Abstract
Plant cell fate determination depends on the relative positions of the cells in developing organisms. The shoot epidermis, the outermost cell layer of the above-ground organs in land plants, protects plants from environmental stresses. How the shoot epidermis is formed only from the outermost cells has remained unknown. Here we show that when inner leaf mesophyll cells are exposed to the surface, these cells show up-regulation of ATML1, a master regulator for epidermal cell identity in Arabidopsis thaliana. Epidermal cell types such as stomatal guard cells regenerate from young inner-lineage tissues that have a potential to accumulate ATML1 protein after epidermal injury. Surgical analyses indicate that application of pressure to the exposed site was sufficient to inhibit ATML1 derepression in the outermost mesophyll cells, suggesting this process requires pressure release. Furthermore, pharmacological analyses suggest that ATML1 derepression in the outermost mesophyll cells require cortical microtubule formation, MAPK signaling and proteasome activity. Our results suggest that surface-positional cues involving mechanical signaling are used to restrict ATML1 activity to the outermost cells and facilitate epidermal differentiation.
Similar content being viewed by others
Introduction
Many plant cells maintain their ability to be reprogrammed and develop into different cell types. Specifically, they can acquire totipotency after exogenous phytohormone- or injury-induced de-differentiation1. Cell ablation experiments in the meristem have shown that eliminated cell types are regenerated from invading neighboring cells of other identities, which suggests that plant cell fate can be re-specified according to the position of the cell, regardless of its lineage, during regeneration2,3,4. The epidermis is one of the well-known cell types that are specified in a position-dependent manner. The shoot epidermis is formed from the outermost cell layer of land plants and is characterized by anticlinal cell division patterns, cuticle deposition and the presence of specialized cell types, such as stomata and trichomes5,6. More than 40 years ago, Stewart and Dermen showed that when epidermal cells underwent unusual periclinal cell divisions, the outer daughter cells maintained epidermal cell identity and the inner daughter cells were differentiated into mesophyll cells7. This report suggests that the “outermost” cell position is essential for acquiring and maintaining epidermal cell identity and also implies that gene activities promoting epidermis or mesophyll fate might be changed in response to the displacement of the cells. However, the molecular mechanisms underlying the position-dependent cell fate change have remained unknown. In the last two decades, several regulators for epidermal cell identity have been identified in Arabidopsis thaliana. ARABIDOPSIS THALIANA MERISTEM L1 LAYER (ATML1), which encodes an HD-ZIP class IV transcription factor, is expressed preferentially in the outermost cells and promotes epidermal cell identity8,9,10,11 (Supplementary Fig. 1a). Mutations in ATML1 and its closest homologue, PROTODERMAL FACTOR2 (PDF2), caused the formation of leaves lacking an epidermis8. In addition, constitutive expression of ATML1 induced ectopic epidermis-related traits in the inner tissues of cotyledons and leaves9,10. These results suggest that ATML1 is a master transcription factor for epidermal cell identity. Therefore, it is expected that the outermost cell-specific activity of ATML1 is responsible for the single epidermal layer formation in Arabidopsis thaliana. Indeed, we have reported that ATML1 activity is restricted to the outermost cells by both transcriptional activation and post-transcriptional repression12. However, it is still unclear how transcription of ATML1 in the outermost cells is established. Our previous report implied that cells should be located at the outermost positions to maintain ATML1 activity because nuclear ATML1 accumulation was reduced in the inner daughter cells of the epidermis that underwent a rare periclinal division12. So far, however, it has not been experimentally addressed whether the outermost location of cells is sufficient to trigger ATML1 expression or not. In this study, we surgically removed or injured the leaf epidermis and found that ATML1 transcription was upregulated in the mesophyll cells exposed to the outermost position. We found that exogenous application of mechanical pressure represses ATML1 upregulation in the mesophyll, suggesting that this process requires pressure release. In addition to the pressure release, cortical microtubule formation, MAPK cascade and proteasome activity were also necessary for the ATML1 derepression in the mesophyll cells. Moreover, injury of outer tissues in young leaves induced epidermis regeneration from inner-lineage cells that can accumulate ATML1 protein.
We have provided molecular and mechanical bases to explain epidermal/mesophyll cell fate changes associated with changes in relative cell positions. Our results suggest that surface-positional cues involving mechanical signals facilitate transcriptional derepression of ATML1 in the outermost cells to generate the epidermal barrier at the interface between plants and their environments to survive terrestrial conditions.
Results
ATML1 expression was increased in mesophyll cells exposed to the surface position
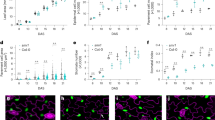
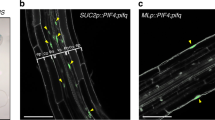
To test the dependence of ATML1 transcription on the cell position, we surgically removed the leaf epidermis and exposed subepidermal mesophyll cells to the outermost position. To observe ATML1 transcriptional activity, we used gATML1-nls-3xGFP plants, in which triple GFP fusion protein with an SV40 nuclear localization signal (NLS) is produced under the control of the native ATML1 regulatory sequence12 (Supplementary Fig. 1b). Weak gATML1-nls-3xGFP signals were detected in mesophyll cells right after removal of the epidermis, which suggests that ATML1 was weakly transcribed in mesophyll cells (Fig. 1a). One day after epidermis removal, GFP signals were increased in the mesophyll cells exposed to the surface position (Fig. 1b, m, n).
a–f The outermost mesophyll cells of gATML1-nls-3xGFP (a, b), proATML1-nls-3xGFP (c, d) and 101×6-nls-3xGFP (e, f) leaves. The outermost mesophyll cells of 10-day-old plants were observed right after (a,c,e) and one day after (b, d, f) removal of the epidermis (0 dar and 1 dar, respectively; dar, day after removal of the epidermis). White arrowheads indicate ATML1-positive nuclei. g Schematic drawing of a transverse leaf section with a part of abaxial (lower) epidermis removed. h, i Inner mesophyll cells (h) and the outermost mesophyll cells (i) of a 10-day-old proATML1-nls-3xGFP seedling at 1 dar. j Schematic drawing of a leaf transverse section with a part of abaxial epidermis injured (highlighted in red). k, l The epidermis of a 9-day-old proATML1-nls-3xGFP leaf was damaged with a needle, and mesophyll (k) and epidermal (l) cells were observed one day after injury. The red dashed region in k mesophyll cells beneath the damaged epidermis; the red lined region in l the dead epidermis. Experiments in a–f, h, i, k, l were repeated three times with similar results. m–o The relative proportion of mesophyll cells showing GFP signals above the threshold was quantified in 10-day-old gATML1-nls-3xGFP (two independent lines; m, n) and proATML1-nls-3xGFP (o) seedlings at 0 dar and 1 dar. n = nine biologically independent leaves except for 1 dar in o (n = ten). Two-tailed Wilcoxon rank-sum test was used (*P < 0.05; **P < 0.01). In the box plots, the 25th percentile, the 50th percentile (central value) and the 75th percentile are marked by horizontal lines within the box. The ends of the whiskers indicate the maximum and minimum values within 1.5 x IQR from the box ends. Outliers are shown above the whiskers. Green, GFP; magenta, FM4-64; white, SR2200. Scale bars: 20 µm.
Next, we examined whether known regulatory sequences are sufficient for the ATML1 upregulation in mesophyll cells. First, we utilized proATML1-nls-3xGFP plants, in which the nls-3xGFP reporter gene was expressed under the 3.4-kb ATML1 promoter sequence13 (Supplementary Fig. 1b). Increase in GFP signals was detected in mesophyll cells of proATML1-nls-3xGFP plants one day after removal of the leaf epidermis, which suggests that the ATML1 promoter contains cis-regulatory sequences sufficient for the surgery-induced activation of ATML1 expression in mesophyll cells (Fig. 1c, d, o). Previously, we have shown that a 101-bp sequence within the ATML1 promoter is sufficient for the expression in the outermost cells of the embryos13. Therefore, we next examined whether the 101-bp sequence is sufficient also for the ATML1 upregulation in mesophyll cells or not. We removed the leaf epidermis of 101×6-nls-3xGFP plants, which produced the nuclear-localized triple GFP reporter under the control of six tandem repeats of the 101-bp sequence13 (Supplementary Fig. 1b). Increase in GFP signals was not detected one day after removal of the epidermis (4 of 4 independent lines; Fig. 1e, f), which suggests that regulatory sequences other than the 101-bp sequence are required for ATML1 induction in mesophyll cells after surgery.
Next, to examine whether ATML1 expression was increased only in the mesophyll cells located at the outermost position, we cleared proATML1-nls-3xGFP leaves with a clearing reagent, ClearSee14. proATML1-nls-3xGFP signals were detected in the outermost mesophyll cells but not in the inner mesophyll cells one day after removal of the epidermis (Fig. 1g–i and Supplementary Fig. 2a, b). These results suggest that ATML1 transcription is activated specifically in the outermost mesophyll cells in a position-dependent manner.
To examine whether complete removal of the epidermis is required for ATML1 activation in the mesophyll cells, we injured epidermal tissues. When epidermal cells of the first or second leaves in 4- or 9-day-old seedlings were damaged with a needle, an increase in ATML1 promoter activity was detected in the subepidermal mesophyll cells beneath the damaged epidermis (10 of 12 and 9 of 10 leaves for 4- and 9-day-old seedlings, respectively; Fig. 1j–l, Supplementary Fig. 2c, d and Supplementary Fig. 3). These results suggest that the epidermal cell injury was sufficient to trigger ATML1 induction in the underlying mesophyll cells.
ATML1 promoter activity was increased in mesophyll cells of a JA-insensitive mutant after removal of the epidermis
After removal or injury of the epidermis, the mesophyll cells should be exposed to increased environmental stress; exposure to air (e.g., oxidation) and dehydration were feasible candidates for the triggers of ATML1 expression. However, ATML1 upregulation was detected even when seedlings were grown in liquid medium after removal of the epidermis (see below), which suggests that oxidation and dehydration are not required for the ATML1 induction.
Epidermis peeling might induce a wound response in underlying mesophyll cells, which could be a trigger of the ATML1 induction. Jasmonate (JA) is known to mediate the wound response; CORONATINE INSENSITIVE 1 (COI1), encoding an F-box protein, is a central component that promotes JA-mediated gene expression and is required for the wounding response15,16. ATML1 upregulation was also detected in mesophyll cells of the coi1 mutant, which suggests that COI1-mediated JA-signaling is not required for the ATML1 induction in mesophyll cells although we cannot exclude the possibility that JA-independent wounding response might be involved in the ATML1 induction (Supplementary Fig. 4).
The ATML1 induction in mesophyll cells after the injury was repressed by mechanical pressure
We noticed that mesophyll cells were often deformed and protruded from the leaf surface after epidermis removal (Fig. 2a and Supplementary Fig. 5). This observation implies that mesophyll cells are pressed by the epidermis and are released from the pressure after removal of the epidermis. Therefore, we examined whether the release from mechanical constraints is involved in the ATML1 induction or not. To this end, we placed the epidermis-peeled proATML1-nls-3xGFP leaf between two coverslips and pressed it by using a paperclip (Fig. 2b). GFP positive cells were reduced in the pressed region compared with the non-pressed region (Fig. 2c, d, g). The cell length along the dorsoventral axis was not significantly different between the pressed outermost mesophyll cells and the intact L2 mesophyll cells overlaid with the epidermis (Supplementary Fig. 6). This observation suggests that our method can provide appropriate pressure to inhibit cell expansion of the outermost mesophyll cells. To examine the correlation between mesophyll cell expansion (i.e. pressure release) and ATML1 induction, we focused on “boundary mesophyll cells” which are located near the boundary between peeled and unpeeled regions but are still overlaid by the epidermis. Because these boundary mesophyll cells are expected to be under different levels of pressures from the partially damaged epidermal layer, we used these cells to examine the correlation between cell expansion and ATML1 expression. Indeed, some boundary mesophyll cells showed invasion into the epidermal layer, suggesting a partial release from the pressure (Supplementary Fig. 7a, b). We found that most of those invaded cells showed ATML1 expression, whereas few uninvaded mesophyll cells did (Supplementary Fig. 7c). These data suggest that there is a positive correlation between mechanical de-repression and ATML1 induction. To test the possibility that decreased GFP signals were caused by the impaired viability of mesophyll cells after the pressure treatment, we utilized a transgenic line in which GFP-ATML1 expression is constitutively induced by estradiol treatment12 (RPS5A»GFP-ATML1). RPS5A»GFP-ATML1 seedlings, with epidermis-peeled leaves pressed between coverslips, were grown in the liquid medium supplemented with estradiol. One day after incubation, GFP-ATML1 was induced in mesophyll cells in both pressed and non-pressed regions (12 of 12 plants; Supplementary Fig. 8a, b). To exclude the possibility that liquid medium may improve the cell viability, we also utilized the R2D2 reporter, in which mDII-nls-TdTomato is expressed under the control of the constitutive RPS5A promoter17. The TdTomato signal intensities in the exposed mesophyll cells were not significantly different between the non-pressed and pressed leaves grown on MS-agar plates (Supplementary Fig. 8c–e). These data suggest that the cell viability and general transcription/translation machinery are not impaired by the pressure treatment. Taken together, these results imply that the mechanical constraint by the epidermis is necessary for the repression of ATML1 in mesophyll cells. Because ATML1 expression in the differentiated epidermal cells was not affected by the pressure treatment (20 of 20 leaves; Supplementary Fig. 9), the mechanical pressure may efficiently repress only the de-novo expression of ATML1, as seen in the exposed mesophyll cell.
a Optical transverse section of a 10-day-old proATML1-nls-3xGFP leaf at 1 dar. Red, epidermal cells; blue, mesophyll cells; asterisks, outermost mesophyll cells. b After epidermis peeling, the first or second leaf of a 9-day-old proATML1-nls-3xGFP seedling was placed between two coverslips and pressed with a paperclip. The orange dashed region, pressed region; the blue dashed region, non-pressed region. c, d proATML1-nls-3xGFP signals in the outermost mesophyll cells in the pressed (c) and non-pressed (d) regions at 1 dar. e, f proATML1-nls-3xGFP signals in the outermost mesophyll cells of 10-day-old seedlings grown in 80 mM (e) and 400 mM (f) mannitol-containing liquid MS medium for 24 h after surgery. Experiments in a and c-f were repeated three times with similar results. g, h The relative proportion of mesophyll cells showing GFP signals above the threshold was quantified in the pressed and non-pressed regions (g) and in 80 mM and 400 mM mannitol-treated seedlings (h). n = nine biologically independent leaves except for 80 mM in h (n = eight). Two-tailed Wilcoxon rank-sum test was used (**P < 0.01). In the box plots, the 25th percentile, the 50th percentile (central value) and the 75th percentile are marked by horizontal lines within the box. The ends of the whiskers indicate the maximum and minimum values within 1.5 x IQR from the box ends. Outliers are shown above and below the whiskers. Green, GFP; magenta; FM4-64. Scale bars: 20 µm in a, c–f; 1 mm in b.
Mesophyll cell expansion induced by epidermis-peeling might be caused by high turgor pressure in these cells. To test whether mesophyll cell expansion or high turgor pressure is required for ATML1 derepression, proATML1-nls-3xGFP plants were grown in liquid medium with low or high osmolality after removal of the epidermis. There were fewer GFP-positive mesophyll cells in high osmolality medium containing 400 mM mannitol than in low osmolality medium with 80 mM mannitol (Fig. 2e, f, h). We also performed the same experiments using the RPS5A»GFP-ATML1 line and found that GFP-ATML1 was induced in the outermost mesophyll cells upon estradiol treatment, regardless of mannitol concentration (Supplementary Fig. 8f, g). This result suggests that the mannitol treatment did not impair the cell viability. The high osmolality of the medium, which absorbs water from the cells and inhibits cell expansion, essentially mimicked the presence of overlying epidermal cells. Considering that the pressure treatment significantly decreased cell expansion, as well as, ATML1 expression of exposed mesophyll cells, inhibition of cell expansion or plasma membrane stretching by the mechanical pressure from the epidermis may mediate ATML1 repression in the inner mesophyll cells. Taken together, these results are consistent with the conclusion that the derepression of ATML1 transcription in mesophyll cells after the injury depends on their mechanical environments.
Increase in cortical microtubule formation in mesophyll cells was necessary for the derepression of ATML1 transcription
Because cortical microtubules are aligned along the maximum mechanical stress directions in the shoot apical meristems, changes in mechanical constraints of the mesophyll cells might also affect microtubule organization18. Therefore, we next observed cortical microtubules by using the tubulin marker 35S-GFP-TUA619. In the intact leaves of 6-day-old seedlings, cortical microtubules were detected as foci at the anticlinal cell boundaries of the epidermis but not of subepidermal cells (16 of 17 leaves; Supplementary Fig. 10a,b). These observations suggest that mesophyll cells have less cortical microtubules compared with the epidermis. Consistently, only a few cortical microtubule arrays were detected in mesophyll cells right after the removal of the epidermis. However, the density of cortical microtubules in mesophyll cells was increased three hours after epidermis peeling (Fig. 3a–c). The cortical microtubule arrays were still prominent in the outermost mesophyll cells one day after the removal of the epidermis (21 of 26 leaves; Supplementary Fig. 10c,d). In summary, in regard to the cortical microtubule density, the exposed mesophyll cells resembled the intact epidermal cells more than the inner mesophyll cells of untreated younger leaves.
a, b 35S-GFP-TUA6 signals in mesophyll cells of 9-day-old seedlings were observed right after (a) and three hours after (b) removal of the epidermis (0 har and 3 har, respectively; har, hour after removal of the epidermis). c Quantification of the cortical microtubule (MT) density in mesophyll cells of 9-day-old 35S-GFP-TUA6 seedlings at 0 har and 3 har. n = 36 cells from 12 biologically independent leaves (three cells from each leaf). Two-tailed Welch’s t-test was used (**P < 0.01). d, e GFP-TUA6 protein localization in mesophyll cells of 9-day-old seedlings grown in liquid MS medium supplemented with 0.1% DMSO (d; Mock) or 30 µM oryzalin (e; Oryzalin) for three hours after epidermis removal. f The cortical microtubule density in the outermost mesophyll cells was quantified in mock and oryzalin-treated 35S-GFP-TUA6 leaves at three har. n = 18 cells from six biologically independent leaves (three cells from each leaf). Two-tailed Welch’s t-test was used (**P < 0.01). g, h Mesophyll cells of the epidermis-peeled first or second leaves in 10-day-old proATML1-nls-3xGFP seedlings grown in liquid MS medium supplemented with 0.1% DMSO (g) or 30 µM oryzalin (h) for 24 h after surgery. i, The relative proportion of mesophyll cells showing proATML1-nls-3xGFP signals above the threshold was quantified in mock and oryzalin-treated plants. n = nine biologically independent leaves. Two-tailed Wilcoxon rank-sum test was used (*P < 0.05). j, k GFP-TUA6 protein localization in mesophyll cells of 9-day-old seedlings grown without (j; Non-pressed) or with (k; Pressed) pressure treatment on peeled leaves for three hours after surgery. Each experiment was repeated three times for a, b, g, h and twice for d, e, j, k with similar results. l The cortical microtubule density was quantified in the outermost mesophyll cells grown for 3 h without or with pressure treatment after surgery. n = 36 cells from 12 biologically independent leaves for non-pressed leaves and n = 33 cells from 11 biologically independent leaves for pressed leaves. Three cells from each leaf were examined. Two-tailed Welch’s t-test was used (ns, P ≥ 0.05). For all the box plots, the 25th percentile, the 50th percentile (central value) and the 75th percentile are marked by horizontal lines within the box. The ends of the whiskers indicate the maximum and minimum values within 1.5 x IQR from the box ends. Outliers are shown above the whiskers. Green, GFP in a, b, d, e, g, h, j, k; magenta, chlorophyll autofluorescence in a, b, d, e, j, k and FM4-64 in g, h. Scale bars: 5 µm in a, b, d, e, j, k and 20 µm in g, h.
Next, we tested whether or not ATML1 derepression is detected when the cortical microtubule formation is disrupted. Treatment with oryzalin, which disrupts microtubules, severely reduced the density of microtubules in exposed mesophyll cells (Fig. 3d–f). proATML1-nls-3xGFP-positive mesophyll cells were also decreased in seedlings treated with oryzalin for 24 h after surgery (Fig. 3g–i). These observations suggest that the increase of cortical microtubule formation is required to derepress ATML1 promoter activity in mesophyll cells. We also examined whether cortical microtubule formation in mesophyll cells is affected by the mechanical pressure. We pressed leaves of 35S-GFP-TUA6 plants after the removal of the epidermis and observed cortical microtubules in mesophyll cells. The density of cortical microtubules was similarly increased in both the pressed and non-pressed regions three hours after epidermis removal (Fig. 3j–l). This observation suggests that the pressure treatment does not affect cortical microtubule formation in mesophyll cells. In addition, the increased cortical microtubules in the pressed regions, which showed decreased ATML1 induction, indicate that cortical microtubule formation is necessary but not sufficient for ATML1 derepression in the mesophyll cells.
MAPK cascade and protein degradation might be necessary for the ATML1 derepression
To examine signaling pathways involved in the de-novo ATML1 induction, we looked for other inhibitors that reduce the induction of proATML1-nls-3xGFP signals in mesophyll cells. We identified two inhibitors, PD98059 and MG132, that each decreased the proportion of proATML1-nls-3xGFP positive mesophyll cells after epidermis removal (Fig. 4a–c, g, h). PD98059 is known as a MAPKK inhibitor20,21 and MG132 is a proteasome inhibitor22,23. This finding therefore implies that MAPK signaling and proteasome-mediated protein degradation are necessary for the ATML1 derepression in the mesophyll cells. We also examined whether these inhibitors affect the formation of cortical microtubules in mesophyll cells. The PD98059 treatment only slightly reduced the density of cortical microtubules and the MG132 treatment did not affect the formation of cortical microtubules in the outermost mesophyll cells after removal of the epidermis (Fig. 4d–f, i, j). These observations suggest that MAPK signaling and proteasome activities are not essential for the cortical microtubule formation in these cells.
a–c Mesophyll cells of the epidermis-peeled first or second leaves in 10-day-old proATML1-nls-3xGFP seedlings grown in liquid MS medium supplemented with 0.1% DMSO (a, Mock), 25 µM MAPKK inhibitor PD98059 (b, PD98059) or 10 µM proteasome inhibitor MG132 (c, MG132) for 24 h after surgery. d–f GFP-TUA6 protein localization in the outermost mesophyll cells of 9-day-old seedlings grown in liquid MS medium supplemented with 0.1% DMSO (d), 25 µM PD98059 (e) or 10 µM MG132 (f) for three hours after surgery. Each experiment was repeated three times for a–e and twice for f with similar results. g, h The relative proportion of the outermost mesophyll cells showing proATML1-nls-3xGFP signals above the threshold was quantified in mock, PD98059 or MG132-treated seedlings at 1 dar. n = nine biologically independent leaves. Two-tailed Wilcoxon rank-sum test was used (**P < 0.01). i, j The cortical microtubule density in the outermost mesophyll cells was quantified in mock, PD98059 or MG132-treated 35S-GFP-TUA6 leaves at 3 har. n = 54 cells from 18 biologically independent leaves for i, n = 27 cells from nine biologically independent leaves for Mock in j and n = 30 cells from ten biologically independent leaves for MG132 in j. Three cells from each leaf were examined. Two-tailed Welch’s t-test was used (**P < 0.01; ns, P ≥ 0.05). For all the box plots, the 25th percentile, the 50th percentile (central value) and the 75th percentile are marked by horizontal lines within the box. The ends of the whiskers indicate the maximum and minimum values within 1.5 x IQR from the box ends. Outliers are shown above the whiskers. Green, GFP in a–f; magenta, FM4-64 in a–c and chlorophyll autofluorescence in d–f. Scale bars: 20 µm in a–c and 5 µm in d–f.
Oryzalin, PD98059 and MG132 did not reduce proATML1-nls-3xGFP signals in the leaf epidermis or embryos (Supplementary Fig. 11). ATML1 expression is initiated in the one-cell stage embryo and is stably maintained by a positive feedback through the L1 box, an ATML1 binding site, in the normal developmental conditions13,24. Therefore, target components of oryzalin, PD98059 and MG132 may be required for de-novo expression of ATML1 during mesophyll cell re-specification but not for the already established expression of ATML1 in the outermost cells.
Outer tissue injury caused epidermis regeneration from the inner-lineage tissue of the leaf
The derepression of ATML1 in the outermost mesophyll cells might result in epidermal cell differentiation in these cells. Therefore, we investigated whether the ATML1-positive mesophyll cells can change their fate and regenerate the epidermis after removal or injury of the overlying epidermis. In the original experimental conditions, we did not find an epidermal-related trait in the outermost mesophyll cells even when plants were grown for seven days after removal of the epidermis (Supplementary Fig. 12a). This might be because ATML1 protein accumulation was inhibited in the inner-lineage cells as demonstrated previously in the embryos12. Therefore, we checked ATML1 protein accumulation in mesophyll cells using the gATML1-3xGFP reporter, in which the 3xGFP-ATML1 fusion gene was expressed under the native ATML1 regulatory sequence12 (Supplementary Fig. 1b). To easily spot the location of the mesophyll cells beneath the damaged epidermal cells, we injured trichomes on the first or second leaves of 9-day-old gATML1-3xGFP seedlings (Fig. 5a). One day after the surgery, ATML1 protein was not detected in mesophyll cells beneath the damaged trichomes (39 of 39 trichomes; Supplementary Fig. 12b, c). This observation might suggest that mesophyll cells used in our experiments were fully differentiated and did not have a potential to change their cell fates. To test this possibility, we next utilized younger seedlings to examine ATML1 protein accumulation. ATML1 protein was detected in the nuclei of mesophyll cells beneath the killed trichomes of 6-day-old gATML1-3xGFP seedlings (20 of 45 trichomes; Fig. 5b). This observation implies that mesophyll cells of younger (i.e. 6-day-old) seedlings have a weaker inhibitory effect on ATML1 protein accumulation and could be re-specified into epidermal cells. To observe cell identity change in mesophyll cells after epidermal cell death, we damaged, with a needle, wider regions of the abaxial surface of the first or second leaves in 6-day-old proATML1-nls-3xGFP seedlings. Accumulation of propidium iodide (PI), a dead cell stain, in the nuclei and the absence of proATML1-nls-3xGFP expression right after the injury confirmed the epidermal cell death in the damaged region (Supplementary Fig. 13a). Five days after the injury, most of the leaf surface cells expressed proATML1-nls-3xGFP, suggesting the regeneration of epidermal cells. We noticed that dents were formed on the leaf surface, presumably in the damaged region (Fig. 5c, d). In the dent region, ATML1-positive cells with small lobes, which are characteristics of young pavement cells, were observed (19 of 22 leaves; Supplementary Fig. 13b). These ATML1-positive cells showed plastid autofluorescence similar to that seen in the intact pavement cells (Supplementary Fig. 13c–e). Stomatal guard cell formation was also observed in the damaged region (17 of 25 leaves; Supplementary Fig. 13b). In order to know whether trichome cells can be also regenerated after epidermis injury, we damaged the trichome-rich adaxial leaf surface. In the damaged region we found trichome-like cells that expressed the trichome marker GLABRA2 (GL2) but were less branched and not surrounded by typical accessory cells, suggesting that they were generated from an unusual developmental process (Supplementary Fig. 14a)25. Taken together, these observations suggest that three epidermal cell types, pavement cells, guard cells and trichomes, were regenerated after epidermal cell injury.
a Schematic drawing of a transverse leaf section showing trichome injury. b ATML1 protein accumulation in mesophyll cells beneath the killed trichomes. After trichomes of the first or second leaves in 6-day-old gATML1-3xGFP seedlings were damaged with a needle, the seedlings were grown for 24 h. Left, leaf epidermis with a killed trichome (arrowhead); right, mesophyll cells beneath the epidermis of the same leaf as in the left panel. c, d Stomata formation from the inner lineage tissues of the leaves after outer tissue injury. After the outer tissues of leaves in 6-day-old proATML1-nls-3xGFP seedlings were damaged with a needle, the seedlings were grown for five days. c shows an optical transverse section of the leaf. Note that inner guard cells and their surrounding cells expressed the proATML1-nls-3xGFP marker. In d the mesophyll-derived inner stoma in c is shown in 3D models reconstructed from optical sections. The left model is cut in half to generate middle and right models. Experiments in b, c were repeated three times with similar results. e Schematic model for molecular mechanisms underlying the derepression of ATML1 in the outermost cells. It has been suggested that the epidermis is under lateral tension29. After removal of the epidermis, the outermost mesophyll cells are expanded vertically, which suggests that plasma membrane of the outermost mesophyll cells is under the same mechanical environment as the epidermal cells (i.e., stretched laterally). Recent studies suggest that stretching of the plasma membrane promotes the activity of certain plasma membrane channels30. Therefore, we speculate that lateral tension to the outermost cells might stretch plasma membrane and activate unknown plasma membrane factors and their downstream signaling pathways that lead to derepression of ATML1. In this model, cortical microtubules formed after cell exposure to the organ surface may act as scaffolds to recruit unknown factors (blue rectangles) that promote epidermal cell specification. Those epidermal fate-promoting factors are activated beneath the plasma membrane by plasma membrane stretch and turn on the downstream signaling that promotes ATML1 transcription. MAPK signaling and the proteasome might be required to activate those plasma membrane factors or downstream factors. Blue, inner guard cells; the red region, presumably damaged epidermal tissues. Green, GFP in b, c; white, PI in b and SR2200 in c. Scale bars: 20 µm.
We noticed that stomata were formed in the inner tissues below the damaged region after abaxial tissue injury, as revealed by 3D reconstruction images (6 of 29 leaves; Fig. 5c, d). Those inner guard cells and their surrounding cells expressed the proATML1-nls-3xGFP marker (Fig. 5c). The inner guard cell formation was also induced when the adaxial outer tissues of the first or second leaves were damaged, as evidenced by KAT1-GUS guard cell marker expression (Supplementary Fig. 14b)26. We then looked for other epidermal cell types formed in the inner tissues after epidermal injury. Intact pavement cells show dot-like small plastid autofluorescence whereas mesophyll cells and guard cells show stronger and larger autofluorescence signals from chloroplasts (Supplementary Fig. 13c, d). In the damaged leaves, we found ATML1-positive inner cells that showed small plastid autofluorescence characteristic of the pavement cells (36 of 216 cells; Supplementary Fig. 14c). The epidermal cell regeneration in the inner tissues supports the ideas that inner mesophyll cells were re-specified-into-epidermal-cells when the outer tissues of the leaves were damaged.
Older mesophyll cells, in which ATML1 protein accumulation is blocked, could not be re-specified as epidermis, suggesting a role of ATML1 in promoting epidermal cell regeneration from mesophyll cells. To further evaluate the necessity of ATML1 for the re-specification of mesophyll cells into the epidermis, we used an atml1 null mutant allele (atml1-3)27. The outer tissues of the first and second leaves of 6-day-old atml1-3 seedlings were killed with a needle, which was confirmed by PI staining (Supplementary Fig. 15a). After five days, we found that the outermost cells of atml1-3 in the damaged region often protruded and were morphologically different from the epidermis (Supplementary Fig. 15b, c). Plastids in these protruded cells were larger than those of the intact pavement cells (Supplementary Fig. 15b, d), suggesting a failure in epidermal cell regeneration. In addition to the atml1-3 mutant, we also utilized the RPS5A»ATML1-SRDX line, in which expression of ATML1 downstream genes is repressed upon the estradiol treatment28. ATML1-SRDX was induced just after the first or second leaves of 6-day-old seedlings were damaged and the treated seedlings were grown for another five days. In ATML1-SRDX induced leaves, cells in the damaged region protruded from the surface and formed callus-like tissues (22 of 32 leaves), which was not observed in control leaves (0 of 46 leaves). To evaluate the efficiency of epidermal regeneration, we used the guard cell formation in the inner tissues as an indicator of mesophyll cell re-specification. This method helped us to distinguish regenerated epidermal cells from the original epidermis. While injury-induced inner guard cell formation was found in DMSO-treated control leaves (11 of 46 leaves), it was not detected in ATML1-SRDX induced leaves (0 of 32 leaves; Supplementary Fig. 15e). Taken together, these results suggest that activation of ATML1 and/or its target genes is required for the regeneration of the epidermal cells from the inner lineage cells.
In summary, we conclude that position- and age-dependent transcriptional/post-transcriptional regulations of ATML1 determine the potential for epidermal cell regeneration in plants.
Discussion
Forty-five years ago, sector analyses in periclinal chimeric albino leaves suggested that L1-derived cells displaced to the L2 cell layer develop into mesophyll cells7. However, it has not been tested whether or not mesophyll-derived cells can acquire an epidermal cell fate when displaced to the outermost positions. The epidermal cell regeneration from the inner-lineage cells after epidermal cell injury in the present study strongly supports the idea that the “cell position” is important to determine epidermal or mesophyll cell identity. We propose that ATML1 derepression is one of the molecular mechanisms that explain this cell fate change. First, de-novo transcriptional derepression of the master gene ATML1 preceded the epidermal fate acquisition in inner-lineage cells. Second, young mesophyll cells that can accumulate ATML1 protein in the nuclei are re-specified as epidermis. Third, nuclear accumulation of ATML1 is decreased in the epidermis-derived cells displaced to the inner cell layer after periclinal cell division12. Lastly, repression of ATML1 and/or its target genes impaired epidermal cell regeneration from the mesophyll cells. We found that cortical microtubules, MAP kinases, and proteasomes are candidate components of signaling pathways that induce ATML1 transcription in the outermost mesophyll cells. Moreover, our results imply that the mechanical pressure from the overlying epidermis is involved in the repression of ATML1 in the mesophyll cells. Mechanical environments are different between the outermost and inner tissues; the outermost tissue is stretched and the inner tissue is compressed, which may be used as positional cues29. We assume that the mechanical pressure exerted by the epidermis inhibits cell expansion as well as ATML1 upregulation in the mesophyll cells. First, mesophyll cells expanded outward when the overlying epidermis was removed, suggesting that mesophyll cells were pressed by mechanical pressures exerted by the epidermis. Second, mesophyll cell expansion after epidermal cell removal was positively correlated with ATML1 induction. Third, when the cell expansion of exposed mesophyll cells was prevented by the mannitol treatment or pressure treatment with a paper clip, ATML1 upregulation was repressed in those mesophyll cells. These results are taken as evidence that mesophyll cell expansion, triggered by the removal of the epidermis, is at least one of the causes of de-novo ATML1 expression during regeneration. However, we cannot exclude the possibility that other signals caused by the damaged epidermis (e.g., wounding response) are also involved in ATML1 de-repression. Moreover, our results are based on observations during epidermis regeneration. Therefore, epidermal cell specification or maintenance during normal development may use different molecular mechanisms. We made a working model for de-novo ATML1 induction in the mesophyll cells by integrating our findings and ideas (Fig. 5e). In this model, we propose that cortical microtubules recruit unknown factors near the plasma membrane. The lateral stretch in the outermost cells activates those factors beneath the plasma membrane, leading to the derepression of ATML1. The MAPK pathway and proteasome activity are required for this process (Fig. 5e). This study not only revealed molecular targets for epidermal/mesophyll cell fate change in the leaves – a long-standing question – but also provides new mechanistic insights and a powerful tool to investigate position-dependent cell fate decision in plants.
Methods
Plants materials and growth conditions
proATML1-nls-3xGFP, 101×6-nls-3xGFP, gATML1-nls-3xGFP, RPS5A»GFP-ATML1, RPS5A»ATML1-SRDX, R2D2, KAT1-GUS, GL2-GUS, atml1-3 and 35S-GFP-TUA6 plants have been described previously10,12,13,17,19,25,26,27,28. coi1 (SALK_035548) was obtained from the ABRC. Seeds were sown on Murashige and Skoog (MS) plates supplemented with 1% sucrose and solidified with either 0.4% phytagel or 1% agar. After incubation at 4˚C for more than two days, plates were moved to the growth chamber at 22 ˚C. The day when plates were transferred to the growth chamber is defined as day 0. After two or three weeks, seedlings were moved to soil and grown at 18 or 22 ˚C. Plants in Supplementary Fig. 3, 6, 8c, d, f, g, 9, 10, 13, 14 and 15 were grown on MS-agar plates under 16 h light condition and otherwise grown on MS-phytagel plates under continuous light.
Surgical epidermis removal and injury
For epidermis removal, the first or second leaves of 9-day-old seedlings were used. The abaxial epidermis of the rosette leaf was removed by the following method. The outer tissue of the petiole was cut transversely with an injection needle under a dissection microscope. The cut edge was picked up with forceps and pulled toward the tip of the leaf to peel the epidermis. For the pressure treatment, peeled or intact leaves were placed between two cover slips and pressed by using a paper clip (28 mm in length, Kohnan). After the surgical treatment, seedlings were grown on MS plates or in liquid MS medium in the growth chamber at 22˚C. For the coi1 mutant, we utilized F3 generation seeds collected from plants heterozygous for the coi1 mutation and homozygous for proATML1-nls-3xGFP. F3 plants without carrying the coi1 mutation were used as a control (WT).
For epidermis injury, the first or second leaves of 4-, 6- or 9-day-old seedlings were used. The abaxial or adaxial surface of the leaves was gently scratched with a needle (Roboz, RS-6064) under a dissection microscope to damage the outer tissues of the leaves. For the detection of ATML1 protein accumulation, trichomes of the leaves were pricked with a needle under a dissection microscope. After damaging the epidermis, the seedlings were grown on MS plates for indicated days in the growth chamber at 22 ˚C under constant light or 16 h light condition. RPS5A»ATML1-SRDX seedlings were grown on 0.0005% dimethyl sulfoxide (DMSO) or 0.1 µM estradiol containing MS-agar plates for five days after damaging. For the dead cell detection before regeneration, seedlings were mounted in 10 µg/ml PI solution right after injury and subjected to confocal laser scanning microscopy. After microscopy, the PI-stained seedlings were washed with water and grown on MS plates for five days to observe regeneration.
Chemical treatment
To treat plants with chemicals after removal of the epidermis, seedlings were grown in 80 mM mannitol-containing liquid-MS medium supplemented with chemicals (30 µM oryzalin, 25 µM PD98059, 10 µM MG132, or 10 µM estradiol). All chemicals were dissolved in dimethyl sulfoxide (DMSO) as stock solutions. As a control, the same amount of DMSO was added to liquid-MS medium. Liquid-MS medium containing 400 mM mannitol was used for the hyperosmotic condition. Seedlings were grown in the growth chamber at 22˚C. Ovules were cultured in liquid Nitsch medium (5% trehalose), supplemented with chemicals or DMSO, at 22 ˚C for indicated days12.
Confocal laser scanning microscopy and image analysis
LSM710 (Carl Zeiss), FV1000 (Olympus) and Leica Stellaris (Leica) confocal laser scanning microscopes were used to detect GFP, TdTomato, GUS, plastid autofluorescence, FM4-64, PI and SCRI Renaissance 2200 (SR2200) signals. GUS signals were detected by using the reflection mode on Leica Stellaris. Mesophyll cells after removal of the epidermis were stained with 20 µM FM4-64 (Sigma Aldrich) in MilliQ water or 1 µl/ml SR2200 (Renaissance Chemicals) in phosphate buffered saline (PBS) for more than five minutes. To observe plastid autofluorescence after damaging the outer tissues, leaves were fixed in 4% paraformaldefyde in PBS containing 2 µl/ml SR2200 for more than one hour at room temperature. Leaves were washed twice with PBS before observation. After damaging trichomes, leaves were mounted in 100 µg/ml PI (Nacalai) solution in MilliQ water for the staining of cell walls and dead cells. To observe the inner tissues of leaves, histological sections were made for Supplementary Figs. 6 and 14c, otherwise the ClearSee method was used14. For the histological sectioning, proATML1-nls-3xGFP leaves were fixed in 4% paraformaldehyde in PBS for one hour and washed once with PBS. The leaves were embedded in 4% agarose in PBS and cross sections were made with a vibratome. The cross sections were stained in 1 µl/ml SR2200 in ClearSee or PBS. For the ClearSee method, leaves were submerged in 4% paraformaldehyde in PBS containing 2 µl/ml SR2200 and were placed at room temperature for more than 30 min. The leaves were washed twice with PBS and cleared with the ClearSee solution.
The levels of ATML1 induction and mDII-TdTomato expression were quantified by using Fiji (fiji.sc). To calculate the proportion of GFP-positive cells, z-stack images of the mesophyll cells were obtained. Maximum projection images of the GFP channel were generated from the z-stack series and were used to count the number of nuclei showing GFP signals above the threshold. Maximum projection images of the FM4-64 channel were used to count the total number of the outermost mesophyll cells. The proportion of GFP-positive cells was determined by calculating the number of GFP-positive mesophyll cells per total number of mesophyll cells in the observed field. For calculating the proportion, experiments were repeated three times; two to four leaves were used in each experiment. In total, nine leaves were used for quantification in Figs. 1m, n, 2g, 3i, 4g, h, and Supplementary Fig. 4c. Nine and ten leaves were used in experiments at 0 dar and 1 dar in Fig. 1o, respectively. Eight and nine leaves were used for quantification in 80 mM and 400 mM mannitol-treated plants, respectively. The proportions were normalized within each experiment by the average of the proportions in 1 dar samples for Fig. 1m–o, nonpressed samples for Fig. 2g, 80 mM treated samples for Fig. 2h, mock treated samples for Figs. 3i, 4g, h, and coi1 samples for Supplementary Fig. 4c. For mDII-TdTomato quantification, the focal plane in which the TdTomato signal was the brightest was selected for each nucleus from the z-stack series and used for the measurement of signal intensity. TdTomato signal intensities in randomly selected three to five nuclei were averaged for each leaf. Fifteen non-pressed leaves and nine pressed leaves were used for the quantification.
For cell length measurement, 10 independent leaves were used. Cell lengths of 10 outermost mesophyll cells or 5 mesophyll cells beneath the epidermal cells in each leaf were averaged for Supplementary Fig. 5. For Supplementary Fig. 6, cell lengths of five cells in each category were averaged in each leaf. Data analysis was performed using R (https://www.R-project.org/). Transverse optical sections and 3D reconstruction were made by using Fiji.
To quantify the correlation between cell invasion and ATML1 expression in the boundary mesophyll cells, we used z-stack images of mesophyll cells, which are located near the boundary between peeled/unpeeled regions and are overlaid by the epidermis, in the first or second leaf of 10-day-old proATML1-nls-3xGFP seedlings at 1 dar. We made optical cross-sections to examine whether boundary mesophyll cells are invaded toward the epidermis. When the outermost ends of mesophyll cells reached the middle depth of the overlaying epidermal layer, the mesophyll cells were judged as “invaded”. Seventy-eight cells from eight leaves were used for the quantification.
The density of protruded cells in the damaged region was calculated by dividing the number of protruded cells by the damaged area. We defined the protruded cell as the surface cell that has intercellular gap spaces between adjacent surface cells and the damaged region as the area where the protruded cells and less lobed pavement cell-like cells were detected. For the quantification of proATML1-nls-3xGFP and atml1-3 regeneration, 20 and 41 leaves were used, respectively.
The sizes of the plastids were measured at the focal planes in which the autofluorescence was the largest for each plastid. Three or four plastids were randomly selected in each cell; plastid sizes in three cells of each category were measured and averaged in each leaf. Eight and 16 leaves were used for the quantification in proATML1-nls-3xGFP and the atml1-3 mutant, respectively.
GUS staining
Leaves were submerged in 90% acetone on ice for 30 min and washed twice with sodium phosphate buffer. The leaves were incubated in the GUS reaction buffer [30 mM Na2HPO4, 20 mM NaH2PO4, 1.5 mM K4Fe, 1.5 mM K3Fe, 500 mg/L X-Gluc, 0.1% Triton] at room temperature under vacuum for 1 h. The samples were further incubated at 37˚C until enough GUS signals were detected. After the incubation, the leaves were fixed in 4% paraformaldehyde in PBS at 4 ˚C overnight and washed twice with PBS. The samples were cleared with the ClearSee solution supplemented with 1 µl/ml SR2200.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data that support the findings of this study are included in this article and supplementary figures. Source data are provided with this paper.
References
Steward, F. C., Ammirato, P. V. & Mapes, M. O. Growth and development of totipotent cells: Some problems, procedures, and perspectives. Ann. Bot. 34, 761–787 (1970).
Marhava, P. et al. Re-activation of stem cell pathways for pattern restoration in plant wound healing. Cell 177, 957–969 (2019).
Sena, G., Wang, X., Liu, H.-Y., Hofhuis, H. & Birnbaum, K. D. Organ regeneration does not require a functional stem cell niche in plants. Nature 457, 1150–1153 (2009).
van den Berg, C., Willemsen, V., Hage, W., Weisbeek, P. & Scheres, B. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 378, 62–65 (1995).
Doll, N. M. et al. A two-way molecular dialogue between embryo and endosperm is required for seed development. Science 367, 431–435 (2020).
Takada, S. & Iida, H. Specification of epidermal cell fate in plant shoots. Front Plant Sci. 5, 49 (2014).
Stewart, R. N. & Dermen, H. Flexibility in ontogeny as shown by the contribution of the shoot apical layers to leaves of periclinal chimeras. Am. J. Bot. 62, 935–947 (1975).
Abe, M., Katsumata, H., Komeda, Y. & Takahashi, T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130, 635–643 (2003).
Peterson, K. M. et al. Arabidopsis homeodomain-leucine zipper IV proteins promote stomatal development and ectopically induce stomata beyond the epidermis. Development 140, 1924–1935 (2013).
Takada, S., Takada, N. & Yoshida, A. ATML1 promotes epidermal cell differentiation in Arabidopsis shoots. Development 140, 1919–1923 (2013).
Iida, H. & Takada, S. A Quarter century history of ATML1 gene research. Plants (Basel) 10, 290 (2021).
Iida, H., Yoshida, A. & Takada, S. ATML1 activity is restricted to the outermost cells of the embryo through post-transcriptional repressions. Development 146, dev169300 (2019).
Takada, S. & Jürgens, G. Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development 134, 1141–1150 (2007).
Kurihara, D., Mizuta, Y., Sato, Y. & Higashiyama, T. ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168–4179 (2015).
Chini, A. et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671 (2007).
Sheard, L. B. et al. Jasmonate perception by inositol-phosphate-potentiated COI1–JAZ co-receptor. Nature 468, 400–405 (2010).
Liao, C.-Y. et al. Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 12, 207–210 (2015).
Hamant, O. et al. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008).
Ueda, K., Matsuyama, T. & Hashimoto, T. Visualization of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma 206, 201–206 (1999).
Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J. & Saltiel, A. R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA 92, 7686–7689 (1995).
Kumari, A., Jewaria, P. K., Bergmann, D. C. & Kakimoto, T. Arabidopsis reduces growth under osmotic stress by decreasing SPEECHLESS protein. Plant Cell Physiol. 55, 2037–2046 (2014).
Goldberg, A. L. Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J. Biol. Chem. 271, 27280–27284 (1996).
Weijers, D. et al. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Develop. Cell 10, 265–270 (2006).
Abe, M., Takahashi, T. & Komeda, Y. Cloning and characterization of an L1 layer-specific gene in Arabidopsis thaliana. Plant Cell Physiol. 40, 571–580 (1999).
Szymanski, D. B., Jilk, R. A., Pollock, S. M. & Marks, M. D. Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125, 1161–1171 (1998).
Nakamura, R. L. et al. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 109, 371–374 (1995).
Roeder, A. H., Cunha, A., Ohno, C. K. & Meyerowitz, E. M. Cell cycle regulates cell type in the Arabidopsis sepal. Development 139, 4416–4427 (2012).
Takada, S. Post-embryonic induction of ATML1-SRDX alters the morphology of seedlings. PLoS ONE 8, e79312 (2013).
Galletti, R., Verger, S., Hamant, O. & Ingram, G. C. Developing a ‘thick skin’: a paradoxical role for mechanical tension in maintaining epidermal integrity? Development 143, 3249–3258 (2016).
Hamilton, E. S. et al. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350, 438–441 (2015).
Acknowledgements
We are grateful to Hirofumi Ohmori (Osaka University, Japan) for DNA sequencing, Prof. Dolf Weijers (Wageningen University, The Netherlands) for R2D2 seeds, the Arabidopsis Biological Research Center for coi1 seeds and the Nottingham Arabidopsis Stock Centre for atml1-3 and 35S-GFP-TUA6 seeds. We greatly thank Prof. Tatsuo Kakimoto (Osaka University, Japan) for supporting our project in his lab. We also thank the past and present members of the plant growth and development lab for their helpful comments and discussion. This work was supported by grants from the Japan Society for the Promotion of Science [16J00702 to H.I.; 20657012, 22687003, 23657036, 26440142, 18K06286 and 22K06280 to S.T.], EMBO Postdoctoral Fellowship [ALTF 128-2020 to H.I.] and the European Research Council [ERC-CoG CORKtheCAMBIA, agreement 819422 to A.P.M].
Author information
Authors and Affiliations
Contributions
H.I. and S.T. designed the experiments. H.I. performed the experiments and analysed the data. S.T. and G.J. provided transgenic plants. A.P.M provided the laboratory reagents and equipment. H.I., A.P.M., S.T., and G.J. wrote the manuscript. S.T. supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Miguel Ángel Moreno Risueño, Jian Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iida, H., Mähönen, A.P., Jürgens, G. et al. Epidermal injury-induced derepression of key regulator ATML1 in newly exposed cells elicits epidermis regeneration. Nat Commun 14, 1031 (2023). https://doi.org/10.1038/s41467-023-36731-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-36731-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.