1 annotations. Only genes expressed in at least one of the two compared experimental conditions were further analyzed.
DESeq2 normalized counts were log2 transformed and each Ac-6-FP treated sample was normalized according to the mean between the corresponding VEH log2 normalized counts (i.e., Ly6Chi Ac-6-FP and Ly6Clo Ac-6-FP samples were normalized to respective mean vehicle samples). A paired t test was performed between Ly6Chi Ac-6-FP/mean vehicle ratios and Ly6Clo Ac-6-FP/mean vehicle ratios. Results were considered statistically significant for uncorrected p values ≤0.05 and fold-changes ≥1.5. Enrichment analyses were performed using WebGestaltR44 on KEGG pathways. The sequence datasets have been deposited in the Gene Expression Omnibus with the accession number GSE183906 (Supplementary data 1) [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE183906].
Co-cultures of MAIT cell and macrophages and immunostaining
MAIT cells were isolated from the spleen of B6-MAITCAST mice using Dynabeads™ untouched™ mouse T cells kit, according to the manufacturer’s instructions. MAIT cells were then sorted as CD3+CD4−CD8−GFP+ cells using BD FACSMelody™ cell sorter, following staining with flow cytometry antibodies as described above (Fig. S5). MAIT cells were pre-activated overnight with 10 μg/ml anti-CD3 (145-2C11, #100340) and 2.5 μg/ml anti-CD28 (37.51, #102116) from BioLegend. BMDM were isolated from C57BL/6 J mice femur and tibia bone marrow, differentiated for 7 days in RPMI containing 10%FCS and 1% penicillin/streptomycin, in the presence of L929 media containing M-CSF. BMDM (3 × 104 cells/well) were seeded in μ-slide 18 well ibiTreat (iBidi, 81816) and co-cultured with MAIT (3 × 104 cells/well) for 48 h in the presence of 10 μM of Ac-6-FP or its vehicle, with a 1:1 MAIT:Macrophage ratio. Cytokine-neutralizing experiments were performed by adding 10 μg/ml of mouse anti-IL-17A (TC11-18H10.1, #506945, BioLegend) and/or anti-TNFα (MP6-XT22, #506332, BioLegend) or isotype (RTK2071, #400432, BioLegend) to the culture medium. BMDM were then fixed by 4% paraformaldehyde and blocked in 1%BSA-PBS and 0.2% Triton X100 for 1 h at RT. Cells were incubated with either FITC rat anti-mouse CCR2 (SA203G11, 1/50, #150608, BioLegend) or goat polyclonal anti-mouse CD206 (1/50, #sc-34577, Santa Cruz Biotechnology) for 2 h at RT, followed by donkey anti-goat Alexa555 (1/500, #A21432, Thermofisher Scientific) for 30 min (Table 4). Cells were mounted by mounting medium with DAPI (50011, ibidi). Representative images were acquired using confocal microscope (Zeiss LSM 780). For quantification, 10–12 fields were randomly selected, and mean intensity of CCR2 and CD206 per cell were measured by Image J. No staining signal was observed when omitting the primary antibody. Cells were isolated from female and male mice.
Statistical analysis
The results are expressed as mean ± standard error of the mean (SEM) or median (interquartile range), as indicated. Mann–Whitney test was used to calculate significant levels between the two groups. For comparison of means from multiple groups against one control group, one-way ANOVA followed by Bonferroni’s multiple comparisons post-test analysis was performed. All p values are two-sided, and p-values less than 0.05 were considered to be statistically significant. Each variable achieving a p value < 0.05 was then introduced into a bivariate model. Wilcoxon matched-pairs signed rank test was used to assay the statistical significance of Ac-6-FP treatment on human PCLS compared to vehicle. All the analyses were performed using GraphPad Prism 8.4.3 software (GraphPad Software, Inc., La Jolla, CA). Sample sizes were adequate to detect large effects between groups, as determined by the reproducibility and variability of each particular experiment and limited by the availability of animal samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequence datasets have been deposited in the Gene Expression Omnibus under accession code GSE183906. Other data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Marcellin, P. et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381, 468–475 (2013).
Friedman, S. L. & Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 75, 473–488 (2022).
Mallat, A. & Lotersztajn, S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 305, C789–C799 (2013).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18, 151–166 (2021).
Seki, E. & Schwabe, R. F. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61, 1066–1079 (2015).
Peiseler, M. et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J. Hepatol. 77, 1136–1160 (2022).
Carter, J. K. & Friedman, S. L. Hepatic stellate cell-immune interactions in NASH. Front. Endocrinol. 13, 867940 (2022).
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012).
Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 (2017).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Toubal, A., Nel, I., Lotersztajn, S. & Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19, 643–657 (2019).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010).
Veerapen, N., Hobrath, J., Besra, A. K. & Besra, G. S. Chemical insights into the search for MAIT cells activators. Mol. Immunol. 129, 114–120 (2021).
Eckle, S. B. et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J. Exp. Med. 211, 1585–1600 (2014).
Bottcher, K. et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 68, 172–186 (2018).
Hegde, P. et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 9, 2146 (2018).
Lodder, J. et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy 11, 1280–1292 (2015).
Habib, A. et al. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut 68, 522–532 (2019).
Krenkel, O. et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 67, 1270–1283 (2018).
Cui, Y. et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J. Clin. Invest. 125, 4171–4185 (2015).
Mitchell, C. et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am. J. Pathol. 174, 1766–1775 (2009).
Holland, P. et al. HS1BP3 negatively regulates autophagy by modulation of phosphatidic acid levels. Nat. Commun. 7, 13889 (2016).
Legoux, F., Salou, M. & Lantz, O. MAIT cell development and functions: the microbial connection. Immunity 53, 710–723 (2020).
Yan, J. et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov. 10, 124–141 (2020).
Chen, Z. et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 10, 58–68 (2017).
Toubal, A. et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat. Commun. 11, 3755 (2020).
Seki, E. et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50, 185–197 (2009).
Schwenger, K. J., Clermont-Dejean, N. & Allard, J. P. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP Rep. 1, 214–226 (2019).
Flament, H. et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol. 22, 322–335 (2021).
Wan, J. et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 59, 130–142 (2014).
Wan, J. et al. LC3-associated phagocytosis protects against inflammation and liver fibrosis via immunoreceptor inhibitory signaling. Sci. Transl. Med. 12, eaaw8523 (2020).
Allaire, M., Rautou, P. E., Codogno, P. & Lotersztajn, S. Autophagy in liver diseases: time for translation? J. Hepatol. 70, 985–998 (2019).
Gilgenkrantz, H., Mallat, A., Moreau, R. & Lotersztajn, S. Targeting cell-intrinsic metabolism for antifibrotic therapy. J. Hepatol. 74, 1442–1454 (2021).
Zhang, C. et al. Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc. Natl Acad. Sci. USA 109, 1667–1672 (2012).
Hsu, P. et al. Cardiolipin remodeling by TAZ/tafazzin is selectively required for the initiation of mitophagy. Autophagy 11, 643–652 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Chiot, A. et al. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nat. Neurosci. 23, 1339–1351 (2020).
Gacem, N. et al. ADAR1 mediated regulation of neural crest derived melanocytes and Schwann cell development. Nat. Commun. 11, 198 (2020).
Naro, C. et al. An orchestrated intron retention program in meiosis controls timely usage of transcripts during germ cell differentiation. Dev. Cell 41, 82–93.e84 (2017).
Traore, M. et al. An embryonic CaVbeta1 isoform promotes muscle mass maintenance via GDF5 signaling in adult mouse. Sci. Transl. Med. 11, eaaw1131 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019).
Acknowledgements
This work was supported by grants from INSERM (France), the Université Paris Cité, Labex Inflamex (to S.L.), Inserm-Transfert (CoPOC MAT-PI-17217-A-01 to S.L.), Agence Nationale pour la Recherche (ANR19-CE14-0041-02 to S.L.) and Fondation pour la Recherche Médicale (Equipe FRM EQU202203014642 to S.L.). We are grateful to Drs G. Gauthier and V. Gratio and to J. Da Silva from the flow cytometry platform, N. Sorhaindo from the Biochemistry Platform (Center de Recherches sur l’Inflammation, INSERM U1149, Paris), and O. Thibaudeau from the Plateau de Morphologie (INSERM UMR1152, Paris), for their help in flow cytometry, liver function tests, and histology respectively. This work benefited from equipment and services from the iGenSeq core facility (genotyping and sequencing) at Institut du Cerveau et de la Moëlle épinière, Paris.
Author information
Authors and Affiliations
Contributions
M.M. and P.H., designed, conducted, and analyzed data from mice and cell experiments. A.H. conducted and analyzed data from human PCLS experiments. V.P. designed, supervised, and analyzed data from human liver samples, and helped with clinical insights and discussion. J.W., C.C., R.A.S., M.C., M.A., and T.T.S. contributed to the acquisition of data for in vivo and in vitro experiments. P.G. supervised and analyzed RNA-seq results. O.L. provided B6-MAITCAST and MR1−/− mice and provided feedback. H.G. contributed to the design and analysis of the data. M.M., P.H., A.H., J.W., C.C., E.W., V.P., M.G., P.G., and H.G. contributed to writing and provided feedback. S.L. conceived, designed, provided financial support, and supervised the project.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dan Pellicci, Frank Tacke, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mabire, M., Hegde, P., Hammoutene, A. et al. MAIT cell inhibition promotes liver fibrosis regression via macrophage phenotype reprogramming. Nat Commun 14, 1830 (2023). https://doi.org/10.1038/s41467-023-37453-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-37453-5
This article is cited by
-
Mucosal-associated invariant T cells in infectious diseases of respiratory system: recent advancements and applications
Journal of Inflammation (2024)
-
Immunology of human fibrosis
Nature Immunology (2023)
-
Glycation-driven matrix crosslinking in cirrhosis
Nature Biomedical Engineering (2023)
-
Hepatic inflammatory responses in liver fibrosis
Nature Reviews Gastroenterology & Hepatology (2023)
-
Gut liver brain axis in diseases: the implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.