Abstract
Cardiac valves ensure unidirectional blood flow through the heart, and altering their function can result in heart failure. Flow sensing via wall shear stress and wall stretching through the action of mechanosensors can modulate cardiac valve formation. However, the identity and precise role of the key mechanosensors and their effectors remain mostly unknown. Here, we genetically dissect the role of Pkd1a and other mechanosensors in atrioventricular (AV) valve formation in zebrafish and identify a role for several pkd and piezo gene family members in this process. We show that Pkd1a, together with Pkd2, Pkd1l1, and Piezo2a, promotes AV valve elongation and cardiac morphogenesis. Mechanistically, Pkd1a, Pkd2, and Pkd1l1 all repress the expression of klf2a and klf2b, transcription factor genes implicated in AV valve development. Furthermore, we find that the calcium-dependent protein kinase Camk2g is required downstream of Pkd function to repress klf2a expression. Altogether, these data identify, and dissect the role of, several mechanosensors required for AV valve formation, thereby broadening our understanding of cardiac valvulogenesis.
Similar content being viewed by others
Introduction
Congenital heart disease is the most common congenital disorder in newborns, with an incidence of approximately 1% of live births1. The most frequent congenital heart defects are cardiac valve abnormalities, which often originate from early developmental defects and are caused by mutations in genes conserved across vertebrates2. Cardiac valves are flexible structures that sit at the boundaries between the chambers. Their malfunction, leading to phenotypes such as regurgitation and stenosis, is one of the primary causes of heart failure3. Thus, a better understanding of the causes of congenital valve disorders will help address a major public health issue.
The zebrafish is a model of choice to study valve development because of its fast organogenesis and ease of optical approach implementation4. The zebrafish heart is two-chambered and comprises three types of valves: the sinus venosus, atrioventricular (AV), and outflow tract (OFT) valves5. Cardiac valves differentiate from endocardial cells, which line the lumen of the heart, shortly after the heart starts beating. Their formation depends upon the hemodynamic forces applied on the endocardial layer6,7. Wall shear stress (WSS) and radial wall pressure, which induces endocardial cell stretching, are the main driving force for valve differentiation and elongation8,9,10,11,12. Although the genetics of flow-inducible genes have been extensively studied13,14,15,16,17, relatively little is known about the upstream blood flow-sensing mechanisms.
Endocardial cells are sensitive to the subtle differences in force that accompany heart morphogenesis. Several families of mechanosensitive molecules are involved in vascular development, including ion channels, integrins, junctional complexes, G protein-coupled receptors, and the glycocalyx14. During cardiac valve formation specifically, membrane-localized mechanosensitive channels, which are in direct contact with the blood flow, control valve elongation. Studies in zebrafish have shown that these mechanosensors, Pkd2 and Trpv4 in all cardiac valves and Piezo1 and Piezo2a in the OFT valves, tightly regulate klf2a expression10,11,18, and that they subsequently control the endocardial and myocardial expression of other genes important for valvulogenesis7,19,20,21,22. In addition, it was also recently reported that the adenosine triphosphate (ATP)–dependent purinergic receptor pathway works in parallel to these channels as a blood flow mechanosensor to mediate AV valve formation12. However, the loss of any of these mechanosensors induces only mild phenotypes10,11,12 compared with abnormal fluid flow conditions in the heart6,23, suggesting the existence of additional mechanosensitive molecules required for valve development.
To test this hypothesis, we provide in this study genetic evidence that pkd1a, an orthologue of human PKD1, which is one of the most commonly mutated genes in Autosomal Dominant Polycystic Kidney Disease24, is essential for AV valve elongation in zebrafish. We identify a synergistic contribution of Pkd1a and other Pkd family members, namely Pkd2 and Pkd1l1, in blood flow-driven valve development, independent of their role in cardiac looping. We further show that the Piezo family members Piezo1 and Piezo2a are also important in AV valve formation in addition to their role in OFT valve development and that they cooperate with Pkd1a during this process. Using high-resolution imaging, we find that these pkd genes control calcium influx as well as klf2a and klf2b expression in the AV endocardial cells. Using a pkd phenotypic rescue assay, we identify the Ca2+/calmodulin-dependent protein kinases Camk2g1 and Camk2g2 as intermediates of the Pkd mechanosensory response in AV valve formation. Taken together, our findings identify major regulators of AV valve development, thereby providing new potential therapeutic targets for congenital valve disorders.
Results
Pkd1a is required for atrioventricular valve development
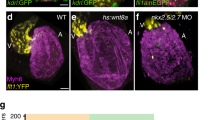
Zebrafish pkd1a mutants display pericardial edema and lymphatic defects at 4 days post-fertilization (dpf) while retaining blood circulation25. To investigate the role of Pkd1a in AV valve development, independently of its function in cardiac and lymphatic morphogenesis, we first characterized the onset of its cardiac phenotype. To do so, we used a previously described mutant allele (hu5855)25 as well as a newly generated mutant allele (bns507) (Fig. S1a). Both mutations lead to a premature termination codon and the disruption of the conserved polycystic cation channel domain (PCCD)26. We found that pkd1a mutants, of both alleles, exhibit wild-type-like morphology (Fig. 1a–c) and cardiac chamber size (Fig. 1d–f) at 78 hours post-fertilization (hpf), but clear defects at 102 hpf, when the cardiac chambers start to collapse (Fig. 1a′–f′) as shown before25. To investigate this phenotype, we first measured the diameter of the cardiac chambers during atrial diastole and systole (Fig. S1b–f) at 78 hpf, and observed no significant differences between pkd1a mutants and wild types (Fig. S1g, h), in contrast to what is observed in tnnt2a mutants, where the atrium is distended27. We then analyzed the blood flow profile across the AV canal in single beating cycles between 54 and 108 hpf, starting with wild-type embryos and larvae. We found that retrograde blood flow movements are almost absent at 102 hpf (Supplementary Movie 3), while strong at 54 and 78 hpf (Supplementary Movies 1, 2), and that following AV valve formation and the reduction of retrograde movements, a no-flow (i.e., no anterograde or retrograde flow) period starts to appear at 78 hpf (Fig. 1g and Supplementary Movie 2). Therefore, we selected 78 hpf to study the pkd1a mutant phenotype, which is the latest stage when pkd1a mutants display a wild-type-like cardiac chamber size. At this stage and at the AV canal, pkd1a mutant larvae, similarly to klf2a; klf2b double mutant larvae, display an increased incidence in retrograde blood flow and a decreased incidence in no-flow (Fig. 1h and Supplementary Movie 4), while maintaining a normal heartbeat (Fig. S1i). We also analyzed AV valve morphology in live pkd1a mutant larvae and found that only 66% exhibited an elongated superior valve leaflet compared with 87% in wild-types (Fig. 1i–k). We then quantified the number of cells present in the superior valve leaflet and found no significant reduction in pkd1a mutants compared with wild type (Fig. 1l–n). Altogether, these data indicate that the Pkd1a function is required for early AV valve development.
a–c′ Wild-type (a-a′) like morphology of pkd1ahu5855 (b-b′) and pkd1abns507 (c-c′) mutants at 78 but not 102 hpf; arrows point to craniofacial defects and pericardial edema. d–f′ Brightfield images of 78 and 102 hpf wild-type (d-d′), pkd1ahu5855 mutant (e-e′), and pkd1abns507 mutant (f-f′) hearts; one time point of a spinning disc movie at atrial diastole; green and red lines outline the heart. g AV flow profile of 54, 78, and 102 hpf wild-type zebrafish; average profile of three cardiac cycles per zebrafish. h AV flow profile of 78 hpf wild-type, pkd1ahu5855 mutant, pkd1abns507 mutant, and klf2a; klf2b double mutant larvae; average profile of three cardiac cycles per zebrafish. i–j′ Confocal imaging of 78 hpf wild-type (i-i′) and pkd1a mutant (j-j′) hearts stained with Bodipy FL C5-Ceramide; i′, j′ are AV canal magnified images of i, j. k Superior AV valve elongation of 78 hpf wild-type and pkd1a mutant larvae. l–m′ Confocal imaging of 78 hpf wild-type (l-l′) and pkd1a mutant (m-m′) hearts immunostained for MHC and Fli1; l′,m′ are AV canal magnified images of l,l. n Superior AV valve cell number in 78 hpf wild types and pkd1a mutants. Hearts imaged in ventral view, anterior to the top, ventricle (V) on the left, and atrium (A) on the right. The center of the box-and-whisker plots represents the median; each of the box-and-whiskers represents a quartile; “+” represents the mean in g, h. The center of the error bar represents the mean in k, n. Error bars indicate s.d in n. P values were calculated using a two-sided Student’s t-test in g, h, n; a two-sided Fisher’s exact test in k; and are relative to wild type. Scale bars: 200 µm in a–c′; 50 µm in d–f′, i, j, l, m; 10 µm in i′, j′, l′, m′. Source data are provided as a Source Data file.
Pkd1a, 2, and 1l1 cooperate to promote AV valvulogenesis
Compared with blood flow-defective conditions, such as those observed in tnnt2a mutants6,10,23, pkd1a mutants display only a mild AV valve phenotype (Fig. 1i–n), suggesting that other mechanosensory genes also regulate AV valve development. Pkd1a has been described to bind Pkd228, another Pkd member involved in AV valve development10. Moreover, Pkd2 has been shown to be complex with Pkd1l1 to control flow mechanosensation in the vertebrate left/right organizer29,30. We thus investigated whether mutant combinations for these three genes displayed a stronger valve phenotype than observed in pkd1a single mutants. For this analysis, we used a previously described pkd2 mutant allele (tc321)31 (Fig. S2a) and generated a frameshift allele for pkd1l1 (bns394) (Fig. S2a′). Both mutations lead to a premature termination codon and the disruption of the PCCD domain26. We found that pkd1l1, but not pkd2, mRNA levels are upregulated in pkd1a mutants (Fig. S2b), suggesting genetic compensation via transcriptional adaptation (TA)32. pkd1l1 mutant animals display the left/right (L/R) asymmetry phenotype previously reported in medaka and mice, with randomized cardiac looping29,30, as do pkd2 mutants31 (Fig. S2c–g). Therefore, every mutant combination that involves pkd2 or pkd1l1 leads to randomized cardiac looping (Fig. S2c–g). As it was previously shown that cardiac looping defects can correlate with early AV valve defects8, we sorted and analyzed only the larvae exhibiting wild-type cardiac looping for all subsequent experiments that involve pkd2 or pkd1l1 mutants. We show that these sorted double and triple pkd mutants display a wild-type-like cardiac chamber size (Fig. 2a–d and Fig. S2h, i), and beating frequency (Fig. S2j) at 78 hpf. When analyzing the AV flow profile of the pkd mutant combinations, we found that all double mutant combinations cause the same retrograde blood flow defects as pkd1a mutations do (Fig. 2e). However, pkd triple mutants exhibit an increased incidence in retrograde blood flow compared with pkd1a; pkd2 double mutants (Fig. 2e and Supplementary Movie 5). Live imaging of the AV valve reveals that superior valve leaflet elongation is almost completely abolished in pkd triple mutant larvae (Fig. 2f–l). We also quantified the number of cells present in the superior valve leaflet and found no significant reduction in pkd double or triple mutants (Fig. 2m–s); in addition, valve luminal and abluminal cells are present in pkd triple mutants (Fig. 2t, u). These data show that Pkd1a, Pkd2, and Pkd1l1 cooperate during AV valve development and are required to promote AV valve elongation.
a–d Brightfield images of 78 hpf wild-type (a), pkd1a mutant (b), pkd1a; pkd2 double mutant (c), and pkd triple mutant (d) hearts; one time point of a spinning disc movie at atrial diastole; green lines outline the heart. e AV flow profile of 78 hpf wild-type, pkd1a mutant, pkd2 mutant, pkd1l1 mutant, pkd double mutant, and pkd triple mutant larvae; average profile of three cardiac cycles per zebrafish. f–k Confocal imaging of 78 hpf wild-type (f), pkd1a mutant (g), pkd double mutant (h–j), and pkd triple mutant (k) hearts stained with Bodipy FL C5-Ceramide; AV canal magnified images. l Superior AV valve elongation of 78 hpf wild-type, pkd1a mutant, pkd double mutant, and pkd triple mutant hearts. m–r Confocal imaging of 78 hpf wild-type (m), pkd1a mutant (n), pkd double mutant (o–q), and pkd triple mutant (r) hearts immunostained for MHC and Fli1; AV canal magnified images. s Superior AV valve cell number in 78 hpf wild types, pkd1a mutants, pkd double mutants, and pkd triple mutants. t, u Confocal imaging of 78 hpf wild-type (t) and pkd triple mutant (u) hearts immunostained for Alcama and Fli1; 10/10 of the wild-type and 9/9 of the pkd triple mutant larvae display luminal and abluminal superior valve cells; AV canal magnified images. Hearts imaged in ventral view, anterior to the top, ventricle (V) on the left and atrium (A) on the right. The center of the box-and-whisker plot represents the median; each of the box-and-whiskers represents a quartile; “+” represents the mean in e. The center of the error bar represents the mean in l, s. Error bars indicate s.d in s. P values were calculated using a two-sided Student’s t-test in e, s; a two-sided Fisher’s exact test in l; and are relative to wild type except when a horizontal bar is used. Scale bars: 50 µm in a–d; 10 µm in f–k, m–r, t, u. Source data are provided as a Source Data file.
Piezo family members are also involved in AV valvulogenesis
Another family of membrane-bound mechanosensors, the Piezo family, has been shown to control OFT valve formation11,18. However, their function in AV valve formation and their interaction with Pkd family members remain unexplored. Here, we have generated mutants for piezo1 (bns340) (Fig. S3a) and piezo2a (bns367) (Fig. S3a′) that carry a deletion of two amino acids in the conserved PFEW domain, which is hypothesized to be involved in channel conductance or gating33. We selected in-frame alleles to try and minimize genetic compensation via TA34, as described for some piezo1 mutants35. We found that piezo1 and piezo2a mRNA are enriched in the AV canal at 78 hpf (Fig. S3k, l) and that piezo1 and piezo2a mRNA levels are unchanged in pkd1a mutants (Fig. S2b). As with pkd2 and pkd1l1 mutants (Fig. S2g), we found that mutants for piezo2a, but not piezo1, exhibit cardiac looping defects (Fig. S3c–g). We thus used larvae exhibiting wild-type cardiac looping for all subsequent experiments involving piezo2a mutants. Furthermore, we found that injecting pkd1a morpholinos (MOs) in piezo2a mutants enhances the cardiac looping defects, in comparison to injecting them into piezo1 mutants (Fig. S3c–g), suggesting cooperation between pkd1a and piezo2a in regulating cardiac morphogenesis. We found that piezo1 and piezo2a mutants exhibit a wild-type-like cardiac chamber size (Fig. 3a–d and Fig. S3h, i), and beating frequency (Fig. S3j) at 78 hpf. We analyzed the AV blood flow profile in piezo1 and piezo2a mutants, and observed an increased incidence in retrograde movements, comparable with those observed in pkd1a mutants (Fig. 3e). Notably, piezo2a; pkd1a double mutants exhibit stronger blood flow defects than the single mutants, suggesting cooperation between pkd and piezo genes during AV valve development (Fig. 3e). Similarly, while piezo1 mutants and piezo2a mutants display valve elongation defects, piezo2a; pkd1a double mutants exhibit stronger defects than the single mutants (Fig. 3f–j). We also quantified the number of cells present in the superior valve leaflet and found no significant reduction in piezo1 mutants, piezo2a mutants, or piezo2a; pkd1a double mutants (Fig. 2k–o). Taken together, our data show that Piezo1 and Piezo2a play an important role during AV valvulogenesis and that Piezo and Pkd family members cooperate in this process.
a–d Brightfield images of 78 hpf wild-type (a), piezo1 mutant (b), piezo2a mutant (c), and piezo2a; pkd1a double mutant (d) hearts; one time point of a spinning disc movie at atrial diastole; green lines outline the heart. e AV flow profile of 78 hpf wild-type, piezo1 mutant, pkd1a mutant, piezo2a mutant, and piezo2a; pkd1a double mutant larvae; average profile of three cardiac cycles per zebrafish. f–i Confocal imaging of 78 hpf wild-type (f), piezo1 mutant (g), piezo2a mutant (h), and piezo2a; pkd1a double mutant (i) hearts stained with Bodipy FL C5-Ceramide; AV canal magnified images. j Superior AV valve elongation of 78 hpf wild types, piezo1 mutants, pkd1a mutants, piezo2a mutants, and piezo2a; pkd1a double mutants. k–n Confocal imaging of 78 hpf wild-type (k), piezo1 mutant (l), piezo2a mutant (m), and piezo2a; pkd1a double mutant (n) hearts immunostained for MHC and Fli1; AV canal magnified images; ventral views, anterior to the top, ventricle (V) on the left and atrium (A) on the right. o Superior AV valve cell number in 78 hpf wild types, piezo1 mutants, pkd1a mutants, piezo2a mutants, and piezo2a; pkd1a double mutants. Hearts imaged in ventral views, anterior to the top, ventricle (V) on the left, and atrium (A) on the right. The center of the box-and-whisker plot represents the median; each of the box-and-whiskers represents a quartile; “+” represents the mean in e. The center of the error bar represents the mean in j, o. Error bars indicate s.d in o. P values were calculated using a two-sided Student’s t-test in e, o; a two-sided Fisher’s exact test in j; and are relative to wild type except when a horizontal bar is used. Scale bars: 50 µm in a–d; 10 µm in f–i, k–n. Source data are provided as a Source Data file.
pkd genes control calcium levels during valvulogenesis
Blood flow regulates intracellular calcium levels in the AV canal via the Trpv4 and Pkd2 mechanosensors to promote valvulogenesis10. We thus investigated whether Pkd1a, 2, and 1l1 also cooperate to control endocardial calcium levels, upstream of AV valve elongation. We first used the fli1a:Gal4FF; UAS:GCamP6s calcium sensor line36 to measure endocardial calcium levels in 78 hpf wild-type larvae, during atrial diastole (Fig. S4a–c) and systole (Fig. S4a′–c′) and found that, as previously reported at 48 hpf10, calcium levels are highest at the AV canal (Fig. S4d and Supplementary Movie 6). Moreover, we also observed higher calcium levels in the presumptive OFT valve area (Fig. S4c and Supplementary Movie 6). We found that both AV and OFT signals were enriched during diastole and systole, without significant differences between the cardiac states (Fig. S4c and Supplementary Movie 6). Strikingly, this calcium elevation was lost specifically in the AV canal in tnnt2a morphants, without a significant decrease in the other cardiac compartments (Fig. 4a–b′, d, e–f′, h and Supplementary Movie 8). pkd triple mutants mimic the tnnt2a morphant condition, with a loss of AV signal while maintaining an elevated calcium level in the ventricle compared with the atrium during atrial diastole (Fig. 4c, d, g, h and Supplementary Movie 7). We then analyzed the expression levels of valve-specific transcription factor genes in the heart. We selected klf2a and klf2b, whose loss is associated with AV valve defects, and which control a large set of endocardial and myocardial genes essential for valve formation7,19,22. We performed Fluorescence In Situ Hybridization (FISH) and observed apparent endocardial upregulation of klf2a (Fig. 5a–d and Fig. S5a–k) and klf2b (Fig. 5e–h and Fig. S5l–s) in pkd triple mutants (Fig. 5c, g), pkd1a; pkd2 double mutants (Fig. S5f, n), and piezo2a; pkd1a double mutants (Fig. S5j, r) compared with pkd1l1 mutants (Fig. 5b, f), pkd2 mutants (Fig. Se, m), pkd1a mutants (Fig. 5i, q), and wild types (Fig. 5a, e and Fig. S5a, d, h, l, p). Notably, the AV signal was stronger in these three conditions compared with the wild types and single mutants (Fig. 5d, h and Fig. S5g, k, o, s). We then performed RT-qPCR on dissected hearts to quantify this differential expression and observed an upregulation of klf2a (Fig. 5i) and klf2b (Fig. 5j) in pkd triple mutants, pkd1a; pkd2 double mutants, and piezo2a; pkd1a double mutants compared with pkd single mutants and wild types. To investigate whether klf2 upregulation could be responsible for the valve elongation phenotype we observed, we injected at the one-cell stage a plasmid encoding UAS:klf2a-p2a-dTomato in nfatc1:Gal4FF positive embryos (Fig. 5k, l). We then sorted the larvae negative for dTomato signal (Fig. 5k) from the positive ones (Fig. 5l) in the superior valve, and found that the positive ones displayed a higher frequency of valve elongation defects than the negative ones. Together, these observations suggest that pkd and piezo genes cooperate to control endocardial calcium levels and negatively regulate the expression of essential AV transcription factor genes.
a–c, e–g Confocal imaging of 78 hpf fli1a:Gal4FF; UAS:GCamP6s wild-type (a, a′, e, e′), tnnt2a morphant (b, b, f, f′) and pkd triple mutant (c, c′, g, g′) hearts during atrial diastole (a–c′) and systole (e–g′); maximum projection of 4D-assembled spinning disc movies. d, h Diameter and endocardial calcium levels along the contracting heart tube in 78 hpf wild-type, tnnt2a morphant, and pkd triple mutant larvae during atrial diastole (d) and systole (h); arrows point to the AV canal calcium peak. Hearts imaged in ventral view, anterior to the top, ventricle (V) on the left, and atrium (A) on the right. Error bars indicate SEM. Scale bars: 50 µm. Source data are provided as a Source Data file.
a–c, e–g Confocal projections of 78 hpf wild-type (a, e), pkd1l1 mutant (b, f), and pkd triple mutant (c, h) hearts stained for MHC, and klf2a (a–c), or klf2b (e–g) expression; ventral views, anterior to the top, ventricle (V) on the left, and atrium (A) on the right. d, h klf2a (d) and klf2b (h) mean fluorescence intensity along the heart tube in 78 hpf wild-type, pkd1l1 mutant, and pkd triple mutant larvae. i, j Relative mRNA levels of klf2a (i) and klf2b (j) in 78 hpf wild-type, pkd1l1 mutant, pkd1a mutant, pkd2 mutant, pkd1a; pkd2 double mutant, pkd triple mutant, and piezo2a; pkd1a double mutant dissected hearts; n = 3 biologically independent samples; Ct values are listed in Supplementary Data 1. k, l Confocal imaging of 78 hpf nfatc1:Gal4FF larvae injected at the one-cell stage with a plasmid encoding a UAS:klf2a-p2a-dTomato, presenting negative (k) or positive (l) superior valve cells, and stained with Bodipy FL C5-Ceramide; 32/34 of the negative larvae and 5/13 of the positive larvae display an elongated superior valve; AV canal magnified images. Hearts imaged in ventral view, anterior to the top, ventricle (V) on the left, and atrium (A) on the right. The center of the error bar represents the mean in i, j. Error bars indicate SEM in d, h; s.d. in i, j. P values were calculated using a two-sided Student’s t-test in i, j and are relative to the wild type. Scale bars: 50 µm in a–c, e–g; 10 µm in k, l. Source data are provided as a Source Data file.
Camk2g is an effector of the Pkd response upstream of klf2a expression
pkd genes control endocardial calcium levels and klf2a expression10. However, the intermediates of this signaling pathway in the AV canal remain unknown. In this context, the Ca2+/calmodulin-dependent (CaM) pathway has been reported to regulate KLF2 mRNA levels in human umbilical vein endothelial cells (HUVECs)37, and CaM protein Kinase II (CaMKII) phosphorylation is reduced in zebrafish pkd2 morphants in two flow responsive tissues, the pronephros38 and the left/right organizer39. Therefore, we decided to test the role of CaMK proteins as potential intermediates of Pkd-driven control of valvulogenesis. In zebrafish, the most strongly expressed camk genes in embryonic endothelial cells are camk2g1 and camk2g240, which we selected for our analysis. CaMKII kinases autophosphorylate at T287 upon calcium stimulation and Camk2gT287D variants are Constitutively Active (CA) even in the absence of calcium38,41. We found that retrograde blood flow movements (Fig. 6a–c) and AV valve elongation defects (Fig. 6d–f) of pkd triple mutants could be partially rescued by injecting mRNA encoding CA zebrafish (z) Camk2g1 or CA human (h) CAMK2G. CA zCamk2g1 overexpression also restored klf2a expression towards wild-type levels in pkd triple mutants (Fig. 6g–i), indicating that Camk2g1 is upstream of klf2a expression during valvulogenesis. To investigate the role of the Camk2g proteins in valve development, we generated frameshift alleles for camk2g1 (bns508) (Fig. S6a) and camk2g2 (bns509) (Fig. S6a′). Both mutations lead to a premature termination codon and the disruption of the conserved CaMKII Association Domain (CaMKII-AD). We analyzed valve-associated defects in camk2g1; camk2g2 double mutants since camk2g2 upregulation can compensate for the loss of camk2g142. We found that camk2g1; camk2g2 double mutants display a wild-type-like cardiac chamber size (Fig. S6b, c), and beating frequency (Fig. S6d), without cardiac looping defects (Fig. 6j, k) at 78 hpf. We then analyzed the AV flow profile as well as valve elongation and found that camk2g1 mutants and camk2g1; camk2g2 double mutants exhibit an increased incidence in retrograde blood flow (Fig. 6j–l) as well as valve elongation defects (Fig. 6m–o). camk2g2 mutants also exhibit valve elongation defects (Fig. 6o) but no increased incidence in retrograde blood flow (Fig. 6i). Altogether, these findings suggest that Camk2g is an important modulator of valvulogenesis downstream of Pkd and upstream of klf2a expression (Fig. 6p).
a, b Brightfield images of 78 hpf hearts from non-injected pkd triple mutant (a) or injected at the one-cell stage with mRNA encoding a CA zCamk2g1 (b). c AV flow profile of 78 hpf pkd triple mutant larvae non-injected or injected at the one-cell stage with mRNA encoding CA zCamk2g1 or CA hCAMK2G. d, e Confocal imaging of hearts from 78 hpf non-injected pkd triple mutant (d) or injected at the one-cell stage with mRNA encoding CA zCamk2g1 (e) and stained with Bodipy. f Superior AV valve elongation of 78 hpf non-injected pkd triple mutants or injected at the one-cell stage with mRNA encoding CA zCamk2g1 or hCAMK2G. g, h Confocal projections of hearts from 78 hpf wild-type (g) and pkd triple mutant injected at the one-cell stage with mRNA encoding CA zCamk2g1 (h), stained for MHC and klf2a expression. i klf2a fluorescence intensity along the heart tube in 78 hpf wild types and pkd triple mutants non-injected or injected at the one-cell stage with mRNA encoding CA zCamk2g1. j, k Brightfield images of 78 hpf wild-type (j) and camk2g1/2 double mutant (k) hearts. l AV flow profile of 78 hpf wild-type, camk2g1 mutant, camk2g2 mutant, and camk2g1/2 double mutant larvae. m, n Confocal imaging of 78 hpf wild-type (m) and camk2g1/2 double mutant (n) hearts stained with Bodipy. o Superior AV valve elongation of 78 hpf wild types and camk2g1/2 mutants. p Model summarizing the mechanosensory control of AV valve elongation. Hearts are oriented, annotated, and imaged as in Fig. 1. The box-and-whisker plots in c, l represent the same type of data as in Fig. 1g. The center of the bar represents the mean in f, o. P values are calculated using a two-sided Student’s t-test in c, l; a Fisher’s exact test in f, o; and are relative to wild type. Error bars indicate SEM in i. Scale bars: 50 µm in a, b, g, h, j, k; 10 µm in d, e, m, n. Source data are provided as a Source Data file.
Discussion
Cardiac valve leaflet formation is sensitive to biomechanical forces generated by blood flow and cardiac muscle contraction6,12,23. Previous studies have shown that membrane-bound mechanosensitive ion channels are involved in valve formation10,11,18. However, the valve phenotypes associated with their loss are not as severe as conditions without blood flow6,23. Using live imaging of double and triple mutant combinations, we uncovered specific mechanosensors with overlapping, or compensatory, functions in AV valve formation.
We first focused on members of the Pkd family described to interact with Pkd2, which is involved in cardiac valve development10,11. Pkd proteins form heteromeric complexes to mediate their membrane localization and function in multiple organs43. Consequently, mutations in partner pairs typically give rise to the same phenotypes42, such as pkd1l1 and pkd2 in the left/right organizer29,30,44. In zebrafish, pkd2 knockdown also shows the same morphological defects as pkd1a and pkd1b double knockdown45, indicating that different heteromeric complexes can be formed between Pkd2 and several Pkd1-like proteins. We, therefore, decided to mutate the known heteromeric partners of Pkd2, namely Pkd1a and Pkd1l128,29,30. We generated double and triple mutants for pkd1a, pkd2, and pkd1l1, and observed valve elongation defects, but no valve cell number differences, in the triple mutants that are similar to those identified in flow-defective zebrafish, with an almost complete absence of the AV valve6,7,23. We hypothesize that Pkd1a, Pkd2, and Pkd1l1 are parts of Pkd heteromeric complexes that control cardiac valve formation through blood flow mechanosensation. In this context, it would be interesting to investigate the function of other Pkd2-like proteins, such as Pkd2l126, that could interact with Pkd1a or Pkd1l1 during cardiac valve development.
Mutations in PKD1 are associated with mitral valve regurgitation in humans24,46. In addition, PKD1l1 mutations are linked to atrioventricular septal malformations that are hypothesized to be a consequence of laterality defects44. Our experiments suggest that patients carrying deleterious mutations in PKD1 or PKD1L1 may have valvular and atrioventricular defects due to early developmental valve malformations, independently of laterality-related disorders. In addition to their role in AV valve formation, further work will be needed to assess whether pkd genes also cooperate during blood flow mechanosensation in other blood vessels endothelial cells47 or lymphatic vessel endothelial cells25,48.
Recent studies have identified a role for mechanosensitive channels of the Piezo family in OFT valve development11,18 and lymphatics valve formation49. Here, we investigated the role of two Piezo family members in AV valve formation and found that mutants for piezo1 and piezo2a display valve elongation defects. Furthermore, we also found that Piezo2a cooperates with Pkd1a during cardiac looping and AV valve development. This cooperation between the Piezo and Pkd families appears to be specific to the AV valve, as Pkd2 does not cooperate with Piezo1 during OFT valve formation11. Future experiments should address the role of the Pkd family members in OFT valve development and evaluate whether Pkd1a or Pkd1l1 cooperate with Piezo family members in the OFT. The expression of piezo1 and piezo2a are restricted to the AV canal in zebrafish embryos18 and larvae (our study). Furthermore, other mechanosensor channels, such as Trpv4, show enrichment in the AV region50. Therefore, it would be interesting to generate fluorescent fusion lines of the mechanosensors essential for cardiac valve formation in order to track their trafficking within endocardial cells and study the signals required for their activation51.
Pkd and Piezo channels have been shown to regulate calcium levels in different tissues52,53. In particular, Pkd2 has been described to partially control calcium enrichment in the AV canal, with pkd2 mutants displaying a similar calcium flux phenotype as the blood flow-defective tnnt2a mutants, cmcl1 morphants, and gata2 morphants10. We found that the pkd genes cooperate to control the intracellular calcium levels in the AV canal during different cardiac states. In parallel with the Pkd and Piezo channels, recent work has identified the ATP–dependent purinergic receptor pathway as a blood flow mechanosensor essential for cardiac valve development12. This pathway is responsible for triggering calcium pulses in the AV canal upon blood flow stimulation, independently of Pkd2 and Piezo proteins12. Therefore, we hypothesize that the generation of AV calcium ion pulses and the AV calcium enrichment are independent processes, the latter requiring Pkd signaling to occur. In this context, it would be interesting to assess whether double and triple mutants of the channels described in our study would display calcium pulse phenotypes in addition to the enrichment defect.
We also found that the pkd genes cooperate to repress the AV endocardial expression of the transcription factor genes klf2a and klf2b, which are essential for valve formation7,19,22. This observation suggests that the expression level of these transcription factors must be tightly regulated and that higher levels of their expression negatively affect AV valve formation. Pkd2 has been reported to positively regulate klf2a expression in the OFT54 and the AV canal10 based on the quantification of a transgenic reporter. This discrepancy with our observations could be explained by a differential role of Pkd2 on klf2a expression in comparison with other Pkd members or by the different experimental approaches used, which could be investigated in future studies.
Although Klf2a targets have been studied in the myocardium and endocardium during zebrafish valve development7,19,20,21,22, the intermediates of Pkd and Klf2a remained unknown. Here, we identified the camk2g genes as upstream mediators of klf2a expression and downstream effectors of Pkd proteins during AV valve elongation. CaMKII proteins are serine/threonine kinases55 involved in a variety of processes during development38,39,41,56. CaMKII kinases phosphorylate their target upon stimulation by intracellular calcium elevation39. We hypothesize that Pkd-mediated AV calcium enrichment triggers the activation of Camk2g1 and Camk2g2, which in turn control klf2a RNA levels in the endocardial cells. Camk2 proteins have not been described to phosphorylate Klf2a. However, the Ca2+/calmodulin-dependent pathway has been shown to regulate KLF2 expression in HUVECs, through the phosphorylation of HDAC5 and the transcription factor MEF237. In zebrafish, protein kinase D2 has been shown to control AV valve formation by regulating Hdac5 activity57. Moreover, Mef2c overexpression results in aberrant klf2a levels in the AV canal58. Hence, it will be interesting to determine whether a Camk2g-Hdac5-Mef2c-Klf2a axis is active in controlling AV valve development in zebrafish. It has been previously hypothesized that Camk2g2 compensates for Camk2g1 to control organ laterality42. However, we did not observe cardiac laterality defects in camk2g double mutants, suggesting that other Camk2 proteins may be involved in this process, and they could also be studied for the control of AV valve formation.
In summary, we propose that members of the pkd and piezo gene families are essential for AV valve formation, through calcium signaling and the calcium-responsive protein Camk2g.
Methods
Zebrafish husbandry
Zebrafish husbandry was performed in accordance with institutional (MPG) and national (German) ethical and animal welfare regulations. Larvae were raised under standard conditions. Adult zebrafish were maintained in 3.5 L tanks at a stock density of 10 zebrafish/L with the following parameters: water temperature: 27–27.5 °C; light/dark cycle: 14/10; pH: 7.0–7.5; conductivity: 750–800 µS/cm. Zebrafish were fed three to five times a day, depending on age, with granular and live food (Artemia salina). Health monitoring was performed at least once a year. All embryos and larvae used in this study were raised at 28 °C and staged at 75% epiboly for synchronization. All procedures performed on animals conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and were approved by the Animal Protection Committee (Tierschutzkommission) of the Regierungspräsidium Darmstadt (reference: B2/1218).
Crispr/Cas9 mutagenesis
Cas9 mRNA in vitro synthesis was performed according to published protocols59. sgRNA design and transcription from an oligonucleotide template were performed as previously described60. RNA injection was performed in a wild-type AB background and mutagenesis efficiency was monitored using a T7 endonuclease assay59. Mutations were then identified through direct sequencing of PCR products in adult F1 animals. The selected Crispr site sequences for our target genes and primers for T7 endonuclease assays are available in Supplementary Data 1.
Zebrafish strains and molecular genotyping
The following published lines were used for this study: klf2abns11 61, klf2bbns12 61, pkd1ahu5855 25, pkd2tc321 31, tnnt2agbt-R14 62, Tg(fli1a:Gal4FF)ubs4 63, Tg(UAS:GCamP6s)nkUAShspzGCaMP6s13a 36, and TgBAC(nfatc1:Gal4)mu286 64. The following mutant lines were newly generated: pkd1abns507, pkd1l1bns394, piezo1bns340, piezo2abns367, camk2g1bns508, and camk2g1bns509.
Given the similarity of the phenotypes observed in the two pkd1a mutant alleles (hu5855 and bns507), all experiments that mention pkd1a−/− were performed interchangeably with one of the two alleles. pkd1abns507 mutants were generated in the pkd2tc321 mutant background because of the proximity of these genes on chromosome 1 (~4 Mb of distance), to facilitate double and triple mutant analysis.
For the experiments with tnnt2a, pkd1a, or pkd2 mutants, zygotic mutants from intercrosses of heterozygous parents were used. tnnt2a mutants were identified based on the silent heart phenotype at 24 hpf65, pkd2 mutants were identified based on their curly tail phenotype at 50 hpf66, and pkd1a mutants were identified after unmounting the larvae used for bodipy and AV flow profile experiments based on their pericardial edema phenotype at 102 hpf25. +/+ and +/− siblings were identified using wild-type and mutant-specific PCR. For the experiments with piezo1, piezo2a, pkd1l1, camk2g1, and camk2g2 mutants, maternal zygotic mutants from an incross of homozygous parents (F3) were used.
For the experiments with pkd1a; pkd2 double mutants, we intercrossed pkd1a+/−; pkd2+/− zebrafish and genotyped the larvae with the pkd2 curly tail phenotype66 for the pkd1a mutant and wild-type alleles. For the experiments with pkd1l1; pkd1a double mutants, we intercrossed pkd1l1−/−; pkd1a+/− zebrafish and genotyped the larvae for the pkd1a mutant and wild-type alleles. For the experiments with pkd1l1; pkd2 double mutants, we intercrossed pkd1l1−/−; pkd2+/− zebrafish and identified the larvae with the pkd2 curly tail phenotype66. For the experiments with pkd triple mutants, we intercrossed pkd1l1−/−; pkd1a+/−; pkd2+/− zebrafish and genotyped the larvae with the pkd2 curly tail phenotype66 for the pkd1a mutant and wild-type alleles. For the experiments with piezo2a; pkd1a double mutants, we intercrossed piezo2a−/−; pkd1a+/− or piezo2a+/−; pkd1a+/− zebrafish and genotyped the larvae for the piezo2a and pkd1a mutant and wild-type alleles. For the experiments with camk2g1; camk2g double mutants, we intercrossed camk2g1−/−; camk2g2+/− zebrafish and genotyped the larvae for the camk2g2 mutant and wild-type alleles. For the experiments with klf2a; klf2b double mutants, we intercrossed klf2a+/−; klf2b−/− zebrafish and identified them after unmounting the larvae used for AV flow profile experiments based on their pericardial edema phenotype at 96 hpf61.
The primers listed in Supplementary Data 1 were used to genotype by PCR the mutant and wild-type alleles described in this study. PCR amplifications were performed with KAPA2G Fast Ready Mix (Sigma) using the following cycling conditions: Initial denaturation 3 min 95 °C; 10 cycles (15 s 95 °C, 30 s 65 °C first to 55 °C last cycle, 30 s 72 °C); 20 cycles (15 s 95 °C, 30 s 55 °C, 30 s 72 °C); final extension 1 min 72 °C.
Plasmid generation
The camk2g1 and klf2a ORF were amplified from a pool of 78 hpf larval cDNA, and cloned into a pCS2+ vector. The T287D mutation was introduced in zcamk2g1-pCS2+ through PCR amplification. hCAMK2GT287D was amplified from a β-actin:GFP- hCAMK2GT287D vector41, and cloned into a pCS2+ vector. klf2a was amplified from klf2a-pCS2+ and cloned into a 5XUAS:p2a-dTomato vector. All primers are listed in Supplementary Data 1, and vector ligation was performed using the in vivo cloning method67.
RNA and morpholino injections
zcamk2g1T287D-pCS2+ and hCAMK2GT287D-pCS2+ were linearized using NotI, and mRNAs were in vitro synthesized using the mMessage mMachine SP6 transcription kit (Ambion). We injected 2 ng of the tnnt2a morpholino65, 5 ng of the pkd1a morpholino (ex8)45, 50 pg of zcamk2g1T287D or hCAMK2GT287D mRNAs, and 13 pg of 5XUAS:klf2a-p2a-dTomato plasmid DNA together with 13 pg of transposase mRNA. All reagents were injected at the one-cell stage at a volume of 1 nl together with 0.2% phenol red.
RT-qPCR analysis
For every condition, RNA was extracted from a pool of 15 dissected hearts (Fig. 5i, j) or from a pool of 10 larvae (Figs. S2b, S3b). RNA was isolated using TRIzol extraction and reverse transcription was performed using the Maxima First Strand cDNA synthesis kit (Thermo Fisher). The results represent biological triplicates of dissected hearts or larvae and two technical duplicates were performed per biological replicate. eef1b2 was used as a reference gene for all conditions. Fold changes were calculated using the 2−ΔΔCt method. The mutant conditions were compared with AB wild types. Animals were genotyped prior to heart dissection (Fig. 5i, j) or RNA extraction from whole larvae (Figs. S2b, S3b). All Ct values and primers are listed in Supplementary Data 1.
RNA in situ hybridizations procedure
Whole-mount RNA in situ hybridizations were performed according to standard protocols68. The in situ probes for piezo1, piezo2a, klf2a, and klf2b were synthesized directly from a PCR product, performed on wild-type AB cDNA at 78 hpf, using an incorporated T7 promoter (Supplementary Data 1). For the FISH experiments, the same protocol was used, with the following modifications: During the first day, larvae were incubated in 1% H2O2 for 30 min. During the second day, larvae were incubated with the primary antibodies anti-DIG-POD (1:5000, Roche 11207733910) and anti-MHC (MF20) (1:500, Invitrogen 14-6503-82) diluted in PBST + 10% Western Blocking reagent (Roche 11921673001). On the third day, after six washes in PBST, larvae were incubated with an amplification solution for 2 min (Akoya Biosciences NEL741001KT). Fluorescein was then added at 1:100 to the amplification solution, then kept in the dark at room temperature for 20 min. The larvae were then incubated for 2 h with the secondary antibody Goat anti-Mouse-Alexa647 (1:500, Invitrogen A21236), and extensively washed in PBST before imaging. In situ hybridizations were imaged using an SMZ25 stereomicroscope (Nikon) with a 2x/0.3 objective (Fig. S3k, l), an LSM 700 confocal laser scanning microscope (Zeiss) with a 40x objective (Fig. 6g, h, Fig. S5a–s), and an LSM 800 Examiner confocal laser scanning microscope (Zeiss) with a 40x objective (Fig. 5a–h). For the FISH experiments, the images represent a maximal projection of ten z-planes (20 µm). The experiments presented in Fig. 5a–h and S5d–s were performed using the same crosses as for the RT-qPCR analysis shown in Fig. 5i, j.
Immunohistochemistry
Whole-mount immunostaining was performed as described previously69, with the following modification: Larvae were fixed in 4% PFA after stopping the heart with 0.2% Tricaine to prevent cardiac collapse during fixation. The primary antibodies used in this study are Mouse anti-MHC (MF20) (1:500, Invitrogen 14-6503-82), Mouse anti-Alcama (1:500, ZN-8, DSHB), and Rabbit anti-Fli1 (1:500, Abcam ab133485), followed by the secondary antibodies: Goat anti-Mouse-Alexa568 (1:500, Invitrogen A11004), Goat anti-Mouse-Alexa647 (1:500, Invitrogen A21236), and Goat anti-Rabbit-Alexa488 (1:500, Invitrogen A110034). All images were analyzed using an LSM 700 confocal laser scanning microscope (Zeiss) with a 40x objective, except for the samples shown in Fig. 2t, u, which were imaged using an LSM 800 Examiner confocal laser scanning microscope (Zeiss) with a 40x objective. For AV cell number quantification, the cells were counted in every plane of a z-stack covering the AV canal. The 78 hpf wild-type dataset (n = 61) is the same in Figs. 1n, 2s, 3o, and represents a pool of pkd1a+/+, pkd1a+/+; pkd2+/+, and camk2g1+/+; camk2g2+/+ larvae. The 78 hpf pkd1a mutant dataset (n = 26) is the same in Figs. 1n, 2s, 3o.
AV flow profile and heart rate quantification
Zebrafish hearts were imaged live using an inverted Cell Observer Spinning Disk microscope with a 25x objective at 240 fps with a pixel width of 1.92 µm. The AV blood flow profile was analyzed using a custom-made ImageJ script that calculates the percentage of anterograde (from the atrium to the ventricle), retrograde (from the ventricle to the atrium), or no-flow (no movement between the atrium and the ventricle) blood movement in a beating cycle. The results were then averaged between 3 beating cycles per embryo/larva. The profiles are represented using a box-and-whisker plot, which depicts the data through their quartiles. The 78 hpf wild-type dataset (n = 217) is the same in Figs. 1g, h, 2e, 3e, 6l, and represents a pool of pkd1a+/+, pkd1a+/+; pkd2+/+, klf2a+/+; klf2b+/+, and camk2g1+/+; camk2g2+/+ larvae. The same dataset was used to measure the heart rate (Figs. S1i, S2j, S3j, S6d). The 78 hpf pkd1a mutant dataset (n = 51) is the same in Figs. 1h, 2e, 3e.
In vivo Bodipy labeling and imaging
Bodipy FL C5-Ceramide (Invitrogen D3521) was added to fish media 1 day prior to imaging at 0.2 µM from a 1 mM stock solution. The larvae were anesthetized in 0.0175% tricaine and their heart stopped using 25 mM BDM (Sigma Aldrich B0753) treatment. Images were then acquired using an LSM 700 confocal laser scanning microscope (Zeiss) using a 40x objective, and the presence of a superior valve leaflet was assessed in every plane of a z-stack covering the AV canal. The 78 hpf wild-type dataset (n = 104) is the same in Figs. 1k, 2l, 3j, 6o, and represents a pool of pkd1a+/+, pkd1a+/+; pkd2+/+, and camk2g1+/+; camk2g2+/+ larvae. The 78 hpf pkd1a mutant dataset (n = 32) is the same in Figs. 1k, 2l, 3j.
4D live imaging of GCamPs calcium sensor
fli1a:Gal4FF; UAS:GCamP6s positive larvae were selected under a fluorescence stereomicroscope. Time-lapse imaging was acquired using a spinning disc microscope at 50 fps with a pixel width of 1.92 µm. Fifty acquisitions were performed for every larva, and the optical plane was moved by 4 µm between movies to cover a z-section depth of 200 µm per heart, starting ventrally. The 50 acquisitions were temporally realigned using a custom-made ImageJ script. We then performed a maximal projection of the z-dimension using ImageJ to visualize the GCamP6s signal in the whole heart, at the atrial diastole and systole states.
Heart segmentation
Heart segmentation was performed on three types of movies: the imaging files generated to measure the AV flow profile (Fig. S1b–e′), the GCamPs 4D movies (Fig. S4a–c′), and the fluorescence in situ z-stack images (Fig. S5a, b). Using a custom-made ImageJ script, we trace two external lines, one on each side of the heart, from the inflow to the outflow, and indicate the AV canal. The script then traces a midline of the heart, calculated to be at mid-distance from the two external lines. The midline tracing is performed independently in the atrium and the ventricle in order to account for the differences in their curvature. The heart is then segmented along the midline by 100 segments, which we use to extract heart diameter and fluorescence intensity parameters from our images. The 78 hpf wild-type (n = 20) and tnnt2a mutant (n = 18) datasets are the same in Figs. S1g, h, S2h, i, S3h, i, S6b, c, and the wild types represent a pool of pkd1a+/+, pkd1a+/+; pkd2+/+, and camk2g1+/+; camk2g2+/+ larvae.
Software, statistics, and reproducibility
Microscopy images were acquired with NIS-Elements 4.30, ZEN 2011, ZEN 2.5 Blue, and ZEN. 3.5 Blue. RT-qPCR data were acquired with Bio-Rad CFX Manager 3.1. AV flow profile analysis, valve elongation quantification, and heart segmentation were performed using Fiji (ImageJ 1.53q). Sequence analysis was performed using ApE (v2.0.54). Data were processed with GraphPad Prism 9 and Microsoft Office 2016. A two-sided Fisher exact test was used to calculate p values for the bodipy experiments and a two-sided Student’s t-test was used to calculate p values for the other experiments. Experiments were performed at least three times independently.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Materials and raw images that support the findings of this study are available upon request to the corresponding authors. Raw data are provided in a Source Data file. Source data are provided with this paper.
References
van der Bom, T. et al. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 8, 50–60 (2011).
Zaidi, S. & Brueckner, M. Genetics and genomics of congenital heart disease. Circ. Res. 120, 923–940 (2017).
Hinton, R. B. & Yutzey, K. E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 73, 29–46 (2011).
Beis, D. et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132, 4193–4204 (2005).
Trinh, L. A. & Stainier, D. Y. Cardiac development. Methods Cell Biol. 76, 455–473 (2004).
Hove, J. R. et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 (2003).
Vermot, J. et al. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 7, e1000246 (2009).
Kalogirou, S. et al. Intracardiac flow dynamics regulate atrioventricular valve morphogenesis. Cardiovasc. Res. 104, 49–60 (2014).
Steed, E., Boselli, F. & Vermot, J. Hemodynamics driven cardiac valve morphogenesis. Biochim. Biophys. Acta 1863, 1760–1766 (2016).
Heckel, E. et al. Oscillatory flow modulates mechanosensitive klf2a expression through trpv4 and trpp2 during heart valve development. Curr. Biol. 25, 1354–1361 (2015).
Duchemin, A. L., Vignes H. & Vermot J. Mechanically activated piezo channels modulate outflow tract valve development through the Yap1 and Klf2-Notch signaling axis. Elife 8, e44706 (2019).
Fukui, H. et al. Bioelectric signaling and the control of cardiac cell identity in response to mechanical forces. Science 374, 351–354 (2021).
Paolini, A. & Abdelilah-Seyfried, S. The mechanobiology of zebrafish cardiac valve leaflet formation. Curr. Opin. Cell Biol. 55, 52–58 (2018).
Duchemin, A. L., Vignes, H., Vermot, J. & Chow, R. Mechanotransduction in cardiovascular morphogenesis and tissue engineering. Curr. Opin. Genet. Dev. 57, 106–116 (2019).
Gunawan, F., Gentile, A., Gauvrit, S., Stainier, D. Y. R. & Bensimon-Brito, A. Nfatc1 promotes interstitial cell formation during cardiac valve development in zebrafish. Circ. Res. 126, 968–984 (2020).
Gunawan, F. et al. Focal adhesions are essential to drive zebrafish heart valve morphogenesis. J. Cell Biol. 218, 1039–1054 (2019).
Rasouli, S. J. & Stainier, D. Y. R. Regulation of cardiomyocyte behavior in zebrafish trabeculation by Neuregulin 2a signaling. Nat. Commun. 8, 15281 (2017).
Faucherre, A. et al. Piezo1 is required for outflow tract and aortic valve development. J. Mol. Cell Cardiol. 143, 51–62 (2020).
Fontana, F. et al. Antagonistic activities of Vegfr3/Flt4 and Notch1b fine-tune mechanosensitive signaling during zebrafish cardiac valvulogenesis. Cell Rep. 32, 107883 (2020).
Goddard, L. M. et al. Hemodynamic forces sculpt developing heart valves through a KLF2-WNT9B paracrine signaling axis. Dev. Cell 43, 274–289.e275 (2017).
Steed, E. et al. klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. Nat. Commun. 7, 11646 (2016).
Faggianelli-Conrozier N. et al. Complementary functions of the mechanosensitive factors egr1, klf2b and klf2a instruct the endocardial program. Preprint at bioRxiv https://doi.org/10.1101/718643 (2019).
Bartman, T. et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2, E129 (2004).
Lumiaho, A. et al. Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am. J. Kidney Dis. 38, 1208–1216 (2001).
Coxam, B. et al. Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Rep. 7, 623–633 (2014).
England, S. J., Campbell, P. C., Banerjee, S., Swanson, A. J. & Lewis, K. E. Identification and expression analysis of the complete family of zebrafish pkd genes. Front. Cell Dev. Biol. 5, 5 (2017).
Liu, L. et al. Combinatorial genetic replenishments in myocardial and outflow tract tissues restore heart function in tnnt2 mutant zebrafish. Biol. Open 8, bio046474 (2019).
Qian, F. et al. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 16, 179–183 (1997).
Field, S. et al. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development 138, 1131–1142 (2011).
Kamura, K. et al. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development 138, 1121–1129 (2011).
Schottenfeld, J., Sullivan-Brown, J. & Burdine, R. D. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134, 1605–1615 (2007).
El-Brolosy, M. A. et al. Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193–197 (2019).
Bagriantsev, S. N., Gracheva, E. O. & Gallagher, P. G. Piezo proteins: regulators of mechanosensation and other cellular processes. J. Biol. Chem. 289, 31673–31681 (2014).
Jakutis, G. & Stainier, D. Y. R. Genotype-phenotype relationships in the context of transcriptional adaptation and genetic robustness. Annu. Rev. Genet. 55, 71–91 (2021).
Kok, F. O. et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 32, 97–108 (2015).
Muto, A. et al. Activation of the hypothalamic feeding centre upon visual prey detection. Nat. Commun. 8, 15029 (2017).
Wang, W. et al. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 115, 2971–2979 (2010).
Rothschild, S. C., Francescatto, L., Drummond, I. A. & Tombes, R. M. CaMK-II is a PKD2 target that promotes pronephric kidney development and stabilizes cilia. Development 138, 3387–3397 (2011).
Francescatto, L., Rothschild, S. C., Myers, A. L. & Tombes, R. M. The activation of membrane targeted CaMK-II in the zebrafish Kupffer’s vesicle is required for left-right asymmetry. Development 137, 2753–2762 (2010).
Gauvrit, S. et al. HHEX is a transcriptional regulator of the VEGFC/FLT4/PROX1 signaling axis during vascular development. Nat. Commun. 9, 2704 (2018).
Rothschild, S. C. et al. Tbx5-mediated expression of Ca(2+)/calmodulin-dependent protein kinase II is necessary for zebrafish cardiac and pectoral fin morphogenesis. Dev. Biol. 330, 175–184 (2009).
Rothschild, S. C. et al. Genetic compensation of γ CaMKII, an evolutionarily conserved gene. Gene 742, 144567 (2020).
Semmo, M., Köttgen, M. & Hofherr, A. The TRPP subfamily and polycystin-1 proteins. Handb. Exp. Pharm. 222, 675–711 (2014).
Sutters, M. & Germino, G. G. Autosomal dominant polycystic kidney disease: molecular genetics and pathophysiology. J. Lab Clin. Med. 141, 91–101 (2003).
Mangos, S. et al. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis. Model Mech. 3, 354–365 (2010).
Vetrini, F. et al. Bi-allelic mutations in PKD1L1 are associated with laterality defects in humans. Am. J. Hum. Genet. 99, 886–893 (2016).
Goetz, J. G. et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep. 6, 799–808 (2014).
Shin, M. et al. Valves are a conserved feature of the zebrafish lymphatic system. Dev. Cell 51, 374–386.e375 (2019).
Nonomura, K. et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc. Natl Acad. Sci. USA 115, 12817–12822 (2018).
Vignes, H. et al. Extracellular mechanical forces drive endocardial cell volume decrease during zebrafish cardiac valve morphogenesis. Dev. Cell 57, 598–609.e595 (2022).
Yaganoglu, S. et al. GenEPi: Piezo1-based fluorescent reporter for visualizing mechanical stimuli with high spatiotemporal resolution. Preprint at bioRxiv https://doi.org/10.1101/702423 (2019).
Vasileva, V. Y., Sultanova, R. F., Sudarikova, A. V. & Ilatovskaya, D. V. Insights into the molecular mechanisms of polycystic kidney diseases. Front. Physiol. 12, 693130 (2021).
Shah, V., Patel, S. & Shah J. Emerging role of piezo ion channels in cardiovascular development. Dev. Dyn. 251, 276–286 (2021).
Kimelman, D., Smith, N. L., Lai, J. K. H. & Stainier, D. Y. Regulation of posterior body and epidermal morphogenesis in zebrafish by localized Yap1 and Wwtr1. Elife 6, e31065 (2017).
Tombes, R. M., Faison, M. O. & Turbeville, J. M. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene 322, 17–31 (2003).
Easley, C. A. et al. Laminin activates CaMK-II to stabilize nascent embryonic axons. Brain Res. 1092, 59–68 (2006).
Just, S. et al. Protein kinase D2 controls cardiac valve formation in zebrafish by regulating histone deacetylase 5 activity. Circulation 124, 324–334 (2011).
Xue, C. et al. Zebrafish vestigial like family member 4b is required for valvulogenesis through sequestration of transcription factor myocyte enhancer factor 2c. Front. Cell Dev. Biol. 7, 277 (2019).
Jao, L. E., Wente, S. R. & Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl Acad. Sci. USA 110, 13904–13909 (2013).
Hruscha, A. et al. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 140, 4982–4987 (2013).
Rasouli, S. J. et al. The flow responsive transcription factor Klf2 is required for myocardial wall integrity by modulating Fgf signaling. Elife 7, e38889 (2018).
Clark, K. J. et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods 8, 506–515 (2011).
Herwig, L. et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr. Biol. 21, 1942–1948 (2011).
Pestel, J. et al. Real-time 3D visualization of cellular rearrangements during cardiac valve formation. Development 143, 2217–2227 (2016).
Sehnert, A. J. et al. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 31, 106–110 (2002).
Brand, M. et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development 123, 129–142 (1996).
Watson, J. F. & García-Nafría, J. In vivo DNA assembly using common laboratory bacteria: a re-emerging tool to simplify molecular cloning. J. Biol. Chem. 294, 15271–15281 (2019).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008).
Juan, T. et al. Myosin1D is an evolutionarily conserved regulator of animal left-right asymmetry. Nat. Commun. 9, 1942 (2018).
Acknowledgements
We thank F. Gunawan for discussions and comments on the manuscript, S. Howard and H-M Maischein for technical support, J. Vermot for providing the pkd2tc321 mutant line, B.M. Hogan for providing the pkd1ahu5855 mutant line, K. Kawakami for providing the Tg(UAS:GCamP6s)nkUAShspzGCaMP6s13a line, and S.C. Rothschild and R.M. Tombes for providing the β-actin:GFP-hCAMK2GT287D vector. This work was supported by a European Molecular Biology Organization Long-Term Fellowship (ALTF 1234-2018) to T.J. and funds from the Max Planck Society to D.Y.R.S.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization (T.J. and D.Y.R.S.), Methodology (T.J.), Investigation (T.J., A.R.d.S., B.C., S.L., and V.C.), Resources (D.Y.R.S.), Writing (T.J. and D.Y.R.S. with inputs from A.R.d.S., B.C., S.L., and V.C.), Supervision (D.Y.R.S.), Project administration and funding acquisition (T.J. and D.Y.R.S.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tzung Hsiai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juan, T., Ribeiro da Silva, A., Cardoso, B. et al. Multiple pkd and piezo gene family members are required for atrioventricular valve formation. Nat Commun 14, 214 (2023). https://doi.org/10.1038/s41467-023-35843-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-35843-3
This article is cited by
-
Highly specific and non-invasive imaging of Piezo1-dependent activity across scales using GenEPi
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.