Abstract
Transition metal-catalyzed enantioselective free radical substitution reactions have recently attracted attention as convenient and important building tools in synthetic chemistry, although construction of stereogenic carbon centers at the propargylic position of propargylic alcohols by reactions with free radicals remains unchallenged. Here we present a strategy to control enantioselective propargylic substitution reactions with alkyl radicals under photoredox conditions by applying dual photoredox and diruthenium catalytic system, where the photoredox catalyst generates alkyl radicals from 4-alkyl-1,4-dihydropyridines, and the diruthenium core with a chiral ligand traps propargylic alcohols and alkyl radicals to guide enantioselective alkylation at the propargylic position, leading to high yields of propargylic alkylated products containing a quaternary stereogenic carbon center at the propargylic position with a high enantioselectivity. The result described in this paper provides the successful example of transition metal-catalyzed enantioselective propargylic substitution reactions with free alkyl radicals.
Similar content being viewed by others
Introduction
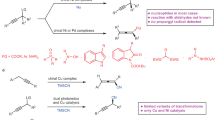
Recently, transition metal-catalyzed enantioselective free radical substitution reactions have gaining attention as versatile synthetic tools, for free radical substitution reactions are more compatible with many functional groups, and tedious protection/deprotection steps can be rather avoided compared to those in ionic substitution reactions such as nucleophilic substitution reactions1,2,3. In addition, radicals are less reactive to Brønsted or Lewis acids, which tend to be required to facilitate dissociation of leaving groups for ionic substitution reactions3. Therefore, free radical substitution reactions provide an attractive approach to deal with substrate limitations for ionic substitution reactions. For instance, transition metal-catalyzed enantioselective allylic substitution reactions with soft nucleophiles (pKa < 25) have been well established as convenient methods to introduce stereogenic carbon centers adjacent to alkene moieties (Fig. 1a)4,5, although reactions with hard nucleophiles (pKa > 25) including alkyl nucleophiles, not activated by functional groups such as methyl derivatives attached with ketone, aldehyde, or ether groups, have been found to be difficult due to the requirement of harsh conditions6. This problem has been very recently shown to be overcome by using free alkyl radicals as the alternative to nucleophiles (Fig. 1b), where the radicals are prepared in situ by the employment of photoredox catalysis7,8,9,10.
a Transition metal-catalyzed enantioselective allylic substitution reactions on treatment with nucleophiles. b Transition metal-catalyzed enantioselective allylic substitution reactions on treatment with free radicals. c Transition metal-catalyzed enantioselective propargylic substitution reactions on treatment with nucleophiles or free radicals. d This work: enantioselective propargylic substitution reactions catalyzed by dual photoredox- and diruthenium system. DC distinct catalyst, LG leaving group, NuH nucleophile, PC photocatalyst.
Similarly to the transition metal-catalyzed enantioselective allylic substitution reactions, transition metal-catalyzed enantioselective propargylic substitution reactions with soft nucleophiles have been very recently developed to introduce stereogenic carbon centers adjacent to alkyne moieties by using ruthenium-11,12,13, copper-14,15,16,17,18,19,20, or nickel-based21,22,23 catalysts (Fig. 1c)24,25,26. However, in contrast to the allylic substitution reactions, application of free radical reactions to the transition metal-catalyzed enantioselective substitution reactions at the propargylic positions of propargylic alcohols or their derivatives has not yet explored, for appropriate methods to control the generation and reactivity of free radicals including inhibition of direct reactions of radicals with alkyne moieties are required. To overcome this problem, we have focused upon the use of two reaction systems: (1) the use of 4-alkyl-1,4-dihydropyridines27,28,29,30 as free radical sources under photoredox conditions, and (2) the use of optically active thiolate-bridged diruthenium complexes [{Cp*RuCl(μ-SR*)}2] (Cp* = η5-C5Me5, R* = chiral substituent)31,32,33,34 as catalysts for enantioselective propargylic substitution reactions (Fig. 1d). Here, 4-alkyl-1,4-dihydropyridines have been shown to supply alkyl radicals to afford alkylated products under photoredox reaction conditions, providing a platform of alkylation reagents designed for the use under milder reaction conditions27,28,29. Application of 4-alkyl-1,4-dihydropyridines can be expanded by dual photoredox- and transition metal-catalyzed systems30,35,36 toward simple alkylation7,8,37,38 and alkylative reactions9,39,40,41,42,43. Regarding the thiolate-bridged diruthenium complexes, which do not catalyze alkylative reactions of alkyne moieties with radicals as known for nickel-based catalysts41,42,43, one ruthenium atom of the diruthenium core works as an electron reservoir for the other substrate-converting ruthenium atom via the electronic communication between the two metals, expected to stabilize not only coordinatively unsaturated species but also radical species31,32. Indeed, reactive intermediates derived from thiolate-bridged diruthenium complexes were shown to capture free radicals44. In total, the strategy to operate enantioselective propargylic alkylation of propargylic alcohols can be summarized as shown in Fig. 1d, where the iridium-based photoredox catalyst generates alkyl radicals from 4-alkyl-1,4-dihydropyridines29,30, and the diruthenium core catalyzes propargylic substitution reactions retaining alkyne moieties unaffected by radicals31,32.
Here, we show the preparation of propargylic alkylated products with a chiral carbon center at the propargylic position via the enantioselective propargylic substitution reaction with free radicals, where the reactivity of radicals is well governed by the dual photoredox- and diruthenium-catalyzed system, providing the successful example of enantioselective propargylic substitution reaction with free radicals.
Results
Reaction optimization
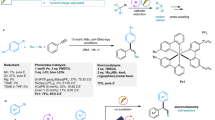
As the beginning, we carried out reactions of 1-phenyl-prop-2-yn-1-ols with substituents introduced at the propargylic position (HC≡CC(OH)(R)Ph, R = H (1), Me (2), or CF3 (3a)) with 1.2 equiv. of 4-benzyl-1,4-dihydropyridine (4a) in the presence of 5 mol% of an optically active thiolate-bridged diruthenium complex [{Cp*RuCl(μ-SR*)}2] ([Ru]−1, SR* = (R)-SCH(Et)C6H3Ph2−3,5)11, 1 mol% of fac-[Ir(ppy)3] (ppy = 2-(pyridyl)phenyl), and 10 mol% of NH4BF4 in 1,2-dichloroethane (ClCH2CH2Cl) at 25 °C for 18 h under visible light irradiation (Fig. 2a and Table 1, entries 1–6). The reaction of 1 with 1.2 equiv. of 4a afforded the corresponding propargylic alkylated product (5) in 42% yield with 29% ee (Table 1, entry 1). Interestingly, addition of 1.2 equiv. of BF3·Et2O increased both the yield and enantioselectivity of 5 up to 73 and 48% ee, respectively (Table 1, entry 2). In contrast, the reaction of 2, a tertiary propargylic alcohol bearing a methyl group introduced at the propargylic position, with 1.2 equiv. of 4a gave only a trace amount of the desired alkylated product (6) under similar reaction conditions even in the presence of 1.2 equiv. of BF3·Et2O (Table 1, entries 3 and 4). The reaction of 3a, another tertiary propargylic alcohol bearing a trifluoromethyl group at the propargylic position, with 1.2 equiv. of 4a gave only a trace amount of the desired alkylated product (7aa) (Table 1, entry 5). However, addition of 1.2 equiv. of BF3·Et2O fairly increased the yield of 7aa up to 47% with a high enantioselectivity such as 86% ee (Table 1, entry 6). These experimental results indicate that introduction of a trifluoromethyl group at the propargylic position is an essential factor to achieve the high enantioselectivity of the propargylic alkylated products.
a Screening of substrates and optimization of the reaction conditions. [Ir], iridium-based photocatalyst; [Ru], ruthenium-based catalyst for propargylic substitution reactions. b Plausible reaction pathways for propargylic substitution reactions of propargylic alcohols with a nucleophile or an alkyl radical catalyzed by a thiolate-bridged diruthenium complex. NuH, nucleophile.
Next, we examined varieties of catalysts for the propargylic substitution reaction (Table 1, entries 6–12). When the reaction of 3a with 1.2 equiv. of 4a was carried out in the presence of 5 mol% of another optically active thiolate-bridged diruthenium complex [{Cp*RuCl(μ-SR*)}2] ([Ru]−2, SR* = (R)-SCH(Et)C6H2Ph3−2,3,5)11 instead of [Ru]−1 as a ruthenium catalyst, isolated yield of 7aa was decreased to 35%, but the enantioselectivity was fairly increased to 93% ee (Table 1, entry 7). Interestingly, yield of 7aa, obtained as a mixture of racemates, increased up to 84% when [{Cp*RuCl(μ-SMe)}2], another thiolate-bridged diruthenium complex with non-chiral centers, was used as the ruthenium catalyst instead of [Ru]−1 or [Ru]−2 (Table 1, entry 8). In addition, several mononuclear and binuclear ruthenium complexes such as [CpRuCl(PPh3)2] (Cp = η5-C5H5), [RuCl2(PPh3)3], [RuCl2(dppe)2], and [{(p-cymene)RuCl(μ-Cl)}2] were further examined as catalysts. However, only a trace amount of 7aa was obtained when [CpRuCl(PPh3)2] was used as a catalyst (Table 1, entry 9), or 7aa was not obtained at all when [RuCl2(PPh3)3], [RuCl2(dppe)2], or [{(p-cymene)RuCl(μ-Cl)}2] was used as a catalyst (Table 1, entries 10–12).
It must be noted that our group has utilized several thiolate-bridged diruthenium complexes as the catalysts for propargylic substitution reactions of propargylic alcohols with nucleophiles. Here, thiolate-bridged diruthenium core containing ruthenium–ruthenium bond has been shown to the key for the catalysis to proceed, where the ancillary ruthenium center of the diruthenium core works as an electron reservoir for the substrate-converting ruthenium center via the electronic communication between the two metals to stabilize reactive intermediates including a coordinatively unsaturated complex (A) obtained by the reaction of the corresponding precursory diruthenium complex ([{Cp*RuCl(μ-SMe)}2] as a typical example) with NH4BF4, a π-alkyne complex (B) obtained via the coordination of a propargylic alcohol (1-phenylprop-2-yn-1-ol as a typical example), a vinylidene complex (C) obtained via the 1,2-migration of the acetylenic proton, an allenylidene complex (D) obtained via the loss of H2O, another vinylidene complex (E) obtained via the attack of a nucleophile at the γ-carbon atom, and another π-alkyne complex (F) obtained via the 1,2-migration of the β-hydrogen atom, which liberates the propargylic substituted product to regenerates the coordinatively unsaturated complex A (solid pathway in Fig. 2b)31,32. The propargylic substitution reactions of propargylic alcohols with alkyl radicals presented in this manuscript have been also shown to be identical to thiolate-bridged diruthenium complexes, and likely follow the reaction pathway similar to Fig. 2b with almost the same reaction pathway for the formation an of allenylidene complex (D) by the reaction of a coordinatively unsaturated species (A) with a propargylic alcohol, but with slightly different reaction pathway for the radical substitution reactions; i.e. capture of an alkyl radical, the addition of a proton, and a single electron transfer occurs against the allenylidene complex D, where radical redox-relay reactions are furnished by the diruthenium core working as an electron pool (dotted pathway in Fig. 2b).
It should be cautioned that starting materials (both 3a and 4a) were recovered for enantioselective propargylic alkylation employing either [Ru]−1 or [Ru]−2 as a ruthenium catalyst (Table 1, entries 6 and 7), thus elongation of the reaction time up to 48 h was examined. When the reaction of 3a with 1.2 equiv. of 4a was carried out in the presence of 5 mol% of [Ru]−2, 1 mol% of fac-[Ir(ppy)3], 10 mol% of NH4BF4, and 1.2 equiv. of BF3·Et2O in ClCH2CH2Cl at 25 °C for 48 h under visible light irradiation, the yield of 7aa was increased up to 81% with a slightly higher enantioselectivity at 94% ee (Table 1, entry 13). Use of other iridium-based photoredox catalysts such as fac-[Ir(Fppy)3] (Fppy = 3,5-difluoro-2-(pyridyl)phenyl) and [Ir(ppy)2(dtbbpy)]PF6 (dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine) instead of fac-[Ir(ppy)3] was also examined, where yields of 7aa became slightly lower with a lower enantioselectivity (Table 1, entries 14 and 15).
Next, we examined optimization of other reaction conditions (Table 1, entries 16–27). When reactions were carried out in other solvents such as CH2Cl2 and Cl(CH2)3Cl, yields of 7aa got lower with a lower enantioselectivity (Table 1, entries 16 and 17) compared to those in ClCH2CH2Cl. Reactions using some other Lewis acids such as B(OH)3, Sc(OTf)3, and BCl3 instead of BF3·Et2O were also examined, but trace amounts of 7aa detectable only by GC-MS were formed in all cases (Table 1, entries 18–20). In addition, reducing the amount of BF3·Et2O down to 10 mol% did not change the enantioselectivity of 7aa with 93% ee but decreased the isolated yield of 7aa to 13% (Table 1, entry 21), demonstrating that the addition of almost a stoichiometric amount of BF3·Et2O is necessary to enhance the reaction. It needs to be comprehended that the present substitution reaction is also the dehydration process, giving a stoichiometric amount of H2O as a byproduct. Indeed, the addition of 1.2 equiv. of H2O was shown to inhibit the reaction (Table 1, entry 22). However, the addition of 2.0 equiv. of anhydrous MgSO4 in the absence of BF3·Et2O did not give the desired product (Table 1, entry 23), while the addition of both 2.0 equiv. of anhydrous MgSO4 and 10 mol% of BF3·Et2O gave 7aa in 15% yield with 92% ee (Table 1, entry 24), demonstrating that main roles of BF3·Et2O may be not only to work as a drying reagent to trap H2O, but also to work as an accelerator for the dehydration process more efficiently than other Lewis acids (B(OH)3, Sc(OTf)3, and BCl3) and anhydrous MgSO4. Finally, additional control experiments were investigated. In the absence of photoredox catalyst but under visible light irradiation (Table 1, entry 25) or in the presence of photoredox catalyst but under dark (Table 1, entry 26), only trace amounts of 7aa were obtained, while formation of 7aa was perfectly not observed in the absence of ruthenium catalyst (Table 1, entry 27), clarifying that the photoredox catalyst, light, and ruthenium catalyst are all necessary to carry out reactions.
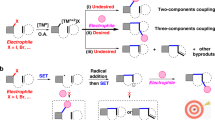
It should be remarked that free alkyl radicals generated from 4-alkyl-1,4-dihydropyridines under photoredox conditions were already employed as alkylation reagents in the dual photoredox- and palladium-catalyzed propargylic substitution reactions of propargylic esters to afford the propargylic alkylated products, although enantioselectivity was not achieved at all37. In this reaction system, both photoredox and palladium catalysts work as photosensitizers under visible light irradiation, where a single electron transfer (SET) between the propargylic esters and excited palladium catalysts occurs to afford the propargylic radicals, which react with the alkyl radicals, generated from 4-alkyl-1,4-dihydropyridines via a SET process with photoredox catalysts, to afford the corresponding propargylic alkylated products. Furthermore, photoinduced enantioselective propargylic substitution reactions were only reported for propargylic cyanation catalyzed by dual photoredox- and copper-catalyzed system, where a SET between the propargylic esters and photoinduced photoredox catalysts occurs to afford the propargylic radicals, which react with the nucleophilic copper cyanate complexes, formed via reactions of chiral copper catalysts and CN– in situ, to afford the corresponding propargylic cyanated products45,46,47 or cyanated allenes48. Thus, the results described in this present manuscript are the successful example of enantioselective propargylic alkylation of propargylic alcohols with free radicals. Moreover, construction a quaternary stereogenic carbon center at the propargylic position was also achieved49.
Substrate scope and application
Taking the reaction conditions of entry 13 in Table 1 as optimized, reactions of 3a with several 4-alkyl-1,4-dihydropyridines (4) to obtain the corresponding propargylic alkylated products were next examined (Fig. 3a). The use of 4-benzyl-1,4-dihydropyridine derivatives, where either electron-donating (4b, Me; 4c, MeO; 4d, OH) or electron-withdrawing substituents (4e, F, 4f, Cl; 4g, Br; 4h, CF3; 4i, MeOC(=O)) were imported at the 4-position of the benzene ring, afforded the corresponding propargylic alkylated products in good to high yields with a high enantioselectivity (7ab, 78% yield, 94% ee; 7ac, 79% yield, 92% ee; 7ad, 70% yield, 92% ee; 7ae, 80% yield, 92% ee; 7af, 74% yield, 90% ee; 7ag, 68% yield, 94% ee; 7ah, 74% yield, 91% ee; 7ai, 75% yield, 96% ee), demonstrating that even phenolic OH group is tolerant (7ad), while the highest enantioselectivity at 96% ee was achieved for that containing benzoate ester (7ai). On the other hand, the use of the 4-benzyl-1,4-dihydropyridine derivatives, where bulky substituents were imported at the 4-position of the benzene ring (4j, tBu; 4k, Ph), afforded the corresponding propargylic alkylated products in good yields with a little lower enantioselectivity (7aj, 70% yield, 84% ee; 7ak, 71% yield, 83% ee). 4-Benzyl-1,4-dihydropyridine derivatives with either electron-donating substituents imported at the 3-position (4l, Me; 4m, F; 4n, Cl; 4o, Br) or the 2-position (4p, Me; 4q, F) of the benzene ring were also applicable to afford the corresponding propargylic alkylated products in good yields with a high enantioselectivity (7al, 73% yield, 90% ee; 7am, 80% yield, 90% ee; 7an, 77% yield, 91% ee; 7ao, 72% yield, 93% ee; 7ap, 74% yield, 90% ee; 7aq, 78% yield, 93% ee).
Scope of alkyl radicals is not limited to substituted benzyl radicals. Indeed, 1,4-dihydropyridine derivatives with arylmethyl groups such as 1-naphthylmethyl (4r), 2-naphthylmethyl (4s), piperonyl (4t), 2-furylmethyl (4u), and 2-thienylmethyl (4v) substituents imported at the 4-position of the dihydropyridine skeleton could be successfully converted into the corresponding propargylic alkylated products in good to high yields with a high enantioselectivity (7ar, 69% yield, 91% ee; 7as, 71% yield, 95% ee; 7at, 66% yield, 91% ee; 7au, 80% yield, 90% ee; 7av, 75% yield, 94% ee), providing a synthetic tool to construct heterocycle-containing alkynes. On the other hand, the use of 1,4-dihydropyridine derivatives with a pyridine-, ether, or amine-containing methyl group, a secondary alkyl group, or a long chain primary alkyl group (R2 = 3-pyridylmethyl, benzyloxymethyl, N,N-dibenzylaminomethyl-, 3-pentyl, or n-nonyl) imported at the 4-position of the dihydropyridine skeleton gave only trace amounts of desired products, leading to failure of their isolation (Fig. 3b), which might be due to either their steric bulkiness or instability of the corresponding alkyl radicals50 generated under photoredox conditions.
Subsequently, propargylic substitution reactions of various propargylic alcohols (3) with 4a were investigated (Fig. 4a). 1,1,1-Trifluoro-2-phenyl-but-3-yn-2-ol derivatives with either electron-donating (3b, Me; 3c, Et; 3d, MeO) or electron-withdrawing substituents (3e, F; 3f, Cl; 3g, Br; 3h, CF3) imported at the 4-position of the benzene ring were successfully alkylated to afford the desired products in good to high yields with a high enantioselectivity (7ba, 81% yield, 90% ee; 7ca, 76% yield, 90% ee; 7da, 71% yield, 91% ee; 7ea, 77% yield, 94% ee; 7fa, 74% yield, 90% ee; 7ga, 70% yield, 92% ee; 7 ha, 83% yield, 93% ee). 1,1,1-Trifluoro-2-phenyl-but-3-yn-2-ol derivatives, where either electron-donating or electron-withdrawing substituents were imported at the 3-position of the benzene ring (3i, Me; 3j, F; 3k, Cl; 3l, Br), were also applicable to afford the corresponding propargylic alkylated products in good yields with a high enantioselectivity (7ia, 67% yield, 90% ee; 7ja, 72% yield, 91% ee; 7ka, 65% yield, 95% ee; 7la, 70% yield, 90% ee).
a Propargylic alkylated products with isolated yield. b Propargylic alcohols with the formation of trace amounts of the desired products detected by GC-MS but not isolable for the reactions of 3 with 4a, or without the formation of the desired products for the reactions of 3 with 4a. c Absolute configuration determination.
Interestingly, not only propargylic alcohols containing phenyl derivatives (3a–3l) but also those containing heterocycles such as 2-furyl and 2-thienyl groups (3m and 3n) were shown to be converted into the corresponding propargylic alkylated products in good yields with a high enantioselectivity (7ma, 76% yield, 96% ee; 7na, 65% yield, 90% ee). However, the reaction of 1,1,1-trifluoro-2-phenyl-but-3-yn-2-ol derivatives (HC≡CC(OH)(CF3)R1) with substituents (Me or F) at the 2-position of the benzene ring (R1 = 2-tolyl or 2-fluorophenyl) or 2-([1,1’-biphenyl]-4-yl)-1,1,1-trifluorobut-3-yn-2-ol (R1 = 2-naphthyl) with 4a gave only trace amounts of the desired products, presumably due to their steric hindrance (Fig. 4b). In addition, formation of the corresponding alkylated products was not observed at all by GC-MS for the reactions of 3-(trifluoromethyl)pent-1-yn-3-ol (HC≡CC(OH)(CF3)Et) with 4a, or of 1,1,1-trifluoro-2-phenylpent-3-yn-2-ol (MeC≡CC(OH)(CF3)Ph) with 4a (Fig. 4b). Namely, introduction of an aromatic moiety (such as substituted phenyl, 2-furyl, or 2-thienyl group) at the propargylic position of propargylic alcohols is necessary for the propargylic alkylation with 4-alkyl-1,4-dihydropyridines under photoredox conditions, while the propargylic moiety of the propargylic alcohols must contain terminal ethynyl group to form ruthenium–allenylidene intermediates.
The reaction of 3l with 4r was further examined to obtain the corresponding propargylic alkylated product (7lr) in 61% yield with a high enantioselectivity at 91% ee (Fig. 4c). The molecular structure of 7lr has been determined after further purification with HPLC and recrystallization from Et2O by a single-crystal X-ray diffraction study, which has clarified that the absolute configuration of 7lr is (R), the chirality of which is same with those obtained for the ruthenium-catalyzed enantioselective propargylic phosphinylation of propargylic alcohols similarly bearing both CF3 and aromatic groups at the propargylic position13,34.
Interestingly, 1,1-difluoro-2-phenyl-but-3-yn-2-ol (8), where CF2H group was introduced at the propargylic position instead of CF3 group, was found to react with 4a to afford the corresponding propargylic alkylated product (9) in 75% yield, although the enantioselectivity at 63% ee was significantly lower than that obtained for 7aa at 94% ee (Fig. 5a), demonstrating that the existence of trifluoromethyl group at the propargylic position plays a critical role for the enantioselective propargylic alkylation34.
a Enantioselective propargylic alkylation of a propargylic alcohol bearing CF2H instead of CF3. b Large-scale preparation of a chiral propargylic alkylated product. c Transformations of a chiral propargylic alkylated product via silver-catalyzed electrophilic bromination with NBS, Sonogashira coupling with PhI, Pd/C-catalyzed hydrogenation with H2, and photoredox- and nickel-catalyzed anti-Markovnikov-type hydroalkylation with 4-alkyl-1,4-dihydropyridine.
The practicality and synthetic potential of the enantioselective propargylic alkylation was then investigated. We first performed a larger-scale reaction of 3a (1.00 mmol) with 4a (1.20 mmol) under the optimized reaction conditions to obtain the propargylic alkylated product 7aa in 75% isolated yield (0.75 mmol) with keeping a high enantioselectivity at 93% ee (Fig. 5b). Further transformations of 7aa using this batch with an enantioselectivity at 93% ee was then examined, because terminal alkyne moieties have a wide range of synthetic utility51. Indeed, AgNO3-catalyzed electrophilic bromination52 of 7aa with NBS (N-bromosuccinimide) afforded the corresponding bromoalkyne (10), while the Sonogashira coupling53 of 7aa with PhI afforded the corresponding internal alkyne (11), both in high yields without any loss of the optical purity of 7aa, providing a synthetic tool for the enantioselective preparation of internal alkynes with propargylic alkyl substituents. Furthermore, Pd/C-catalyzed hydrogenation54 of 7aa gave the corresponding diphenylalkane (12) in 96% yield, while the photoredox- and NiCl2·6H2O-catalyzed hydroalkylation of 7aa with 4a, the synthetic method very recently reported by our group42, afforded anti-Markovnikov-type alkylated alkene (13) in 70% yield as a mixture of (E)- and (Z)-isomers (E/Z = 89/11), all obtained almost without the loss of the optical purity of 7aa (Fig. 5c).
Mechanistic studies
To obtain more information on the enantioselective induction, we have conducted stoichiometric and catalytic reactions of the ruthenium–allenylidene complex [Cp*RuCl(μ-SR*)2Ru{C=C=C(CF3)Ph}Cp*]BF4 (14, SR* = (R)-SCH(Et)C6H3Ph2-3,5), which was originally prepared by the reaction of [Ru]-1 with 3a in the presence of NH4BF4 and anhydrous MgSO413, and now has been shown to be prepared by the similar reaction in the presence of BF3·Et2O as a dehydrating reagent instead of anhydrous MgSO4 (Fig. 6a). When the stoichiometric reaction of 14 with 1.2 equiv. of 4a was carried out in the presence of 1 mol% of fac-[Ir(ppy)3] and 1.0 equiv. of 3b but in the absence of BF3·Et2O in ClCH2CH2Cl at 25 °C for 48 h under visible light irradiation, the propargylic alkylated product 7aa was obtained in 66% yield with 88% ee (Fig. 6b). These results indicate that BF3·Et2O is not concerned with the alkylation of the allenylidene complex but likely with the dehydration process for the formation of allenylidene complex at least for propargylic alcohols containing CF3 group at the propargylic position.
Separately, the reaction of 3a with 1.2 equiv. of 4a in the presence of 5 mol% of 14, 1 mol% of fac-[Ir(ppy)3], and 1.2 equiv. of BF3·Et2O in ClCH2CH2Cl at 25 °C for 48 h was examined to afford 7aa in 85% yield with 85% ee (Fig. 6c). This result has clarified that ruthenium–allenylidene complexes such as 14 worked as possible key reactive intermediates for the propargylic alkylation as have been confirmed for the propargylic substitution reactions of propargylic alcohols with nucleophiles12,13,55.
In order to get more mechanistic information of the reaction pathway, we next investigated the propargylic alkylation of 3a with 4a in the presence of 1.2 equiv. of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) to capture radicals under the optimized reaction conditions. As a result, formation of propargylic alkylation product 7aa was perfectly inhibited, while 1-(benzyloxy)-2,2,6,6-tetramethylpiperidine (15)56, the TEMPO-trapped benzyl adduct, was obtained in 34% yield (Fig. 6d). Formation of other TEMPO-trapped compounds was not observed from the reaction mixture. At present, we cannot exclude the possiblity of the formation of propargylic radicals as reactive intermediates37,45,46,47,48,57,58,59,60, because we used tertiary propargylic alcohols bearing the CF3 moiety as the substrate, where the corresponding propargylic radicals are difficult to be trapped by TEMPO.
Then, Stern–Volmer analysis for emission quenching of fac-[Ir(ppy)3] by 4a, 3a, or the ruthenium–allenylidene complex [Cp*RuCl(μ-SMe)2Ru(C=C=CHPh)Cp*]BF4 (16), prepared by the reaction of [{Cp*RuCl(μ-SMe)}2] with 1 in the presence of NH4BF4 and anhydrous MgSO455, was performed in ClCH2CH2Cl. As a result, the quenching rate constants were calculated to be k4a = (1.10 ± 0.05) × 108 M–1 s–1, k3a = (5.3 ± 0.2) × 107 M–1 s–1 and k16 = (1.72 ± 0.07) × 107 M–1 s–1, respectively (Fig. 7a, see Supplementary Information for calculations). This result revealed that the quenching of photoexcited fac-[Ir(ppy)3] by 4a can occur twice as fast as that by 3a, and 6.5 times as fast as that by 16.
Additional cyclic voltammetric study of fac-[Ir(ppy)3] revealed that the 1st oxidation wave of fac-[Ir(ppy)3] in ClCH2CH2Cl appeared at +0.25 V vs FeCp2+/0 as a reversible process, whereas the 1st reduction wave appeared at –1.60 V vs FeCp2+/0 as an irreversible process (Supplementary Fig. 1a). These correspond to the estimated redox potentials of fac-[Ir(ppy)3]*/fac-[Ir(ppy)3]– at +0.90 V vs FeCp2+/0 and fac-[Ir(ppy)3]+/fac-[Ir(ppy)3]* at –2.25 V vs FeCp2+/0. On the other hand, cyclic voltammogram of 4a exhibited an irreversible oxidation wave at +0.53 vs FeCp2+/0 (Supplementary Fig. 1b), clarifying that photoexcited fac-[Ir(ppy)3] can oxidize 4a to afford the radical cation 4a•+ via a SET process, where the C–C bond scission further occurs to give the benzyl radical and a pyridinium cation (Fig. 7a). As for the propargylic moiety, oxidation of 3a was found to be difficult to occur, but an irreversible reduction wave was observed at –1.36 V vs FeCp2+/0 of 3a in ClCH2CH2Cl (Supplementary Fig. 1c). Thus, the possibility that the reduction of 3a by the photoexcited fac-[Ir(ppy)3] proceeds to afford the radical anion 3a•– via a SET process leading to the formation of a propargylic radical (Fig. 7a), as has been proposed for the formation of propargylic radicals via a SET process of propargylic esters with an excited palladium species37, cannot be excluded based on the Stern–Volmer and cyclic voltammetric studies. However, radical capture experiment by TEMPO partially supports that such a SET process to generate the corresponding propargylic radical was rather limited, but quenching of photoexcited fac-[Ir(ppy)3] by 3a occurred rather via triplet state energy transfer. Similarly, the possibility of the redox of diruthenium complexes by the photoexcited fac-[Ir(ppy)3] cannot be excluded based on the previous CV measurements61,62, whereas quenching experiments have clarified that such a SET process occurs by one order of magnitude less frequently than the SET process between fac-[Ir(ppy)3] and 4a.
A light on/off experiment was further performed for propargylic alkylation of 3a with 4a to afford 7aa, under the typical catalytic reaction conditions. As shown in Fig. 7b, the reaction proceeded smoothly under visible light irradiation, but did not proceed in dark, which evinced the requirement of continuous visible light irradiation is necessary, but less possibility of chain propagation participating in the main reaction pathway. In addition, time course study for propargylic alkylation of 3a with 4a to afford 7aa under the typical catalytic reaction conditions was investigated (Supplementary Fig. 2), which revealed that the present dual photoredox- and ruthenium-catalyzed reaction was shown to follow the zeroth order kinetics at k7aa = (2.33 ± 0.04) × 10–7 M–1 s–1, typical for photochemical reactions under saturation of catalysts with reactants, in the optimized reaction conditions. Independently by using Hg lamp filtered at 440 nm instead of LED light, the quantum yield of alkylation of 3a with 4a under typical reaction conditions was determined to be Φ = 0.0502 ± 0.0003 by chemical actinometry (Supplementary Fig. 3). Both the quantum yield measurement as well as light on/off experiment have clarified that the catalytic formation of 7aa proceeds rather via sequential redox pathways, but not via a radical chain process63,64. Thus, the alkyl radicals in situ are mainly formed by the SET process between an excited-state photoredox catalyst and 4-alkyl-1,4-dihydropyridines28,29,65.
To obtain more information on the radical reactions mediated by ruthenium–allenylidene complexes, we performed DFT calculations for the reaction of the achiral diruthenium–allenylidene complex I with the benzyl radical (Bn•) and the pyridinium cation (PyH+) (pKa = 2.96 in H2O)66 generated via the scission of 4-benzyl-1,4-dihydropyridine 4a, and the reduced iridium(II) species fac-[Ir(ppy)3]– in ClCH2CH2Cl to afford a vinylidene complex, aromatized pyridine (Py), and the regenerated iridium(III) catalyst fac-[Ir(ppy)3] (Fig. 8a; the Cartesian coordinates of the 3D structures of all species are given in the Supplementary Data 1). Here, the attack of the benzyl radical (Bn•) to the γ-carbon (Cγ) atom in the monocationic diruthenium–allenylidene complex I gives the monocationic diruthenium–alkynyl radical complex III via the formation of the reactant complex II and the transition state TSII-III. The Gibbs free energies (ΔG298K) of II, TSII-III, and III relative to the initial state (I + Bn•) are 4.0, 7.5 and −24.8 kcal/mol, respectively (Fig. 8a and Supplementary Fig. 5 for energy diagram and structures, respectively), indicating that these reaction steps smoothly proceed. The results of IRC calculations for TSII-III are shown Supplementary Fig. 6. The change in the Mulliken spin density of atoms (Supplementary Fig. 6b) shows that the radical center moves from the carbon atom of the benzyl radical to the allenylidene region along the reaction coordinate (ruthenium and Cβ atoms). The SOMO, which has in-phase overlapping between the Cγ atom in the allenylidene complex and the carbon atom in benzyl radical, is gradually stabilized along the reaction coordinate, and the MO becomes doubly occupied (Supplementary Fig. 7). The spin density surface of III (Supplementary Fig. 8) also demonstrates delocalization of spin density, indicating the importance of the diruthenium system.
The monocationic diruthenium–alkynyl radical complex III is then exergonically reduced by 2[Ir(ppy)3]– to form the neutral diruthenium–alkynyl complex IV (III + fac-[Ir(ppy)3]− (ΔG298K = −24.8 kcal/mol) → IV + fac-[Ir(ppy)3] (ΔG298K = − 92.9 kcal/mol)), while III is endergonically protonated by protonated pyridine to afford the dicationic diruthenium–vinylidene complex VI (III + PyH (ΔG298K = − 24.8 kcal/mol) → VI + Py (ΔG298K = − 9.5 kcal/mol)). Thus, it is more likely that III is first reduced to afford IV, and then IV is protonated to afford the diruthenium–vinylidene complex V (ΔG298K = − 98.5 kcal/mol). The protonation of IV was calculated to be exergonic by 5.6 kcal/mol. Our present DFT calculations indicate that the attack of benzyl radical to the Cγ atom in the ruthenium–allenylidene complex followed by the reduction and protonation easily gives the ruthenium–vinylidene complex.
It should be carefully paid attention that two reaction pathways can be possible for the formation of the vinylidene complex V from the allenylidene complex I: one is the attack of Bn• to the allenylidene complex I to form the monocationic alkynyl radical complex III at first, followed by the one-electron reduction by fac-[Ir(ppy)3]– to generate the vinylidene complex V (upper pathway in Fig. 8b) as has been shown by the DFT calculations in Fig. 8a; the other is one-electron reduction of the allenylidene complex I by fac-[Ir(ppy)3]– to form the neutral alkynyl radical complex VII at first, followed by the attack of Bn• (lower pathway in Fig. 8b). Indeed, mononuclear ruthenium allenylidene complexes were known to be reduced by strong reducing reagents such as cobaltocene to afford the radical species67, which were known to react with radical reagents68,69. However, our group previously found that the thiolate-bridged diruthenium complex [{Cp*RuCl(μ-SMe)}2] underwent reductive coupling of propargylic alcohols including 1 to afford 1,5-hexadiynes under reductive conditions44. Here, a neutral alkynyl radical complex VII was generated in situ by the reaction of I with a trace amount of oxygen, which reacted with another radical species in the presence of a proton source to afford the corresponding propargylic homo-coupled product (Fig. 8b). However, such compounds were not obtained at all, both for the reaction of 3a with 4a (Table 1, entry 8) or for the reaction of 1a with 4a in the present reaction conditions except for using [{Cp*RuCl(μ-SMe)}2] as a ruthenium catalyst instead of [Ru]-1 or [Ru]-2. The corresponding propargylic radicals were also not trapped by TEMPO (Fig. 8b), which may support the reaction pathway via the attack of benzyl radical at first, followed by the one-electron reduction. However, we cannot exclude the possibility of the other reaction pathway only by the TEMPO experimental result.
Based on the mechanistic studies, a plausible catalytic reaction pathway can be drawn as depicted in Fig. 9a, containing two catalytic cycles: the photoredox catalytic cycle and the ruthenium catalytic cycle. In the photoredox cycle, the iridium catalyst fac-[Ir(ppy)3] ([Ir]) is excited under visible light irradiation to afford a photoexcited iridium catalyst ([Ir]*). Then, a SET process between [Ir]* and 4-alkyl-1,4-dihydropyridine (4) occurs to give the reduced iridium catalyst ([Ir]–), an alkyl radical (R•), and a pyridinium cation (PyH+) via C−C bond scission. On the other hand, in the ruthenium cycle, coordination of the propargylic alcohol (3) to the coordinatively unsaturated species (G), obtained by the reaction of [Ru]-1 or [Ru]-2 with NH4BF4, occurs first to afford the π-alkyne complex (H), leading to the formation of the vinylidene complex (I) via 1,2-proton transfer. Then, dehydration accelerated by BF3·Et2O occurs to give the allenylidene complex (J). Then, alkyl radical attacks at the γ-position of the allenylidene ligand of J from Re face to afford the alkynyl radical complex (K). The alkynyl radical complex K, which is stabilized by the diruthenium core acting as an electron pool to furnish the radical redox reaction, is then reduced by the iridium(II) species [Ir]– through single electron transfer to afford the alkynyl complex (L). Then protonation at the β-position occurs, presumably by the pyridinium cation PyH+ to afford the vinylidene complex (M), followed by the proton transfer to afford the π-alkyne complex (N). Finally, ligand exchange with propargylic alcohol 3 gives the product (7) and regenerates the starting π-alkyne complex H via the formation of coordinatively unsaturated complex G.
As for the asymmetric induction, the enantioselectivity of the products presented in this manuscript is the same as that observed for the enantioselective propargylic phosphinylation of propargylic alcohols bearing both aryl and CF3 moieties at the propargylic position with phosphorus-centered nucleophiles in the presence of [Ru]-1 or [Ru]-2 as a catalyst13. The mechanism of asymmetric induction has been recently elucidated in details by DFT calculations for the enantioselective nucleophilic propargylic substitution reaction of 3a with a nucleophile in the presence of [Ru]-2 as a catalyst, which has revealed the existence of intramolecular π/π and C–H/F interactions among the allenylidene, the optically active thiolate, and pentamethylcyclopentadienyl ligands in the diruthenium–allenylidene complex34. Accordingly, alkyl radicals can almost only approach from the Re face of the diruthenium–allenylidene intermediate (from backward in Fig. 9b), similarly to that proposed for the enantioselective propargylic substitution reactions of derived propargylic alcohols bearing both aryl and CF3 moieties with nucleophiles34, leading to the formation of (R)-isomer as the major product.
Discussion
We have developed the photoredox- and diruthenium-catalyzed enantioselective propargylic alkylation of propargylic alcohols with 4-alkyl-1,4-dihydropyridines under ambient conditions and visible light irradiation. Especially, we have succeeded in the construction of a quaternary stereogenic carbon center at the propargylic position. This is the first successful example of transition metal-catalyzed enantioselective propargylic substitution reactions with free radicals, demonstrating that free radicals generated by the photoredox catalysis can act as formal nucleophilic reagents to afford substituted products that cannot be achieved by reactions with ionic nucleophiles. The key reaction step is the attack of an alkyl radical to the γ-carbon of the ruthenium-allenylidene complex bearing optically active thiolate ligands. The dual photoredox- and diruthenium-catalytic system has also controlled the radical reactions with the photoredox catalyst generating free radicals and the diruthenium catalyst capturing free radicals, keeping the concentration of free radicals constantly low. The usage of 4-alkyl-1,4-dihydropyridine skeletons as milder alkylation reagents has successfully expanded toward the enantioselective propargylic substitution reactions, adding an entry for enantioselective radical processes1,2. We believe this work provides another fundamental method for organic syntheses under mild reaction conditions.
Methods
General procedure for the preparation of chiral propargylic alkylation products
In an oven dried 20 mL Schlenk flask were placed [Ru]-2 (6.3 mg, 0.0050 mmol) and NH4BF4 (1.1 mg, 0.010 mmol) under N2. Anhydrous ClCH2CH2Cl (2.0 mL) was added, and then the mixture was magnetically stirred at room temperature for 30 min. Then, 1,1,1-trifluoro-2-phenylbut-3-yn-2-ol (3a) (20.0 mg. 0.10 mmol), diethyl 4-benzyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate (4a) (41.2 mg, 0.12 mmol), fac-[Ir(ppy)3] (0.7 mg, 0.0011 mmol), and BF3·Et2O (15 μL, 17 mg, 0.12 mmol) were added under N2 at room temperature. The reaction flask was placed in an As One LTB-125 constant low temperature water bath set at 25 °C, and was illuminated from the bottom of the bath with an Aitech System TMN100 × 120–22WD 12 W white LED lamp (400–750 nm) at a distance of ~2 cm from the light source for 48 h. The volatiles were removed in vacuo, and the residue was purified by column chromatography (SiO2) with n-hexane as an eluent to afford (R)-(2-(trifluoromethyl)but-3-yne-1,2-diyl)dibenzene ((R)-7aa) as a colorless oil (22.2 mg, 0.081 mmol, 81% yield based on the amount of 3a). The enantiomeric excess of 7aa was determined by HPLC analysis; DAICEL Chiralpak OJ-H, hexane/iPrOH = 99/1, flow rate = 0.5 mL/min, λ = 220 nm, retention time: 14.6 min (major) and 19.9 min (minor), 94% ee.
Data availability
The crystallographic data for the structure generated in this study have been deposited at the Cambridge Crystallographic Data Centre under deposition number CCDC 2172478 ((R)-7lr). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/.
References
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Kazmaier, U. Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis (Top Organomet. Chem. 38). (Springer, 2012).
Pàmies, O. et al. Recent advances in enantioselective Pd-catalyzed allylic substitution: from design to applications. Chem. Rev. 121, 4373–4505 (2021).
Huang, H.-M., Bellotti, P. & Glorius, F. Transition metal-catalysed allylic functionalization reactions involving radicals. Chem. Soc. Rev. 49, 6186–6197 (2020).
Zhang, H.-H., Zhao, J.-J. & Yu, S. Enantioselective allylic alkylation with 4-alkyl-1,4-dihydro-pyridines enabled by photoredox/palladium cocatalysis. J. Am. Chem. Soc. 140, 16914–16919 (2019).
Crisenza, G. E. M., Faraone, A., Gandolfo, E., Mazzarella, D. & Melchiorre, P. Catalytic asymmetric C–C cross-couplings enabled by photoexcitation. Nat. Chem. 13, 575–580 (2021).
Xue, S., Limburg, B., Ghorai, D., Benet-Buchholz, J. & Kleij, A. W. Asymmetric synthesis of homoallylic alcohols featuring vicinal tetrasubstituted carbon centers via dual Pd/photoredox catalysis. Org. Lett. 23, 4447–4451 (2021).
Song, C., Zhang, H.-H. & Yu, S. Regio- and enantioselective decarboxylative allylic benzylation enabled by dual palladium/photoredox catalysis. ACS Catal. 12, 1428–1432 (2022).
Inada, Y., Nishibayashi, Y. & Uemura, S. Ruthenium-catalyzed asymmetric propargylic substitution reactions of propargylic alcohols with acetone. Angew. Chem. Int. Ed. 44, 7715–7717 (2005).
Kanao, K., Tanabe, Y., Miyake, Y. & Nishibayashi, Y. Intramolecular edge-to-face aromatic π−π interaction in optically active ruthenium−allenylidene complexes for enantioselective propargylic substitution reactions. Organometallics 29, 2381–2384 (2010).
Liu, S., Tanabe, Y., Kuriyama, S., Sakata, K. & Nishibayashi, Y. Ruthenium-catalyzed enantioselective propargylic phosphinylation of propargylic alcohols with phosphine oxides. Angew. Chem. Int. Ed. 60, 11231–11236 (2021).
Detz, R. J., Delville, M. M. E., Hiemstra, H. & van Maarseveen, J. H. Enantioselective copper-catalyzed propargylic amination. Angew. Chem. Int. Ed. 47, 3777–3780 (2008).
Hattori, G., Matsuzawa, H., Miyake, Y. & Nishibayashi, Y. Copper-catalyzed asymmetric propargylic substitution reactions of propargylic acetates with amines. Angew. Chem. Int. Ed. 47, 3781–3783 (2008).
Hattori, G. et al. Copper-catalyzed enantioselective propargylic amination of propargylic esters with amines: copper−allenylidene complexes as key intermediates. J. Am. Chem. Soc. 132, 10592–10608 (2010).
Li, R.-Z., Liu, D.-Q. & Niu, D. Asymmetric O-propargylation of secondary aliphatic alcohols. Nat. Catal. 3, 672–680 (2020).
Wen, Y.-H., Zhang, Z.-J., Li, S., Song, J. & Gong, L.-Z. Stereoconvergent propargylic alkylation of enals via cooperative NHC and copper catalysis. Nat. Commun. 13, 1344 (2022).
Pu, X., Dang, Q.-D., Yang, L., Zhang, X. & Niu, D. Doubly stereoconvergent construction of vicinal all-carbon quaternary and tertiary stereocenters by Cu/Mg-catalyzed propargylic substitution. Nat. Commun. 13, 2457 (2022).
Qian, H.-D. et al. Catalytic asymmetric vinylogous and bisvinylogous propargylic substitution. J. Am. Chem. Soc. 144, 15779–15785 (2022).
Watanabe, K. et al. Nickel-catalyzed asymmetric propargylic amination of propargylic carbonates bearing an internal alkyne group. Org. Lett. 20, 5448–5451 (2018).
Chang, X., Zhang, J., Peng, L. & Guo, C. Collective synthesis of acetylenic pharmaceuticals via enantioselective nickel/Lewis acid-catalyzed propargylic alkylation. Nat. Commun. 12, 299 (2021).
Hu, Q., He, Z., Peng, L. & Guo, C. Combining nickel and squaramide catalysis for the stereodivergent α-propargylation of oxindoles. Nat. Synth. 1, 322–331 (2022).
Roh, S. W., Choi, K. & Lee, C. Transition metal vinylidene- and allenylidene-mediated catalysis in organic synthesis. Chem. Rev. 119, 4293–4356 (2019).
Tsuji, H. & Kawatsura, M. Transition-metal-catalyzed propargylic substitution of propargylic alcohol derivatives bearing an internal alkyne group. Asian Org. Chem. 9, 1924–1941 (2020).
Nishibayashi, Y. Development of asymmetric propargylic substitution reactions using transition metal catalysts. Chem. Lett. 50, 1282–1288 (2021).
Nakajima, K., Nojima, S., Sakata, K. & Nishibayashi, Y. Visible-light-mediated aromatic substitution reactions of cyanoarenes with 4-alkyl-1,4-dihydropyridines through double carbon–carbon bond cleavage. ChemCatChem 8, 1028–1032 (2016).
Gu, F., Huang, W., Liu, X., Chen, W. & Cheng, X. Substituted Hantzsch esters as versatile radical reservoirs in photoredox reactions. Adv. Synth. Catal. 360, 925–931 (2018).
Wang, P.-Z., Chen, J.-R. & Xiao, W.-J. Hantzsch esters: an emerging versatile class of reagents in photoredox catalyzed organic synthesis. Org. Biomol. Chem. 17, 6936–6951 (2019).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Ammal, S. C., Yoshikai, N., Inada, Y., Nishibayashi, Y. & Nakamura, E. Synergistic dimetallic effects in propargylic substitution reaction catalyzed by thiolate-bridged diruthenium complex. J. Am. Chem. Soc. 127, 9428–9438 (2005).
Sakata, K. & Nishibayashi, Y. Mechanism and reactivity of catalytic propargylic substitution reactions via metal–allenylidene intermediates: a theoretical perspective. Catal. Sci. Technol. 8, 12–25 (2018).
Sakata, K., Goto, Y. & Nishibayashi, Y. Enantioselectivity in ruthenium-catalyzed propargylic substitution reactions of propargylic alcohols with acetone: a DFT study. Chem. Asian J. 16, 3760–3766 (2021).
Sakata, K., Uehara, Y., Kohara, S., Yoshikawa, T. & Nishibayashi, Y. Effect of propargylic substituents on enantioselectivity and reactivity in ruthenium-catalyzed propargylic substitution reactions: a DFT study. ACS Omega 7, 36634–36642 (2022).
Allen, A. E. & MacMillan, D. W. C. Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci. 3, 633–658 (2012).
Kim, U. B., Jung, D. J., Jeon, H. J., Rathwell, K. & Lee, S. Synergistic dual transition metal catalysis. Chem. Rev. 120, 13382–13433 (2020).
Zhou, Z.-Z., Jiao, R.-Q., Yang, K., Chen, X.-M. & Liang, Y.-M. Photoredox/palladium co-catalyzed propargylic benzylation with internal propargylic carbonates. Chem. Commun. 56, 12957–12960 (2020).
Guo, Q. et al. Visible-light promoted regioselective amination and alkylation of remote C(sp3)-H bonds. Nat. Commun. 11, 1463 (2020).
Nakajima, K., Nojima, S. & Nishibayashi, Y. Nickel- and photoredox-catalyzed cross-coupling reactions of aryl halides with 4-alkyl-1,4-dihydropyridines as formal nucleophilic alkylation reagents. Angew. Chem. Int. Ed. 55, 14106–14110 (2016).
Nakajima, K., Guo, X. & Nishibayashi, Y. Cross-coupling reactions of alkenyl halides with 4-benzyl-1,4-dihydropyridines associated with E to Z isomerization under nickel and photoredox Catalysis. Chem. Asian J. 13, 3653–3657 (2018).
Zhang, Y., Tanabe, Y., Kuriyama, S. & Nishibayashi, Y. Cooperative photoredox- and nickel-catalyzed alkylative cyclization reactions of alkynes with 4-alkyl-1,4-dihydropyridines. J. Org. Chem. 86, 12577–12590 (2021).
Zhang, Y., Tanabe, Y., Kuriyama, S. & Nishibayashi, Y. Photoredox- and nickel-catalyzed hydroalkylation of alkynes with 4-alkyl-1,4-dihydropyridines: ligand-controlled regioselectivity. Chem. Eur. J. 28, e202200727 (2022).
Zhou, Z.-Z. et al. Photoredox/nickel dual-catalyzed regioselective alkylation of propargylic carbonates for trisubstituted allenes. Chem. Commun. 57, 9390–9393 (2021).
Onodera, G., Nishibayashi, Y. & Uemura, S. Ruthenium-catalyzed reductive coupling reaction of propargylic alcohols via hydroboration of allenylidene intermediates. Organometallics 25, 35–37 (2006).
Lu, F.-D. et al. Asymmetric propargylic radical cyanation enabled by dual organophotoredox and copper catalysis. J. Am. Chem. Soc. 141, 6167–6172 (2019).
Lu, R. et al. Enantioselective copper-catalyzed radical cyanation of propargylic C–H bonds: easy access to chiral allenyl nitriles. J. Am. Chem. Soc. 143, 14451–14457 (2021).
Wang, F., Chen, P. & Liu, G. Copper-catalysed asymmetric radical cyanation. Nat. Synth. 1, 107–116 (2022).
Liu, Q., Zheng, J., Zhang, X. & Ma, S. Photo and copper dual catalysis for allene syntheses from propargylic derivatives via one-electron process. Nat. Commun. 13, 3302 (2022).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Miroshnichenko, E. A. et al. Radical reorganization energies. Russ. J. Phys. Chem. B 13, 225–230 (2019).
Stang, P. J. & Diederich, F. Modern Acetylene Chemistry (Wiley, 2008).
Jiang, H, Zhu C. & Wu, W. Preparation of haloalkynes. In Haloalkyne Chemistry (eds. Jiang, H, Zhu C. & Wu, W.) 5–7 (Springer, 2016).
Sonogashira, K. Palladium-catalyzed alkynylation: Sonogashira alkyne synthesis. Handb. Organopalladium Chem. Org. Synth. 1, 493–529 (2002).
King, A. O. & Shinkai, I. Palladium on carbon. e-EROS Encycl. Reagents Org. Synth. https://doi.org/10.1002/047084289X.rp006 (2001).
Nishibayashi, Y. et al. Ruthenium-catalyzed propargylic substitution reactions of propargylic alcohols with oxygen-, nitrogen-, and phosphorus-centered nucleophiles. Chem. Eur. J. 11, 1433–1451 (2005).
Yasu, Y., Koike, T. & Akita, M. Visible light-induced selective generation of radicals from organoborates by photoredox catalysis. Adv. Synth. Catal. 354, 3414–3420 (2012).
Schley, N. D. & Fu, G. C. Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation. J. Am. Chem. Soc. 136, 16588–16593 (2014).
Huo, H., Gorsline, B. J. & Fu, G. C. Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles. Science 367, 559–564 (2020).
Smith, S. W. & Fu, G. C. Nickel-catalyzed asymmetric cross-couplings of racemic propargylic halides with arylzinc reagents. J. Am. Chem. Soc. 130, 12645–12647 (2008).
Oelke, A. J., Sun, J. & Fu, G. C. Nickel-catalyzed enantioselective cross-couplings of racemic secondary electrophiles that bear an oxygen leaving group. J. Am. Chem. Soc. 134, 2966–2969 (2012).
Miyake, Y., Endo, S., Nomaguchi, Y., Yuki, M. & Nishibayashi, Y. Novel monophosphido-bridged diruthenium complexes: efficient preparative method and catalytic activity toward reactions of propargylic alcohols with aromatic compounds. Organometallics 27, 4017–4020 (2008).
Miyake, Y., Endo, S., Yuki, M., Tanabe, Y. & Nishibayashi, Y. Synthesis and reactivity of hybrid phosphido- and thiolato-bridged diruthenium complexes. Organometallics 27, 6039–6042 (2008).
Cismesia, M. A. & Yoon, T. P. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 6, 5426–5436 (2015).
Kärkäs, M. D., Matsuura, B. S. & Stephenson, C. R. J. Enchained by visible light–mediated photoredox catalysis: radical-chain processes can dominate the kinetics of photogenerated radical catalysis. Science 349, 1285–1286 (2015).
Nakajima, K., Miyake, Y. & Nishibayashi, Y. Synthetic utilization of α-aminoalkyl radicals and related species in visible light photoredox catalysis. Acc. Chem. Res. 49, 1946–1956 (2016).
Ong, K. C., Douglas, B. & Robinson, R. A. Dissociation constants of pyridinepentacarboxylic acid and other pyridinecarboxylic acids. J. Chem. Eng. Data 11, 574–576 (1966).
Rigaut, S., Maury, O., Touchard, D. & Dixneuf, P. H. New paramagnetic ruthenium complexes via one-electron reduction of metallacumulenes. Chem. Commun. 37, 373–374 (2001).
Rigaut, S., Monnier, F., Mousset, F., Touchard, D. & Dixneuf, P. H. Ruthenium acetylide oxidation: from stable radicals to allenylidene synthesis via γ-elimination of H+. Organometallics 21, 2654–2661 (2002).
Rigaut, S. et al. Bis-allenylidene metal complex and unique related radical with delocalization of one electron over both trans carbon-rich chains. J. Am. Chem. Soc. 126, 4072–4073 (2004).
Acknowledgements
We acknowledge Grants-in-Aid for Scientific Research (Grants 20H05671, 20K05680, 20K21203, and 22K19041) from JSPS and MEXT. Y.Z. is a recipient of the JSPS Research Fellowships for Young Scientists.
Author information
Authors and Affiliations
Contributions
Y.Z., Y.T., and Y.N. designed and analyzed the experiments. Y.Z. ran all experiments. Y.T. conducted X-ray crystallographic and CV measurements. K.S. conducted DFT calculations. Y.Z., Y.T., K.S., and Y.N. wrote the manuscript. Y.Z., Y.T., S.K., K.S., and Y.N. contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Tanabe, Y., Kuriyama, S. et al. Interplay of diruthenium catalyst in controlling enantioselective propargylic substitution reactions with visible light-generated alkyl radicals. Nat Commun 14, 859 (2023). https://doi.org/10.1038/s41467-023-36453-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-36453-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.