Abstract
Trypanosoma brucei is a protozoan parasite that causes human African trypanosomiasis. Its major surface antigen VSG is expressed from subtelomeric loci in a strictly monoallelic manner. We previously showed that the telomere protein TbRAP1 binds dsDNA through its 737RKRRR741 patch to silence VSGs globally. How TbRAP1 permits expression of the single active VSG is unknown. Through NMR structural analysis, we unexpectedly identify an RNA Recognition Motif (RRM) in TbRAP1, which is unprecedented for RAP1 homologs. Assisted by the 737RKRRR741 patch, TbRAP1 RRM recognizes consensus sequences of VSG 3’UTRs in vitro and binds the active VSG RNA in vivo. Mutating conserved RRM residues abolishes the RNA binding activity, significantly decreases the active VSG RNA level, and derepresses silent VSGs. The competition between TbRAP1’s RNA and dsDNA binding activities suggests a VSG monoallelic expression mechanism in which the active VSG’s abundant RNA antagonizes TbRAP1’s silencing effect, thereby sustaining its full-level expression.
Similar content being viewed by others
Introduction
Monoallelic gene expression (MAE), or allelic exclusion, is essential for many organisms. Notable examples include genome imprinting, X chromosome inactivation, and random monoallelic expression of autosomal genes in mammals1. Many genes that undergo MAE encode cell surface receptors. For example, each human and mouse olfactory sensory neuron expresses only one odorant receptor gene1. Several microbial pathogens, including Trypanosoma brucei, employ MAE as part of their antigenic variation strategy2,3,4,5.
Trypanosoma brucei is a protozoan parasite that causes human African trypanosomiasis. It sequentially expresses distinct Variant Surface Glycoproteins (VSGs), its major surface antigen, to evade the host’s immune surveillance. VSG is monoallelically transcribed by RNA polymerase I6 from one of the ~15 nearly identical VSG expression sites (ESs)7,8. In these subtelomeric polycistronic transcription units, VSG is always the last gene located within 2 kb of the telomeric repeats7,8. Parasites that express multiple VSGs are more rapidly eliminated by the host9, underscoring the importance of VSG MAE for T. brucei survival. Many factors affect VSG MAE, such as nuclear lamina, the inositol phosphate pathway2,3, transcription elongation10,11, and a subtelomere and VSG-associated VEX complex12,13,14.
Our previous studies have demonstrated that TbRAP1, a nuclear and essential telomere protein, is a key regulator of VSG MAE15,16,17,18. Depletion of TbRAP1 leads to derepression of silent VSGs up to a thousand-fold15,16,17,18. The TbRAP1-mediated silencing is stronger at loci closer to the telomere than those further away15. We recently reported that TbRAP1 possesses a dsDNA binding activity mediated by its R/K patch (737RKRRR741) in the DNA binding (DB) domain (aa 734–761)18. This dsDNA binding activity is essential for TbRAP1’s association with the telomere chromatin and TbRAP1-mediated VSG silencing. However, we do not understand the underlying mechanism of how TbRAP1 selectively permits the active VSG to be fully expressed while silencing other VSGs.
Here our NMR studies identify an RNA Recognition Motif (RRM) in TbRAP1, while known RAP1 homologs have not been reported to have any RRM domains. Assisted by the R/K patch, TbRAP1 RRM binds the 16-mer consensus sequence of VSG 3’UTRs19,20 in vitro, while TbRAP1 binds the active VSG RNA in vivo. Strikingly, mutations in the RRM domain that specifically abolish the TbRAP1-VSG RNA interaction lead to an acute decrease in the active VSG RNA level by ~50% and subsequent derepression of all silent VSGs, thus disrupting both aspects of VSG MAE. In contrast, mutations in the R/K patch alone or in both the R/K patch and RRM lead to acute depression of silent VSGs, but the active VSG RNA is only moderately decreased by ~13%. Mechanistically, TbRAP1’s RNA and dsDNA binding activities compete in a substrate concentration-dependent manner. Such competition suggests a mechanism of VSG MAE where the active VSG’s abundant RNA antagonizes TbRAP1’s dsDNA binding-mediated silencing effect at the active VSG locus to sustain its full-level expression.
Results
TbRAP1 interacts with the active VSG RNA in vivo
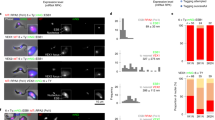
RAP1 homologs have been identified from kinetoplastids to mammals21. None of the known RAP1 homologs has been reported to have any RNA binding activity. We previously found that TbRAP1 does not bind the telomeric repeat-containing RNA (TERRA) in RNA IP experiments22,23,24. However, to our great surprise, we found that TbRAP1 interacts with the active VSG RNA (Fig. 1a, b). RNA crosslinking immunoprecipitation (RNA CLIP) assays were performed in TbRAP1F2H+/- cells that express VSG2 as the major surface antigen (Table 1), in which one TbRAP1 allele is deleted and the other has an N-terminal FLAG-HA-HA (F2H) tag17. Quantitative RT-PCR (qRT-PCR) analysis detected significantly more active VSG2 RNA in the TbRAP1 CLIP product than in the negative control IgG CLIP product (Fig. 1a). RNAs of the telomerase reverse transcriptase (TbTERT25), small nuclear RNA gene activation protein 50 (SNAP50), and Protein Kinase A catalytic subunit (PKAC1) were also examined in CLIP products. Approximately the same amount of TbTERT, SNAP50, and PKAC1 RNAs were detected in both TbRAP1 and IgG CLIP products (Fig. 1a). Therefore, TbRAP1 interacts with the active VSG RNA but not TbTERT, SNAP50, or PKAC1 RNAs. We also performed RNA CLIP in PVS3-2/OD1-1 cells (Table 1) that express VSG9 as the major surface antigen15. Again, qRT-PCR detected significantly more VSG9 RNA in the TbRAP1 CLIP product than in the IgG control (Fig. 1b), indicating that TbRAP1 can interact with the active VSG RNA regardless of which VSG is expressed. Therefore, we report for the first time that TbRAP1 is associated with the active VSG RNA in vivo, an unprecedented finding for RAP1 homologs.
a RNA CLIP experiments were performed in TbRAP1F2H+/- cells that express VSG2. qRT-PCR was performed to estimate the amount of the VSG2 RNA and the TbTERT, SNAP50, and PKAC1 RNAs in the RNA CLIP product. Enrichment of the VSG2, TbTERT, SNAP50, and PKAC1 RNAs (CLIP/Input) was calculated for the CLIP experiment using the HA antibody 12CA5 and that using IgG. Relative enrichment was calculated using the enrichment of IgG CLIP as a reference. Average and standard deviation were calculated from three (SNAP50 & PKAC1), five (TbTERT), and seventeen (VSG2) independent experiments. P values of two-sided unpaired t-tests (compared to VSG2 RNA enrichment) are shown. b RNA CLIP was performed in VSG9-expressing PVS3-2/OD1-1 cells using a TbRAP1 rabbit antibody15 and IgG and the enrichment of the VSG9 RNA in the CLIP product was calculated. Average and standard deviation were calculated from three independent experiments. Error bars represent standard deviation. Source data are provided as a Source Data file. c Domain structure of TbRAP1. Inset, an enlarged diagram of the TbRAP1 MybLike domain (aa 639–761)15, which contains an RRM (aa 653–727) and the DNA Binding (DB) domain (aa 734–761)18. Arrowheads mark the conserved F655 and F694 residues. d Superposition of TbRAP1653-727 (green) with the RRM1 domain of hnRNP A1 (orange) bound with a short RNA oligo (golden) [https://doi.org/10.2210/pdb5MPG/pdb]28. Inset highlights that F655 and F694 in TbRAP1 superimpose well with F17 and F59 in hnRNP A1 that form stacking interactions with the RNA substrate.
The TbRAP1 MybLike domain contains an RRM module
To investigate whether the TbRAP1 MybLike domain (aa 639–761) is responsible for binding to the active VSG RNA, we first determined the solution structure of TbRAP1639-761 by NMR spectroscopy (Fig. 1c, Supplementary Fig. 1a, Supplementary Table 1). The N-terminal region of TbRAP1639-761 does not adopt a typical Myb fold but forms a canonical RRM (aa 653–727)26 with the characteristic topology of a four-stranded anti-parallel β-sheet and two α-helices packed behind the β-sheet (Fig. 1d; Supplementary Fig. 1b). The DB domain (aa 734–761) at the C-terminus of TbRAP1639-761 forms a long and flexible loop (Supplementary Fig. 1b, left). In contrast, none of the known RAP1 homologs has been reported to have an RRM domain.
RRM is a conserved structural platform that binds to diverse RNAs and ssDNAs26,27. Sequence analysis shows that TbRAP1 RRM contains the signature RNP1 and RNP2 sequence motifs, with F655 in RNP2 and F694 in RNP1 representing the two conserved aromatic residues critical for substrate binding (Supplementary Fig. 1c)26. TbRAP1 RRM superimposes well with RRM1 of heterogeneous nuclear ribonucleoprotein (hnRNP) A1 bound with an RNA oligo [https://doi.org/10.2210/pdb5MPG/pdb]28 (Fig. 1d), with a Root Mean Square Deviation (RMSD) of ~3.3-3.5 Å for the main chain atoms. Notably, F655 and F694 of TbRAP1 match exactly to F17 and F59 of hnRNP A1 that form stacking interactions with RNA (Fig. 1d). In addition, sequence alignment and structural prediction by AlphaFold229 confirm that RAP1 homologs in representative Trypanosomatida organisms all have a highly conserved RRM (Supplementary Fig. 1d), while vertebrate and fungal RAP1s do not seem to have any RRM domain (Supplementary Fig. 1e). Thus, the RRM domain is uniquely conserved in RAP1 homologs of these microbial parasites but absent in RAP1s from higher eukaryotes.
TbRAP1 RRM binds to the consensus VSG 3’UTR region in vitro
Since TbRAP1639-761 contains an RRM domain plus a flexible DB domain, we then used NMR titration to test whether it binds to the active VSG RNA. We used 34-VSG-UTR, a 34 nt RNA from the 3’UTR of VSG2 that contains the consensus 16-mer found in all VSG 3’UTRs (Supplementary Table 2)19,20. We titrated 34-VSG-UTR into 15N-labeled TbRAP1639-761 (Supplementary Table 3) and observed significant concentration-dependent chemical shifts for RNP1 and RNP2 residues, particularly F655 and F694, in heteronuclear single quantum correlation (HSQC) spectra (Fig. 2a–c). A few residues in the DB domain also showed noticeable chemical shifts, although at much lower magnitudes compared to RNP1 and RNP2 residues (Fig. 2a, b). These results suggest that both the RRM module and the DB domain interact with the 34-VSG-UTR, with RRM playing a major role. Compared to other RRM domains, the chemical shifts induced by 34-VSG-UTR in TbRAP1639-761 are mostly in the slow-to-intermediate exchange region, indicative of a moderate micromolar binding affinity28.
a, d, g 1H-15N HSQC NMR spectra of 15N-labeled TbRAP1639–761 (a), TbRAP1639–733 (d) and TbRAP1639–7332FL (g) in the absence (black) and presence of 34-VSG-UTR in 3× molar excess (red). In (a), residues located in the RRM domain (labeled in green) showed noticeable chemical shifts (arrow) while residues in the DB domain (labeled in blue) did not (underline). In (g), no chemical shifts were observed. b, e Chemical shift differences of individual TbRAP1 residues in NMR titration when TbRAP1639-761 (b) or TbRAP1639-733 (e) was used. Source data are provided as a Source Data file. c Inset of overlaid 1H-15N-HSQC spectra in (a) highlighting chemical shift perturbations for key residues in RRM in the absence (black) and presence of 34-VSG-UTR in 1x (blue), 2x (green) and 3× (red) molar excess. Residues located on RNP1 and RNP2 of RRM, including the conserved F655 and F694 are highlighted in (c). f Insets of overlaid 1H-15N-HSQC spectra of 15N-labeled TbRAP1639–733 in the absence (black) or presence of 34-VSG-UTR (top), (UUAGGG)2 (middle), and 35-random (bottom) in 1x (blue), 2x (green) and 3× (red) molar excess. Highlighted residues are the same as in (c). Only 34-VSG-UTR induced noticeable chemical shifts in the RRM domain. PPM, parts per million.
To further characterize how the RRM and DB domains bind RNA, we did similar NMR titration studies using the RRM-containing TbRAP1639-733, TbRAP1639-7332FL with the two key aromatic residues F655 and F694 of the RRM domain mutated to leucine residues, and TbRAP1639-7615A with the R/K patch in the DB domain mutated to five alanines18 (Supplementary Table 3). For both TbRAP1639-733 and TbRAP1639-761, 34-VSG-UTR induced similar patterns of chemical shift in RNP1 and RNP2 (Fig. 2, a, b, d, e), but the magnitude was smaller for TbRAP1639-733 than for TbRAP1639-761 (Fig. 2, b, c, e, f). NMR titration using TbRAP1639-7615A also showed similar results as TbRAP1639-733 (Supplementary Fig. 1f). However, no chemical shifts were observed for TbRAP1639-7332FL even when 34-VSG-UTR was in 3-fold molar excess (Fig. 2g). These results indicate that RRM alone can bind 34-VSG-UTR, which requires the two conserved aromatic residues F655 and F694, while the DB domain helps to strengthen this binding.
To explore the sequence specificity of TbRAP1 RRM, we tested TbRAP1639-733’s binding to (UUAGGG)2, an oligo that contains the TERRA sequence22,23,24. (UUAGGG)2 did not induce any noticeable chemical shifts when titrated to TbRAP1639-733 (Fig. 2f; Supplementary Fig. 2a), which is consistent with our previous observation that TbRAP1 does not bind TERRA24. We also tested TbRAP1639-733’s binding to 35-random, a 35 nt RNA with a random sequence (Supplementary Table 2) by NMR titration. No noticeable chemical shifts were detected, either (Fig. 2f; Supplementary Fig. 2b). These data suggest that TbRAP1639-733 does bind RNA with certain sequence specificity.
RRM domains are known to recognize short RNA motifs of 2-8 nucleotides26,27. To further map which sequence within 34-VSG-UTR can be recognized by TbRAP1 RRM, we performed NMR titration using 16-VSG-UTR, an oligo that contains only the 16-mer consensus sequence in VSG 3’UTRs (Supplementary Table 2). 16-VSG-UTR and 34-VSG-UTR induced the same pattern of chemical shifts in both TbRAP1639-761 and TbRAP1639-733 (Fig. 2a, b, d, e; Supplementary Fig. 2c–f). Therefore, the 16-mer consensus sequence in VSG 3’UTRs is sufficient to be recognized by TbRAP1 RRM. In addition, 16-VSG-UTR also induced stronger chemical shifts in TbRAP1639-761 than TbRAP1639-733, further validating the supporting role of the DB domain (Supplementary Fig. 2c–f). Furthermore, the magnitude of chemical shifts induced by 16-VSG-UTR for the aromatic residues F655 and F694 in RRM was ~50% lower than those induced by 34-VSG-UTR (Supplementary Fig. 2d, f vs. Fig. 2b, e, respectively). These subtle differences suggest that TbRAP1 RRM may recognize additional sequence motifs in the longer 34-VSG-UTR substrate, which leads to stronger binding and more prominent chemical shifts. Since RRM domains are known to have promiscuous binding activities, it is likely that TbRAP1 RRM can recognize more than one sequence within the VSG RNA.
We also used the fluorescence polarization assay as a biophysical technique to assess the RNA binding activity of TbRAP1. Fluorophore-labeled 16-VSG-UTR was titrated to TbRAP1639-761, TbRAP1639-733, and TbRAP1639-7615A and the estimated binding affinity Kd were ~258, 929, and 969 μM, respectively (Supplementary Fig. 2g–i). These data corroborate our NMR studies to confirm that TbRAP1 RRM recognizes the 16-mer consensus sequence of VSG 3’UTRs. This RNA binding activity requires the two conserved aromatic residues, F655 and F694 in RNP2 and RNP1, respectively, and is enhanced by the DB domain.
We subsequently performed EMSA to validate the TbRAP1 RRM-mediated RNA binding activity. Initially, TrxA-His6 (TH6) or GST-tagged TbRAP1 fragments were used (Supplementary Fig. 3a). TH6-tagged TbRAP1639-761, TbRAP1639-733, and TbRAP1639-7615A (Supplementary Table 3) all bound 170-VSG-UTR, a 170 nt RNA containing the VSG2 3’UTR sequence (Supplementary Table 2), while TH6 alone or TH6-TbRAP1639-7612FA&5A (F655AF694A, 737RKRRR741 mutated to 737AAAAA741, Supplementary Table 3) did not (Supplementary Fig. 3b–d). In addition, GST-TbRAP1414-855 bound this RNA, while GST alone and the GST-tagged duplex telomere DNA-binding TbTRF30 did not (Supplementary Fig. 3b, c, e; Supplementary Table 3).
To examine TbRAP1-specific RNA binding activity without any possible interference by the fusion tag, we cleaved the TH6 tag by 3C and purified tagless TbRAP1 fragments (Supplementary Fig. 3f). Both TbRAP1639-761 and TbRAP1639-733 bound 170-VSG-UTR (Fig. 3a, b) but TbRAP1639-7332FQ (F655QF694Q), TbRAP1639-7332FL (F655LF694L), or TbRAP1639-7332FA (F655AF694A) did not (Fig. 3c, d; Supplementary Fig. 3g; Supplementary Table 3). In addition, more than one TbRAP1639-733 molecule can bind the same 170-VSG-UTR substrate when the protein:RNA ratio is increased (Fig. 3b).
Untagged recombinant TbRAP1639-761 (a, e, j), TbRAP1639-733 (b–d, f–i), TbRAP1639-7332FQ (c, g), and TbRAP1639-7332FL (d, h) were incubated with 170-VSG-UTR (a–d), 170-no-VSG (e–h), 35-VSG-UTR (i), 35-random (i), or 16-VSG-UTR (j) (Supplementary Table 2). The concentration of protein (µM) used in each reaction is indicated on top of each lane. Samples were electrophoresed in 0.8% agarose gels (a–i) or a 1.2% agarose gel (j) in 0.5 x TBE buffer. Source data are provided as a Source Data file.
Unexpectedly, tagless TbRAP1639-761 and TbRAP1639-733 bound 170-no-VSG (Fig. 3e, f; Supplementary Table 2) but none of TbRAP1639-7332FQ, TbRAP1639-7332FL, or TbRAP1639-7332FA did (Fig. 3g, h; Supplementary Fig. 3g). Similarly, TH6-tagged TbRAP1639-761, TbRAP1639-733, and TbRAP1639-7615A also bound 170-no-VSG (Supplementary Fig. 3h, i). TbRAP1639-733 did exhibit higher affinity to 170-VSG-UTR than to 170-no-VSG (Supplementary Fig. 3j), indicating that TbRAP1 RRM prefers the VSG 3’UTR sequence. Nevertheless, the observation that TbRAP1639-733 bound 170-no-VSG (Fig. 3f) seems inconsistent with the fact that TbRAP1639-733 does not bind 35-random in NMR titration (Fig. 2f; Supplementary Fig. 2b). We, therefore, examined whether TbRAP1639-733 binds 35-random in EMSA. 35-VSG-UTR was used as a positive control, which contains both the 9-mer and the 16-mer consensus motifs in VSG 3’UTR (Supplementary Table 2)20. TbRAP1639-733 bound 35-VSG-UTR but not 35-random in EMSA (Fig. 3i), confirming the NMR titration result. RRM domains usually recognize a short RNA sequence of 2-8 nucleotides26,27. It is possible that 170-no-VSG may contain additional sequences that can be recognized by TbRAP1 RRM other than the consensus sequences in VSG 3’UTRs.
We further performed EMSA using the shorter 16-VSG-UTR substrate (Supplementary Table 2), to better explore the sequence specificity of TbRAP1’s RNA binding activity. Interestingly, TbRAP1639-761 clearly bound 16-VSG-UTR (Fig. 3j) but TbRAP1639-733’s binding affinity appears to be too weak to be detected by EMSA. This observation supports our NMR titration results and further validates the importance of the DB domain in the RRM-mediated RNA binding. Additionally, Kd values estimated by EMSA show stronger affinity of TbRAP1639-761 for 35-VSG-UTR than 16-VSG-UTR, which is consistent with our NMR titration results (Supplementary Fig. 2j).
The in vivo TbRAP1-VSG RNA interaction depends on the conserved aromatic residues in RRM
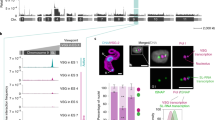
We generated TbRAP1F/mut strains by replacing the WT TbRAP1 allele with various RRM mutants in TbRAP1F/+ cells (Supplementary Fig. 4a, b; Table 1)17. To specifically examine the in vivo RNA binding activities of TbRAP1 mutants, we did RNA CLIP after deleting the loxP-flanked TbRAP1 (the F allele) by Cre, as RRM mutants can interact with WT TbRAP1 through its BRCT domain17. Removal of the TbRAP1 F allele was confirmed by PCR (Supplementary Fig. 4c–g). TbRAP1∆RRM and TbRAP1∆MybL (MybLike deletion)18 were expressed at a subtly lower level than WT TbRAP1 (Supplementary Fig. 4h), while TbRAP1-2FQ, TbRAP1-2FL, TbRAP1-2FA, and TbRAP1-2FA&5A were expressed the same as TbRAP1 (Supplementary Fig. 4i–l). As expected, TbRAP1∆MybL and TbRAP1∆RRM mutants that lack the whole RRM domain lost the TbRAP1-VSG2 RNA interaction (Fig. 4a). Similarly, TbRAP1-2FQ, TbRAP1-2FA, and TbRAP1-2FA&5A did not bind VSG2 RNA, either (Fig. 4a). Interestingly, although TbRAP1-2FL bound significantly lower amount of VSG2 RNA than WT TbRAP1, this mutant appeared to have a smaller RNA binding defect than other mutants (Fig. 4a).
a RNA CLIP experiments were performed in various TbRAP1F/mut strains (expressing VSG2) after a 30-h induction of Cre. The presence of the active VSG2 RNA in the RNA CLIP product was determined by qRT-PCR. The enrichment of VSG2 RNA (CLIP/Input) was calculated for the CLIP experiment using the HA antibody 12CA5 and that using IgG. Relative enrichment was calculated using the enrichment of IgG CLIP as a reference. Average and standard deviation were calculated from two to seventeen independent experiments (the exact number of experiments was indicated in parentheses following each strain name). P values of two-sided unpaired t-tests between the TbRAP1F2H+/- and TbRAP1F/mut are shown on top of corresponding columns. Data for WT TbRAP1 is the same as that in Fig. 1a. b–d ChIP experiments using the HA antibody 12CA5, a TbTRF rabbit antibody30, and IgG were done in TbRAP1F2H+/- cells and Cre-induced (for 30 h) TbRAP1F/2FA&5A (b) TbRAP1F/2FQ (c) and TbRAP1F/2FL (d) cells. Average and standard deviation were calculated from two to five independent experiments (exact number of samples are indicated beneath bottom labels). P values of two-sided unpaired t-tests are shown (ChIP using 12CA5, TbRAP1F/mut vs TbRAP1F2H+/-). Source data are provided as a Source Data file. e IF analyses were done in TbRAP1F2H+/- (top), TbRAP1F/2FQ (middle), and TbRAP1F/2FL (bottom) cells. 12CA5 and a TbTRF chicken antibody15 were used. TbRAP1 genotypes are listed on the left. DNA was stained by DAPI. All images are of the same scale and a size bar is shown in one of the images.
Because TbRAP1 DB enhances the RNA binding activity in vitro, we further examined the effect of DB domain mutations on VSG RNA binding in vivo. We previously reported that TbRAP1∆DB and TbRAP1-5A were expressed at the same level as TbRAP118. Surprisingly, both TbRAP1∆DB and TbRAP1-5A only pulled down background level of VSG2 RNA (Fig. 4a). Since neither mutant is associated with the telomere chromatin18, this observation suggests that being localized at the telomere is a prerequisite for TbRAP1 to bind the active VSG RNA, which has a high concentration only at the active VSG locus.
We also performed Chromatin IP (ChIP) to test whether the RRM domain is necessary for TbRAP1’s localization to the telomere. TbRAP1-2FA&5A did not associate with the telomere chromatin (Fig. 4b), presumably because the 5A mutation already abolished TbRAP1’s DNA binding activities18. In contrast, TbRAP1-2FQ, TbRAP1-2FL, and TbRAP1-2FA still associated with the telomere chromatin (Fig. 4c, d; Supplementary Fig. 4m). Immunofluorescence (IF) analysis further showed that both TbRAP1-2FQ and 2FL were partially colocalized with TbTRF that binds the duplex telomere DNA30 the same way as WT TbRAP1 (Fig. 4e). Hence, in vivo binding of TbRAP1 to the active VSG RNA depends on RRM’s two conserved residues F655 and F694 and the R/K patch within the DB domain. Additionally, the RRM-mediated RNA binding activity is not required for TbRAP1’s association to the telomere chromatin.
TbRAP1’s RNA binding activity is important for VSG MAE and telomere integrity
We examined phenotypes of TbRAP1F/∆RRM, TbRAP1F/2FQ, TbRAP1F/2FL, TbRAP1F/2FA, and TbRAP1F/2FA&5A after a 30–48 h Cre induction (Supplementary Fig. 5a–e). In TbRAP1F/∆RRM, TbRAP1F/2FQ, TbRAP1F/2FA, and TbRAP1F/2FA&5A cells, Cre induction led to an acute growth arrest (Supplementary Fig. 5f–i). However, TbRAP1F/2FL cells showed a slower but not arrested growth phenotype upon Cre induction (Supplementary Fig. 5j), which is consistent with the observation that the 2FL mutant affects the RNA binding less than 2FQ, 2FA, and ∆RRM (Fig. 4a). In addition, substituting an aromatic ring in the phenylalanine residue with a long hydrophobic chain in the leucine residue likely has a weaker effect than substituting it with an alanine.
VSG MAE has two essential aspects: silencing all but one VSGs and a full-level expression of the active VSG. In TbRAP1∆RRM, TbRAP12FQ, and TbRAP12FL mutants, qRT-PCR analysis after the 30-48 h Cre induction detected a significant decrease (~40-60%) in the active VSG2 RNA level, while RNA levels of silent VSGs increased several hundred-fold (Supplementary Fig. 5k–m), indicating that TbRAP1 RRM is essential for both aspects of VSG MAE. The decrease in VSG2 level is particularly striking because the active VSG RNA is ~10,000 fold more abundant than any silent VSG RNA (Fig. 5a)16. Thus, ~50% reduction of the active VSG2 RNA represents a more overwhelming change than the several hundred-fold increase in RNA levels of silent VSGs. This decrease is also in distinct contrast to the phenotype of TbRAP1F/5A and TbRAP1F/∆DB cells that mutated the R/K patch, where silent VSGs were similarly derepressed but the active VSG RNA remained at ~90% of the WT level (Supplementary Fig. 5n, o)18. Interestingly, in TbRAP12FA&5A mutant, the active VSG RNA level was also only decreased to ~87% of the WT level (Supplementary Fig. 5p). Hence, TbRAP1’s RNA binding activity is particularly essential for keeping the active VSG fully transcribed, while mutating the R/K patch leads to a global VSG derepression and renders the TbRAP1-VSG RNA interaction unimportant.
a A diagram illustrating the ~10,000-fold difference between the active VSG RNA amount and any silent VSG RNA amount. Spheres are not drawn to scale. b–e, g qRT-PCR of RNA levels of the active VSG2 (indicated in red), several silent ES-linked VSGs, and chromosome internal TbTERT and tubulin in TbRAP1F/2FQ (b), TbRAP1F/2FL (c), TbRAP1F/2FA (d), TbRAP1F/5A (e), and TbRAP1F/2FA&5A (g) cells. The fold changes in RNA level are shown in the log scale. Average and standard deviation were calculated from two to nine independent experiments (exact number of samples are indicated beneath each column). The change in VSG2 RNA level in these mutants is plotted again in the linear scale in (f). At the 12 h point, derepression of VSG3, 6, and 9 in TbRAP1F/2FQ, TbRAP1F/2FL, TbRAP1F/2FA, and TbRAP1F/2FA&5A cells was compared to that in TbRAP1F/5A by two-sided unpaired student t-tests, and p values of significant differences are indicated on top of corresponding columns in (b–d, g). The changes in the VSG2 RNA level at all time points were compared to that in TbRAP1F/5A cells in the same way. P values of significant differences are indicated on top of corresponding columns in (f). Error bars in (b–g) represent standard deviation. Source data are provided as a Source Data file. h IF analysis of TbRAP1F/2FL cells before and after the Cre induction. Antibodies specifically recognizing VSG6 (green) and VSG3 (red), which were silent in WT cells, were used. DAPI was used to stain DNA. All panels are of the same scale, and a size bar is shown in one of the panels.
We further examined the RNA levels of the active VSG2 at early time points of 12–36 h after Cre induction in TbRAP1F/mut cells, aiming to assess direct effects of TbRAP1 RRM mutations on VSG expression. Western analysis confirmed the decrease of the total TbRAP1 level in these cells (Supplementary Fig. 6a–e). Strikingly, the active VSG2 RNA level showed significant drop by 12 h and continued to decrease over time, dropping to 58%, 68%, and 50% of the WT level by 24 h in TbRAP1F/2FQ, TbRAP1F/2FL, and TbRAP1F/2FA cells, respectively (Fig. 5b–d, f). In contrast, the VSG2 RNA level remained close to the WT level in TbRAP1F/5A (~90% by 30 h after Cre induction) and TbRAP1F/2FA&5A cells (~87% by 36 h after Cre induction) (Fig. 5e–g). Our temporal profiling of the VSG2 RNA level further confirms that TbRAP1 RRM is critical for sustaining full-level expression of the active VSG.
We also examined the RNA levels of several silent VSGs at the time points of 12–36 h after Cre induction in TbRAP1F/mut cells. Notably, derepression of silent VSG 3, 6, and 9 at 12 h after the Cre induction in RRM point mutants was only ~10 fold, significantly milder than the ~100 fold observed in TbRAP1F/5A cells (Fig. 5b–e). The magnitude of depression became similar at later time points of 18, 24, and 36 h (Fig. 5b–e). Nevertheless, both VSG3 and VSG6, two silent VSGs in uninduced TbRAP1F/2FL cells, were expressed simultaneously in individual cells upon Cre induction (Fig. 5h). Overall, these results confirm that disrupting TbRAP1’s RNA binding indeed led to VSG derepression, albeit with a slower kinetic profile compared to mutations in the DB domain.
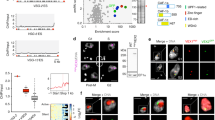
Subsequently, we examined the transcriptome profiles in TbRAP1F/2FQ and TbRAP1F/2FA&5A cells by RNAseq. ~5,000 genes were up-regulated and 200-1500 genes were down-regulated in the TbRAP1F/2FQ and TbRAP1F/2FA&5A cells (Supplementary Fig. 6f, g). A large number of VSG genes and pseudogenes were up-regulated in both mutants, including all silent VSGs in bloodstream form VSG ESs (Supplementary Figs. 7a, b and 8). GO term analysis indicated that significantly derepressed genes are predominantly involved in host immune response evasion (Supplementary Fig. 7c). We also estimated the VSG2 RNA half-life in TbRAP1F/∆MybL, TbRAP1F/∆RRM, and TbRAP1F/2FQ cells. The VSG2 RNA levels were examined by qRT-PCR after various lengths of time of Actinomycin D treatment, but the half-life of VSG2 RNA did not change in RRM mutants (Supplementary Fig. 9).
We previously showed that TbRAP1 suppresses VSG switching by maintaining genome integrity at the telomere and subtelomere23. Since TbRAP1-2FL is viable, we estimated the VSG switching rate in TbRAP1-/2FL cells (Table 1), which is twice as high as that in WT cells (Fig. 6a), suggesting that the TbRAP1’s RNA binding activity also helps suppress VSG switching. In addition, the level of γH2A, an indicator of DNA damage31, was increased mildly (Fig. 6b), and significantly more γH2A was associated with the telomere chromatin in Cre-induced TbRAP1F/2FL cells (Fig. 6c). TbRAP1∆RRM, TbRAP1-2FQ, TbRAP1-2FA, and TbRAP1-2FA&5A mutants exhibited a strong growth arrest phenotype (Supplementary Fig. 5f–i), which prevented us from determining the VSG switching rate in these mutants. Therefore, we examined the γH2A levels. An increased level of γH2A was observed in Cre-induced TbRAP1F/2FQ, TbRAP1F/2FA&5A, TbRAP1F/2FA, and TbRAP1F/∆RRM cells (Fig. 6d, e; Supplementary Fig. 6h–i), indicating that these mutants had more DNA damage. Particularly, we observed an increased amount of γH2A associated with the telomere chromatin (Fig. 6f) and the active ES (Fig. 6g) in TbRAP1F/2FQ cells after the Cre induction, indicating that the TbRAP1’s RNA binding activity is also critical for telomeric and subtelomeric integrity. Telomeric DNA breaks, particularly those at the active VSG vicinity, can lead to cell death in >80% of parasites32, which can explain why RRM mutants have growth defects.
a TbRAP1-/2FL exhibits an increased VSG switching rate. Average and standard deviation were calculated from three (WT) and four (TbRAP1-/2FL) independent experiments. P values of two-sided unpaired t-tests are shown (TbRAP1-/2FL vs TbRAP1+/+). b, d, e Western analyses to examine the γH2A protein level in WT cells before and after phleomycin treatment (as a positive control) and in TbRAP1F/2FL (b), TbRAP1F/2FQ (d), and TbRAP1F/2FA&5A (e) cells before and after a 30–48 h Cre induction. A γH2A rabbit antibody23 and the tubulin antibody TAT-149 were used. Molecular marker was run on the left lane in each gel and their sizes are indicated on the left. c, f ChIP using the γH2A rabbit antibody and IgG in TbRAP1F/2FL (c) and TbRAP1F/2FQ (f) cells after a 30 h Cre induction followed by Southern blotting using a telomere and a tubulin probe. Blots were exposed to a phosphorimager. Images were quantified using ImageQuant and average and standard deviation were calculated from two (γH2A antibody, (TTAGGG)n probe in TbRAP1F/2FL cells) or three (all other samples) independent experiments in (c) and three independent experiments in (f). P values of two-sided unpaired t-tests (mutant vs. control cells) are shown. g ChIP using a γH2A rabbit antibody and IgG in TbRAP1F/2FQ cells followed by quantitative PCR using primers specific to the indicated active and silent ES loci. SNAP50 is a chromosome internal gene. Average enrichment (ChIP/Input) was calculated from three independent experiments. P values of two-sided unpaired t-tests (γH2A ChIP products, +Cre vs. -Cre) are shown. Error bars in (a, c, f, g) represent standard deviation. Source data are provided as a Source Data file.
TbRAP1 binds DNA and RNA in a mutually exclusive manner
Our NMR structure of TbRAP1639-761 shows that the DB domain forms a long and flexible loop that does not contact the RRM module (Supplementary Fig. 1b). Thus, it is theoretically possible for TbRAP1 to bind DNA and RNA simultaneously. To test this possibility, we conducted EMSA assays using both dsDNA and RNA substrates. We first confirmed that TbRAP1639-761 bound a duplex telomeric DNA probe, 100-ds(TTAGGG) (Fig. 7a; Supplementary Table 2)18. However, when non-radiolabeled 170-VSG-UTR and radiolabeled 100-ds(TTAGGG) were both incubated with TbRAP1639-761, no ternary complex of TbRAP1-RNA-DNA was observed (Fig. 7b). Instead, the amount of TbRAP1-DNA complex gradually decreased in the presence of an increasing amount of 170-VSG-UTR (Fig. 7b). Similarly, when non-radiolabeled 100-ds(TTAGGG) and radiolabeled 170-VSG-UTR were both incubated with TbRAP1639-761, no ternary complex was observed while the amount of TbRAP1-RNA gradually decreased with increasing amount of 100-ds(TTAGGG) (Fig. 7c). EMSA estimated that the Kd values for binding either 100-ds(TTAGGG) or 170-VSG-UTR by TbRAP1639-761 are comparable in the range of ~100–300 nM (Fig. 7d), thus allowing two-way competition. To investigate whether such competition applies to shorter DNA or RNA substrates, we further compared TbRAP1639-761 binding on 80-dsDNA and 81-VSG-UTR (Supplementary Table 2), as the shortest ssDNA and dsDNA that TbRAP1 can bind is ~60 nt and 60 bp, respectively18. TbRAP1639-761 bound 80-dsDNA (Fig. 7e) as expected18. When an increasing amount of 81-VSG-UTR was added to the reaction using radiolabeled 80-dsDNA as the substrate, no ternary complex of TbRAP1-RNA-DNA was observed, but 81-VSG-UTR competed away TbRAP1639-761’s binding on 80-dsDNA (Fig. 7f). Therefore, DNA and RNA bind to TbRAP1 in mutually exclusive and competitive manner due to their overlapping binding site and comparable binding affinities.
EMSA experiments were performed using TbRAP1639–761. Radiolabeled 100-ds(TTAGGG) (a, b), 170-VSG-UTR (c), and 80-dsDNA (e, f) were used as the binding substrates. Non-radiolabeled 170-VSG-UTR (b), 100-ds(TTAGGG) (c), and 81-VSG-UTR (f) were used as competitors. The concentration of proteins (µM) used in each experiment is indicated on top of each lane in (a) and (e). 4.7 µM (b), 2.35 µM (c), and 0.5 µM (f) of TbRAP1639–761 was used in each competition reaction. The molar excess of the competitor is indicated on top of each lane in (b, c, f). Samples were electrophoresed in 0.8% agarose gels in 0.5x TBE buffer. d TbRAP1639-761’s affinities to 100-ds(TTAGGG) and 170-VSG-UTR (Kd values) were estimated by EMSA. Average and standard deviation were calculated from four (for 100-ds(TTAGGG)) or eight (for 170-VSG-UTR) independent experiments. Source data are provided as a Source Data file.
Discussion
Our NMR studies reveal that TbRAP1 MybLike folds into an RRM module with a canonical βαββαβ topology, in addition to a C-terminal flexible loop corresponding to the DB domain18. Similar to other RRM domains, the four-stranded β-sheet in TbRAP1 RRM is a functional RNA binding site, with conserved residues F655 and F694 poised to form stacking interactions with RNA substrates26,27. Hence, TbRAP1 is different from known RAP1 homologs, which do not contain RRM or possess any RNA binding activity. Thus, our study uncovers an additional important function of this essential telomere protein.
We have validated the RNA binding activity of TbRAP1 RRM by NMR titration, fluorescent polarization, and EMSA in vitro, which is also confirmed by RNA CLIP in vivo. In vitro, TbRAP1 RRM alone can bind the 16-mer consensus sequence of VSG 3’UTRs20. This binding explains why TbRAP1 interacts with all types of active VSG RNA in vivo. Moreover, RRM domains are known to be promiscuous and capable of recognizing many RNA sequence motifs. As a result, it is probable that TbRAP1 may bind to RNA sequences aside from the consensus 16-mer of VSG 3’UTRs. Indeed, our NMR titration data shows that TbRAP1 RRM bound to 35-VSG-UTR with stronger affinity than 16-VSG-UTR, and TbRAP1 RRM also binds a 170-nt long RNA without any VSG2 3’UTR sequence in the EMSA experiment. In addition, it is possible that TbRAP1 RRM may recognize the structural features of VSG 3’UTRs, as they have been predicted to form a common secondary structure20. Future experiments are needed to determine additional RNA sequences, both within and outside VSG RNAs, that are recognized by TbRAP1 RRM.
Interestingly, we found that TbRAP1 DB enhances the RRM-mediated RNA binding activity. This effect is particularly significant for recognizing short RNA oligos in vitro, such as 16-VSG-UTR. As the DB domain is a long and flexible loop with little inter-domain interaction with RRM, it is possible that RRM and DB contact different parts of the RNA substrate independently. This bi-valent binding mode may achieve higher binding affinity than RRM alone. This combinatorial effect to enhance RNA binding affinity has been reported in other RNA binding proteins. In particular, many RNA splicing proteins such as FUS and hnRNPU contain intrinsically disordered arginine-rich RS or RGG repeats adjacent to well-folded RNA-binding domains such as RRM or Zinc Finger (ZF) domains33,34. These disordered motifs are capable of sequence-independent RNA interaction and have been reported to synergize with RRM or ZF to enhance overall RNA binding34,35. Additionally, the dual roles of the DB domain in mediating both TbRAP1’s DNA and RNA binding offers a mechanistic underpinning for mutually exclusive and concentration-dependent competition between the two activities.
Examination of VSG RNA levels reveals an essential role of TbRAP1 RRM in maintaining the full-level expression of the active VSG, which is a critical aspect of VSG MAE. We observed a striking decrease of ~50% in the active VSG RNA level in RRM point mutants. For two reasons, this decrease most likely did not result from a reduced amount of available RNA polymerase I for transcribing the active VSG, even though silent VSGs were derepressed globally. Firstly, TbRAP1 DB mutants such as ∆DB and 5A only affect the active VSG RNA level subtly, even though silent VSGs were derepressed up to ~1000 fold. Secondly, at 12 h post Cre-induction, the active VSG RNA level in RRM mutants is already significantly lower than that in the 5A mutant, while silent VSGs have not been derepressed to the same extent as that in the 5A mutant. Furthermore, the decreased active VSG RNA level in RRM point mutants is not caused by RNA processing, as the half-life of the active VSG RNA is not affected by RRM mutations, and TbRAP1 is primarily a nuclear protein as shown in IF analyses (Fig. 4e)15.
The notable phenotype we observed for active VSG is closely related to the moderate binding affinity of the TbRAP1-VSG RNA interaction and its competition with the DB-mediated DNA binding activity. Our EMSA studies reveal that TbRAP1’s RNA and DNA binding activities are mutually exclusive and compete in a substrate concentration-dependent manner. Thus, the formation of TbRAP1-RNA and TbRAP1-DNA complexes depends on the relative abundance of RNA as opposed to DNA. At silent ESs, VSGs are not transcribed, and the VSG RNA level is very low. TbRAP1 binds dsDNA by its DB domain and establishes/maintains proper VSG silencing (Fig. 8). In contrast, at the active ES, VSG is highly transcribed by RNA polymerase I, representing ~10% of total RNA6,16,36. As nascent VSG RNA is colocalized with the active ES when examined by IF/FISH37, the local concentration of the active VSG RNA is expected to greatly exceed that of the local dsDNA. Hence, at the active VSG locus, TbRAP1 binds the VSG RNA via its RRM domain instead, disrupting the silencing effect mediated by its dsDNA binding activity (Fig. 8). Therefore, while TbRAP1’s dsDNA binding activity silences VSGs globally, its RNA binding activity selectively sustains the full-level VSG expression at the active VSG locus.
Dark lines with terminal bars represent repressive effect. Thicker line represents stronger effect. Red curved lines represent nascent VSG2 RNA. Red star and green hexagon represent the RNA and dsDNA binding activities of TbRAP1, respectively. Binding VSG2 RNA competes TbRAP1’s ability to bind local telomeric DNA.
The moderate RNA binding activity is also consistent with the observation that both TbRAP1∆DB and TbRAP1-5A mutants lose their interaction with the active VSG RNA in vivo, although these mutants bind various RNA substrates in vitro. Presumably, a high concentration of the active VSG RNA is essential for the TbRAP1-VSG RNA interaction. Disrupting TbRAP1’s DNA binding activity removes TbRAP1 from the telomere and away from nearby ESs18, including the active VSG locus, the only nuclear location where a high concentration of VSG RNA is expected. Thus, TbRAP1∆DB and TbRAP1-5A mutants likely do not get access to a high concentration of VSG RNA and bind it. This prerequisite presumably increases the specificity of TbRAP1’s RNA binding activity in vivo, and TbRAP1 is unlikely to interact with random RNA if it is not associated with the chromatin. In addition, in TbRAP1∆DB and TbRAP1-5A mutants18, the TbRAP1-VSG RNA interaction is no longer required for a high level of expression of the active VSG, as the TbRAP1-mediated silencing effect is abolished in the first place18. This further supports our hypothesis that TbRAP1’s RNA binding mainly antagonizes TbRAP1’s dsDNA binding-mediated silencing effect (Fig. 8).
TbRAP1 RRM mutants are defective in VSG silencing even though its dsDNA binding activity is intact. Such VSG derepression displayed subtly slower kinetics than in the 5A mutant with disrupted DNA binding. T. brucei has been reported to sense the decreased VSG translation and induce VSG mRNA synthesis36. It is possible that, in TbRAP1 RRM mutants, the decreased level of VSG expression induced silent VSG derepression through this sensing mechanism. Conversely, the TbRAP1-VSG RNA interaction may send a direct signal to allow the silencing of other VSGs.
Among examples of MAE, underlying molecular mechanisms are highly process-dependent. In Borrelia bacteria38 and Pneumocystis yeast39, the MAE of their major surface antigen genes requires a unique expression site and is achieved by transcribing only the expression site-resident allele. In P. falciparum, histone modification and pairing of var gene intron and promotor play important roles in var gene MAE40. Our study uncovers the competition between TbRAP1’s RNA-binding and DNA-binding activities as a mechanism of VSG MAE, prompting more detailed investigations and facilitating a deeper understanding of antigenic variation in T. brucei. Notably, the RAP1 RRM domain is highly conserved among Trypanosomatids but absent in higher eukaryotes. With this feature and activity likely conserved in Trypanosomatida organisms, TbRAP1 can serve as a promising target for antiparasitic agents.
Methods
T. brucei strains and plasmids
All T. brucei strains used in this study (Table 1) are derived from bloodstream form Lister 427 cells that express the T7 polymerase and the Tet repressor (Single Marker, aka SM)41. All strains express VSG2 except that PVS3-2/OD1-1 expresses VSG915. All T. brucei cells were cultured in the HMI-9 medium supplemented with 10% FBS and appropriate antibiotics.
TbRAP1F/+ was established previously and described in ref. 17. All TbRAP1F/mut strains were established using the same strategy. N-terminal F2H- and NLS-tagged TbRAP1-2FA&5A, TbRAP1-5A, TbRAP1∆DB, and TbRAP1∆RRM, F2H-tagged TbRAP1-2FQ, TbRAP1-2FL, and TbRAP1-2FA flanked by sequences upstream and downstream of the TbRAP1 gene, together with a PUR marker, were cloned into pBluescript-SK to generate respective targeting constructs. Mutant targeting plasmids were digested with SacII before transfecting the TbRAP1F/+ cells to generate respective TbRAP1F/mut strains, which were confirmed by Southern and sequencing analyses.
Bacterial expression plasmids used in this study are listed in Supplementary Table 3.
Quantitative real-time PCR (qRT-PCR)
For qRT-PCR, total RNA was isolated from T. brucei cells using RNAstat-60 (TelTest, Inc.), treated by DNase (Qiagen), and purified using the RNeasy column (Qiagen). cDNA was synthesized using a random hexamer and the MMLV reverse transcriptase (Promega) according to the manufacturer’s manual. cDNA and γH2A ChIP product were analyzed by real-time PCR on a CFX Connect (Bio-Rad) using SsoAdvanced Universal SYBR® Green Supermix (Bio-Rad) according to the manufacturer’s manual. rRNA level was measured and used as a loading control. Data acquired on CFX Connect were processed using MS Excel and Graphpad Prism. qPCR primer sequences are listed in Supplementary Table 4.
Chromatin Immunoprecipitation (ChIP)
200 million cells were cross-linked by 1% formaldehyde for 20 min at RT with constant mixing, and the cross-linking was stopped by 0.1 M Glycine. Chromatin was sonicated by a BioRuptor for 6 cycles (each 30 sec sonication and 30 sec rest) at the high level to get DNA fragments of ~500 bp on average. After saving a small amount of sonicated sample as the input fraction, the sample was equally divided into three fractions, each incubating with 1 µg of HA monoclonal antibody 12CA5 (MSKCC Antibody & Bioresource Core), TbTRF rabbit antibody30, or IgG conjugated with Dynabeads-Protein G (ThermoFisher) for three hs at 4 °C. In γH2A ChIP, the total lysate was equally divided into two fractions, each incubating with 1 µg of γH2A rabbit antibody23 or IgG conjugated with Dynabeads-Protein G. After washing, IPed products were eluted from the beads and DNA was isolated from the products followed by Southern slot blot hybridization or quantitative PCR analysis.
Recombinant protein expression and purification
All recombinant proteins used in this study are listed in Supplementary Table 3.
Recombinant protein expression constructs were transformed into various E. coli strains for optimum expression (Supplementary Table 3). Protein samples used for EMSA studies were expressed in standard LB media. Proteins used for acquiring 15N HSQC NMR spectrum were expressed in M9 minimal media, with 15N labeled ammonium chloride (15N,98%+) (Cambridge Isotope Laboratories, Inc.) as nitrogen source and D-Glucose (Cambridge Isotope Laboratories, Inc.) as carbon source. Protein expression was induced by IPTG. TrxA-His6 (TH6)-tagged proteins were purified with His•bind resin (Millipore) or NiNTA agarose (Qiagen) according to the manufacturer’s protocol. GST-tagged proteins were purified with Glutathione Sepharose 4 Fastflow beads (GE) according to manufacturer’s protocol. Purified proteins were dialyzed in dialysis buffer (20 mM HEPES pH 7.9, 100 mM KCl, 0.1 mM EDTA, 1 mM PMSF, 15% Glycerol, and 1 mM DTT) at 4 °C overnight. Affinity purified TH6-tagged proteins were dialyzed in 3C protease reaction buffer (50 mM Tris pH 8, 150 mM NaCl). A total of 4 mg of dialyzed protein was digested with 140 units of Pierce™ HRV 3C Protease (ThermoFisher Scientific) at 4 °C overnight with nutation. The digestion mix was passed through NiNTA agarose column (Qiagen) to remove TH6 and 3C-His6. Tagless protein was collected from the flow-through fraction and concentrated using a Centricon (Millipore).
Fluorescence polarization
Fluorescence polarization (FP) experiments were conducted in black 384-well microplates with triplicates. To determine the equilibrium dissociation constant (Kd) between 16-VSG-UTR and each of the recombinant TbRAP1639-761, TbRAP1639-7615A, and TbRAP1639-733 proteins, serial ten two-fold dilutions of the proteins were prepared in the binding buffer (20 mM sodium phosphate, 50 mM KCl, 5% glycerol, pH 7.0). The highest protein concentration started at 1200 μM, and 5’ 6-carboxyfluorescein (6-FAM) labeled 16-VSG-UTR (Sigma-Aldrich) was diluted to 50 nM. 40 µl protein-RNA mixtures were incubated for 20 min at room temperature. FP signals were detected at 25 °C using a CLARIOstar (BMG LABTECH, Germany) multi-mode microplate reader equipped with polarization filters with excitation wavelengths at 482 nm (482–16 mode) and emission wavelengths at 530 nm (530–40 mode), respectively. The anisotropy value was obtained with a unit of millipolarization (mP). Focus and gain were adjusted by a reference well containing FAM-labeled RNA only. The Kd value was analyzed by fitting a nonlinear regression curve with one site-specific binding mode in GraphPad Prism.
Electrophoretic mobility shift assay (EMSA)
Purified recombinant proteins were incubated with 0.3 nM radiolabeled 170-VSG-UTR/170-no-VSG (Fig. 3a–h; Fig. 7c; Supplementary Fig. 3g), 1.5 nM radiolabeled 35-VSG-UTR/35-random (Fig. 3i), 10 nM radiolabeled 16-VSG-UTR (Fig. 3j), or 1.2 nM 170-VSG-UTR/170-no-VSG (Supplementary Fig. 3b–e, h, i) in 15 µl of 1 X RNA EMSA buffer (20 mM HEPES pH 7.9, 235 mM KCl, 1 mM MgCl2, 0.1 mM EDTA, 100 ng/µl BSA, 5% Glycerol, 1 mM DTT) at room temperature for 30 mins. Samples were electrophoresed in a 0.8% agarose (Fig. 3, a–i; Fig. 7; Supplementary Fig. 3e, g), a 1.2% agarose (Fig. 3j), or a 5% native polyacrylamide gel (Supplementary Fig. 3b–d, h, i) in 0.5 X TBE running buffer. Gels were dried and exposed to a phosphorimager.
Probe preparation for EMSA
RNA probes were in vitro transcribed from 120 ng of template DNA using the Maxiscript T7/SP6 transcription kit (ThermoFisher) according to the manufacturer’s protocol. Radiolabeled RNA was gel-purified by 10% denaturing PAGE. Purified RNA was resuspended in 40 µl of RNase-free ddH2O. 35-VSG-UTR, 35-random, and 16-VSG-UTR (Supplementary Table 2) were synthesized by IDT and end-labeled by radioactive ATP using T4 polynucleotide kinase (NEB).
A total of 150 ng of double-stranded linear DNA was radiolabeled using the Klenow fragment (NEB) and 32P alpha dCTP in a 50 μl of reaction (50 mM Tris pH 6.8, 10 mM Magnesium acetate, 0.1 mM DTT, 0.05 mg/ml BSA, 0.6 mM dNTPs without dCTP) at room temperature for 60 mins. The radiolabeled probe was purified by 3 ml Sephadex G-50 column and precipitated overnight in 0.2 M sodium acetate pH 5.5/Ethanol followed by washes with 70% Ethanol and resuspension in 50 μl of ddH2O.
K d Calculation
Densitometry data from various EMSA gels were obtained from ImageQuant (GE). Titration curves were generated by plotting protein concentration vs percentage shift of the radiolabeled probe. The Kd value was analyzed by fitting a nonlinear regression curve with one site-specific binding mode in GraphPad Prism. TbRAP1 fragments without any tag was used in EMSA for calculating Kd. The protein purity is >90% (Supplementary Fig. 3f).
NMR spectroscopy
The concentrations of TbRAP1639-761 were 0.1 mM for the 15N-HSQC spectra, 0.6 mM for 2D 1H-1H-NOESY and 1.0 mM for 15N-NOESY, HNCACB, CACB(CO)NH, 13C-HSQC and 13C-NOESY experiments. NMR samples were prepared in 100 mM phosphate buffer (Na2HPO4-NaH2PO4, pH 6.5) with 90% H2O/10% D2O or 99.9% D2O. NMR spectra were acquired on Varian Inova 500, 750, or 800 MHz spectrometers at 298 K. The data were processed using NMRPipe42 and analyzed using Sparky43 and CCPN44. Backbone and side-chain resonance assignment were achieved via the standard heteronuclear triple resonance correlation experiments using 15N, 13C-double labeled TbRAP1639-761. Interproton distance restraints were generated from 2D/3D NOESY experiments using a mixing time of 100 ms. Hydrogen bond restraints were generated base on the nuclear overhauser enhancement (NOE) patterns and derived from Talos45. Initial structure models were generated using CNSsolve46 using interproton distance, dihedral angle, and hydrogen bond restraints. Final structure refinement was performed using Xplor-NIH 3.347 using an implicit solvent potential48. Ten best structures of TbRAP1639-761 without restraint violation were selected.
NMR titration assay
15N-HSQC spectra were acquired with 0.1 mM 15N-labeled protein samples in 20 mM sodium sulfate, 150 mM NaCl, 1 mM EDTA, and 1 mM DTT at pH 6.5. NMR titrations were performed by adding unlabeled concentrated RNA (1-5 mM) to 15N labeled protein (0.1 mM) gradually. NMR spectra were acquired on Varian Inova 800 MHz spectrometer at 293 K.
RNA cross-linking immunoprecipitation (CLIP)
500 million cells suspended in 1 X TDB (5 mM KCl, 80 mM NaCl, 1 mM MgSO4, 20 mM Na2HPO4, 2 mM NaH2PO4, 20 mM glucose) were UV crosslinked (800 mJ) in a UV Stratalinker 2400 (Stratagene). Cells were then harvested by centrifugation, resuspended in the IP buffer (10 mM Tris•Cl pH 8.0, 150 mM NaCl, 0.1% NP-40, 1 X Protease inhibitor cocktail (Roche), 40 units RNaseIn, 1% TritonX-100, 0.1% SDS, 100 µM TLCK, 1 µM Pepstatin A), and incubated on ice for 30 mins. Samples were centrifuged at 15,800 x g and 4 °C for 15 mins and the supernatant is collected as the lysate. 10% of the lysate was saved as input. The rest lysate was equally divided into two fractions, each incubating with 1.2 µg of monoclonal HA antibody 12CA5 (MSKCC Antibody & Bioresource Core)/TbRAP1 rabbit antibody15,17,18 or IgG conjugated with Dynabeads Protein-G (ThermoFisher) at 4 °C for 3 h with rotation. The IPed products were washed with wash buffer (10 mM Tris•Cl pH 8.0, 120 mM NaCl, 0.2% NP-40, 1% TritonX-100, 0.1% SDS) three times followed by washing with 1 X PBS once. After washing, the IP products were treated with proteinase K (200 µg) for 30 mins at 50 °C shaking at 450 rpm in a Thermomixer. RNA was then isolated from the IPed products using RNA STAT-60 (Tel-Test, Inc.) followed by DNase treatment and RNA purification over an RNeasy column (Qiagen). Reverse transcription was done using MMLV (Promega) according to the manufacturer’s protocol followed by quantitative PCR using primers specific to various genes (Supplementary Table 4) and SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad).
Determination of VSG2 mRNA stability
TbRAP1F/mut cells with and without the Cre expression (induced by adding 100 ng/ml Doxycycline for 29 h) were treated with 10 µg/ml Actinomycin D (Sigma) for 0, 15, 30, 45, 90, 120, or 150 mins. 40 million cells were harvested at each time point for isolation of total RNA using RNA STAT-60 (Tel-Test, Inc.). RNA samples were treated with DNase and purified on an RNeasy column (Qiagen). Quantitative RT-PCR was done the same way as described above. Data were processed using MS Excel and Graphpad Prism.
Immunofluorescence (IF) analysis
Cells were fixed with 2% formaldehyde at RT for 10 min, permeabilized in 0.2% NP-40/1 X PBS at RT for 8 min, blocked by 1 X PBS/0.2% cold fish gelatin/0.5% BSA at RT twice, each for 10 min, followed by incubation with the primary antibody (12CA5 was diluted 1:2 K; TbTRF, TbRAP1, VSG6 rabbit antibodies and VSG3 monoclonal antibody were diluted 1:1 K; TbTRF chicken antibody was diluted 1:200) at RT for 2 h and the secondary antibody at RT for 1 h. Cells were then washed with 1 X PBS/0.2% cold fish gelatin/0.5% BSA and 1 X PBS followed by staining with 0.5 µg/ml DAPI before mounting coverslips on slides. Images were taken by a DeltaVision Elite deconvolution microscope. Images were deconvolved using SoftWoRx.
VSG switching assay
TbRAP1+/+ and TbRAP1-/2FL cells were first cultured for ~10.5 population doublings. At the end of culturing, 30 million cells were incubated with 10 µg of VSG2 monoclonal Antibody (IgM, MSKCC Antibody & Bioresource Core) on ice for 15 min. After washing 3 times with growth medium, cells were incubated with MACS beads conjugated with a rat anti-mouse IgM antibody (Miltenyi) on ice for 15 min followed by washing with growth medium twice. The mixture was then loaded onto an LD column, and cells in the flow-through fraction were collected and plated on 96-well dishes. 1/6 of the collected cells (equivalent to 5 millions of initial cell population) were evenly distributed onto three 96-well dishes. Similarly, 1/3 (equivalent to 10 millions of initial cell population) and 1/2 (equivalent to 15 millions of initial cell population) of the collected cells were evenly distributed into six and eight 96-well dishes, respectively. All recovered colonies were tested again by western slot blot using a VSG2 rabbit antibody (without the cross-reaction portion, 1:10,000), and VSG2-positive clones were excluded from switchers. Raw switching frequency was calculated by dividing the number of true switcher colonies by the initial cell number. To determine plating efficiency, cells were plated at 1 cell/well concentration onto 3 X 96-well plates. Plating efficiency was calculated by dividing the number of colonies grown up by 288. The final switching rate was calculated by normalizing raw switching frequency with plating efficiency and divided by the number of population doublings. Data were processed using MS Excel and Graphpad Prism.
RNAseq
The Cre expression was induced by adding doxycycline in TbRAP1F/2FQ and TbRAP1F/2FA&5A cells for 30 h before total RNA was isolated and purified through RNeasy columns (Qiagen). All RNA samples were run on a BioAnalyzer 2100 (Agilent Technologies) using the Agilent RNA 6000 nano kit to verify the RNA quality before sent to Novogene for library preparation and RNA high throughput sequencing, which was the same as described in18.
RNAseq Data Analysis
RNAseq data were analyzed by Novogene:
Quality control
Raw reads of fastq format were first processed through Novogene perl scripts. In this step, clean reads were obtained by removing reads containing adapters, reads containing poly-N and low-quality reads. At the same time, Q20, Q30 and GC content of the clean reads were calculated. All downstream analyses were based on the clean reads with high quality.
Reads mapping to the reference genome
The T. brucei lister 427 genome TriTrypDB-45_TbruceiLister427_2018_Genome.fasta and its annotation TriTrypDB-45_TbruceiLister427_2018.gff were downloaded from the TriTryp DB and used as reference. Index of the reference genome was built using hisat2 2.1.0 and paired-end clean reads was aligned to the reference genome using HISAT2.
Quantification of gene expression level
HTSeq v0.6.1 was used to count the read numbers mapped to each gene. FPKM of each gene was calculated based on the length of the gene and the reads count mapped to this gene.
Differential expression analysis
Differential expression analysis of two conditions/groups (three biological replicates per condition) was performed using the DESeq R package (1.18.0). DESeq provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value <0.05 found by DESeq were assigned as differentially expressed.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The coordinates of the TbRAP1 RRM structures generated in this study have been deposited in the Protein Data Bank (PDB) with the PDB identifier 7XRW and Biological Magnetic Resonance Data Bank (BMRB) with the identifier 36489. The NMR titration data generated in this study have been deposited at BMRB with the identifier 30936. The RNAseq data generated in this study have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE193394. Source data are provided with this paper. Reagents generated in this study are available upon request from the corresponding authors. Source data are provided with this paper.
References
Khamlichi, A. A. & Feil, R. Parallels between Mammalian Mechanisms of Monoallelic Gene Expression. Trends Genet 34, 954–971 (2018).
Cestari, I. & Stuart, K. Transcriptional regulation of telomeric expression sites and antigenic variation in trypanosomes. Curr. Genomics 19, 119–132 (2018).
Gunzl, A., Kirkham, J. K., Nguyen, T. N., Badjatia, N. & Park, S. H. Mono-allelic VSG expression by RNA polymerase I in Trypanosoma brucei: expression site control from both ends? Gene 556, 68–73 (2015).
Guizetti, J. & Scherf, A. Silence, activate, poise and switch! mechanisms of antigenic variation in Plasmodium falciparum. Cell Microbiol 15, 718–726 (2013).
Frank, M. & Deitsch, K. Activation, silencing and mutually exclusive expression within the var gene family of Plasmodium falciparum. Int. J. Parasitol. 36, 975–985 (2006).
Gunzl, A. et al. RNA polymerase I transcribes procyclin genes and variant surface glycoprotein gene expression sites in Trypanosoma brucei. Eukaryot. Cell 2, 542–551 (2003).
Hertz-Fowler, C. et al. Telomeric expression sites are highly conserved in Trypanosoma brucei. PLoS ONE 3, e3527 (2008).
Müller, L. S. M. et al. Genome organization and DNA accessibility control antigenic variation in trypanosomes. Nature 563, 121–125 (2018).
Aresta-Branco, F., Sanches-Vaz, M., Bento, F., Rodrigues, J. A. & Figueiredo, L. M. African trypanosomes expressing multiple VSGs are rapidly eliminated by the host immune system. Proc. Natl Acad. Sci. USA 116, 20725–20735 (2019).
Vanhamme, L. et al. Differential RNA elongation controls the variant surface glycoprotein gene expression sites of Trypanosoma brucei. Mol. Microbiol. 36, 328–340 (2000).
Kassem, A., Pays, E. & Vanhamme, L. Transcription is initiated on silent variant surface glycoprotein expression sites despite monoallelic expression in Trypanosoma brucei. Proc. Natl Acad. Sci. USA 111, 8943–8948 (2014).
Faria, J. et al. Monoallelic expression and epigenetic inheritance sustained by a Trypanosoma brucei variant surface glycoprotein exclusion complex. Nat. Commun. 10, 3023 (2019).
Faria, J. et al. Spatial integration of transcription and splicing in a dedicated compartment sustains monogenic antigen expression in African trypanosomes. Nat. Microbiol 6, 289–300 (2021).
Glover, L., Hutchinson, S., Alsford, S. & Horn, D. VEX1 controls the allelic exclusion required for antigenic variation in trypanosomes. Proc. Natl Acad. Sci. USA 113, 7225–7230 (2016).
Yang, X., Figueiredo, L. M., Espinal, A., Okubo, E. & Li, B. RAP1 is essential for silencing telomeric variant surface glycoprotein genes in Trypanosoma brucei. Cell 137, 99–109 (2009).
Pandya, U. M., Sandhu, R. & Li, B. Silencing subtelomeric VSGs by Trypanosoma brucei RAP1 at the insect stage involves chromatin structure changes. Nucleic Acids Res 41, 7673–7682 (2013).
Afrin, M., Kishmiri, H., Sandhu, R., Rabbani, M. A. G. & Li, B. Trypanosoma brucei RAP1 has essential functional domains that are required for different protein interactions. mSphere 5, e00027–20 (2020).
Afrin, M. et al. TbRAP1 has an unusual duplex DNA binding activity required for its telomere localization and VSG silencing. Sci. Adv. 6, eabc4065 (2020).
Cross, G. A. M., Kim, H. S. & Wickstead, B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol. Biochem Parasitol. 195, 59–73 (2014).
Ridewood, S. et al. The role of genomic location and flanking 3’UTR in the generation of functional levels of variant surface glycoprotein in Trypanosoma brucei. Mol. Microbiol. 106, 614–634 (2017).
Li, B. & Zhao, Y. Regulation of antigenic variation by Trypanosoma brucei telomere proteins depends on their unique DNA binding activities. Pathogens 10, 967–986 (2021).
Rudenko, G. & Van der Ploeg, L. H. Transcription of telomere repeats in protozoa. EMBO J. 8, 2633–2638 (1989).
Nanavaty, V., Sandhu, R., Jehi, S. E., Pandya, U. M. & Li, B. Trypanosoma brucei RAP1 maintains telomere and subtelomere integrity by suppressing TERRA and telomeric RNA:DNA hybrids. Nucleic Acids Res. 45, 5785–5796 (2017).
Saha, A. et al. TbTRF suppresses the TERRA level and regulates the cell cycle-dependent TERRA foci number with a TERRA binding activity in its C-terminal Myb domain. Nucleic Acids Res. 49, 5637–5653 (2021).
Dreesen, O., Li, B. & Cross, G. A. M. Telomere structure and shortening in telomerase-deficient Trypanosoma brucei. Nucleic Acids Res. 33, 4536–4543 (2005).
Maris, C., Dominguez, C. & Allain, F. H. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 272, 2118–2131 (2005).
Daubner, G. M., Cléry, A. & Allain, F. H. T. RRM–RNA recognition: NMR or crystallography… and new findings. Curr. Opin. Struct. Biol. 23, 100–108 (2013).
Beusch, I., Barraud, P., Moursy, A., Cléry, A. & Allain, F. H. Tandem hnRNP A1 RNA recognition motifs act in concert to repress the splicing of survival motor neuron exon 7. Elife 6, e25736 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Li, B., Espinal, A. & Cross, G. A. M. Trypanosome telomeres are protected by a homologue of mammalian TRF2. Mol. Cell Biol. 25, 5011–5021 (2005).
Glover, L. & Horn, D. Trypanosomal histone gammaH2A and the DNA damage response. Mol. Biochem Parasitol. 183, 78–83 (2012).
Glover, L., Alsford, S. & Horn, D. DNA break site at fragile subtelomeres determines probability and mechanism of antigenic variation in African trypanosomes. PLoS Pathog. 9, e1003260 (2013).
Ozdilek, B. A. et al. Intrinsically disordered RGG/RG domains mediate degenerate specificity in RNA binding. Nucleic Acids Res. 45, 7984–7996 (2017).
Järvelin, A. I., Noerenberg, M., Davis, I. & Castello, A. The new (dis)order in RNA regulation. Cell Commun. Signal 14, 9 (2016).
Loughlin, F. E. et al. The solution structure of FUS bound to RNA reveals a bipartite mode of RNA recognition with both sequence and shape specificity. Mol. Cell 73, 490–504. e6 (2019).
Maudlin, I. E., Kelly, S., Schwede, A. & Carrington, M. VSG mRNA levels are regulated by the production of functional VSG protein. Mol. Biochem. Parasitol. 241, 111348 (2020).
Navarro, M. & Gull, K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 414, 759–763 (2001).
Dai, Q. et al. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol. Microbiol 60, 1329–1343 (2006).
Schmid-Siegert, E. et al. Mechanisms of surface antigenic variation in the human pathogenic fungus Pneumocystis jirovecii. MBio 8, e01470–17 (2017).
Deitsch, K. W., Lukehart, S. A. & Stringer, J. R. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat. Rev. Microbiol 7, 493–503 (2009).
Wirtz, E., Leal, S., Ochatt, C. & Cross, G. A. M. A tightly regulated inducible expression system for dominant negative approaches in Trypanosoma brucei. Mol. Biochem Parasitol. 99, 89–101 (1999).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Lee, W., Tonelli, M. & Markley, J. L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31, 1325–1327 (2015).
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins: Struct., Funct., Bioinforma. 59, 687–696 (2005).
Shen, Y., Delaglio, F., Cornilescu, G. & Bax, A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44, 213–223 (2009).
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D. Biol. Crystallogr 54, 905–921 (1998).
Schwieters, C. D., Bermejo, G. A. & Clore, G. M. Xplor-NIH for molecular structure determination from NMR and other data sources. Protein Sci. 27, 26–40 (2018).
Tian, Y., Schwieters, C. D., Opella, S. J. & Marassi, F. M. A practical implicit solvent potential for NMR structure calculation. J. Magn. Reson 243, 54–64 (2014).
Woods, A. et al. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93, 491–500 (1989).
Acknowledgements
We thank Dr. Donny Licatolasi, Dr. Anton Komar, Dr. Kurt Runge, and Catherine Z. Wang for their comments on the manuscript. This work is supported by an NIH R01 grant AI066095 (Li), an NIH S10 grant S10OD025252 (Li), Research Grants Council grants PolyU 151062/18 M, 15103819, 15106421, R5050-18 and AoE/M-09/12 (Zhao), Shenzhen Basic Research Programs of China JCYJ20170818104619974 & JCYJ20210324133803009 (Zhao). Shenzhen Basic Research Program of China JCYJ20220818100215033 (Zhang). Research Grants Council grant C4041-18E (Wong, Zhang, Zhao). The publication cost is partly supported by GRHD at CSU and by PolyU.
Author information
Authors and Affiliations
Contributions
Study conception and design: B.L. and Y.Z.; Data collection: A.K.G., M.A., X.Y., A.S., S.A.S., X.P., Z.J., and B.L.; Data analysis and interpretation of results: B.L., Y.Z., K.B.W., and M.Z.; Draft manuscript preparation: B.L. and Y.Z.; Funding acquisition: B.L., Y.Z., K.B.W., and M.Z.; Supervision: B.L., Y.Z., and M.Z. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daniela Rhodes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaurav, A.K., Afrin, M., Yang, X. et al. The RRM-mediated RNA binding activity in T. brucei RAP1 is essential for VSG monoallelic expression. Nat Commun 14, 1576 (2023). https://doi.org/10.1038/s41467-023-37307-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-37307-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.