Abstract
Hydrogen (H2) is expected to play a crucial role in reducing greenhouse gas emissions. However, hydrogen losses to the atmosphere impact atmospheric chemistry, including positive feedback on methane (CH4), the second most important greenhouse gas. Here we investigate through a minimalist model the response of atmospheric methane to fossil fuel displacement by hydrogen. We find that CH4 concentration may increase or decrease depending on the amount of hydrogen lost to the atmosphere and the methane emissions associated with hydrogen production. Green H2 can mitigate atmospheric methane if hydrogen losses throughout the value chain are below 9 ± 3%. Blue H2 can reduce methane emissions only if methane losses are below 1%. We address and discuss the main uncertainties in our results and the implications for the decarbonization of the energy sector.
Similar content being viewed by others
Introduction
Commitments to reach net-zero carbon emissions have drawn renewed attention to hydrogen (H2) as a low-carbon energy carrier1,2. Currently, H2 is mostly used as an industrial feedstock, and its global production has a high carbon footprint because it relies almost entirely (≈95%) on fossil fuels1. However, many technologies to produce H2 with a lower carbon footprint are available1. Among these, low-carbon H2 can be produced from water electrolysis powered by renewable energy (green H2) or from methane reforming coupled with carbon capture and storage (blue H2). H2 fuel may be especially important to decarbonize energy and transport sectors where direct electrification is complicated, like heavy industry, heavy-duty road transport, shipping, and aviation1. H2 is also being considered for storing renewable energy1. As a result of this potential, countries accounting for more than a third of the world’s population have developed national strategies for large-scale H2 production1,2.
Even if a more hydrogen-based economy would reduce CO2 emissions and improve air quality3, it would also increase the H2 emissions into the atmosphere. The H2 molecule is very small and difficult to contain, so it is still largely unknown how much H2 will leak in future value chains. H2 emissions will also occur due to venting, purging, and incomplete combustion4,5,6. This potential increase in H2 emissions has received relatively little attention to date because H2 is neither a pollutant nor a greenhouse gas (GHG). However, it has been long known7,8,9,10 that H2 emissions may exert a significant indirect radiative forcing by perturbing the concentration of other GHG gases in the atmosphere. This indirect GHG effect of H2 calls for a detailed scrutiny of the global H2 budget and the environmental consequences of its perturbation11,12.
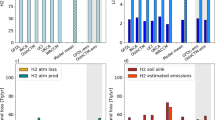
H2 is the second most abundant reactive trace gas in the atmosphere, after methane, with an average concentration of around 530 ppbv13. H2 sources include both direct emissions (≈45% of total sources) and production in the troposphere from the oxidation of volatile organic compounds (≈25%) and methane (≈30%)11,14. The main H2 sinks are the uptake by soil bacteria (70–80% of total tropospheric removal) and the atmospheric reaction with the radical OH (20–30%), which is responsible for the indirect GHG effect of H2. H2’s reaction with the OH radical tends to increase tropospheric methane (CH4) and ozone (O3), which are two potent greenhouse gases. It also increases stratospheric water vapor, which is associated with stratospheric cooling and tropospheric warming8,15. Recent global climate models have estimated that hydrogen has an indirect radiative forcing of around 1.314–1.816 10−4 W m−2 ppb\({}_{{{{{{{{\rm{v}}}}}}}}}^{-1}\), and a global warming potential (GWP) that lies in the range 11 ± 5 for a 100-year time horizon16. Hence, H2 emissions are far from being climate neutral, and their largest impact is related to the perturbation of atmospheric CH414,16, the second most important anthropogenic GHG.
The tropospheric budgets of H2 and CH4 are deeply interconnected (Fig. 1). First, the removal of both gases from the atmosphere is controlled by their reaction with OH, which is the dominant sink (≈90%) for atmospheric methane17,18. An increase in the concentration of tropospheric H2 may reduce the availability of OH, consequently weakening CH4’s removal and increasing CH4’s lifetime and abundance14,19. Second, methane is a primary precursor of hydrogen. Namely, CH4 oxidation results in the production of formaldehyde, whose photolysis produces H2. Firn-air records suggest that the increase in H2 over the 20th century can be largely explained by the increase in CH4 concentration20.
Sketch of H2 and CH4 tropospheric budgets and their interconnections: (1) the competition for OH; (2) the production of H2 from CH4 oxidation; (3) the potential emissions [minimum-maximum] due to a more hydrogen-based energy system. Flux estimates (Tg/year) are from refs. 11,18. Arrows are scaled with mass flux intensity, CH4 scale being 10 times narrower than H2 scale. On a per-mole basis, H2 consumes only around 3 times less OH than CH4. ppq = part per quadrillon (10−15). a top-down estimate including also minor atmospheric sinks (<10%). b range obtained as a difference between total and fossil fuel emissions18.
Additionally, H2 and CH4 are linked at the industrial level. Around 60% of global H2 production is currently produced from steam methane reforming (gray H2) and is responsible for 6% of global natural gas use1. In the next decade, steam methane reforming coupled with carbon capture and storage will likely remain the dominant technology for large-scale H2 production (blue H2), since facilities for H2 production from renewable sources (green H2) will require time to become operational and economically favorable2.
Since CH4 is the second-largest contributor to atmospheric warming since the beginning of the industrial era and there are global efforts to mitigate its atmospheric levels21, it is crucial to quantify the response of atmospheric CH4 to increasing H2 production.
We analyze this problem through a simple atmospheric model that captures the interaction between H2 and CH4 (“Methods”). The investigation of the transient dynamics (“Methods”) shows that any H2 emissions pulse to the atmosphere leads to a small transient growth of atmospheric CH4 whose effects last for several decades. In the next sections, we focus on how the equilibrium concentrations of tropospheric H2 and CH4 would respond to scenarios of continuous emissions from an energy system where part of the fossil fuel energy share is replaced by green or blue H2. The analysis emphasizes how atmospheric CH4 could either decrease or increase, mainly depending on the H2 production pathway and the amount of H2 lost to the atmosphere. The latter is defined through the hydrogen emission intensity (HEI), namely the percentage of H2 produced that is lost to the atmosphere. Specifically, we find a critical HEI above which the CH4 atmospheric burden rises despite the lower fossil fuel use. We assess the critical factors and the main uncertainties in the quantification of this critical HEI. We finally discuss how our results can help better inform policymakers regarding the trade-off associated with different scenarios of hydrogen production and use.
Results
Emission scenarios
Here we investigate how the tropospheric burdens of methane and hydrogen would be affected by the transition to a more hydrogen-based energy system, wherein hydrogen replaces part of the current fossil fuel energy (≈490 ExJ in 201922). To achieve this goal, we estimate the CH4 and H2 source changes, \({{\Delta }}{S}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\) and \({{\Delta }}{S}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\), where Δ indicates the difference to the current tropospheric conditions (“Methods”). This fossil fuel displacement reduces both CH4 and H2 sources (Fig. 1). The rise in H2 production causes additional H2 emissions due to intentional (e.g., venting) and unintended (e.g., fugitive) losses, and possibly CH4 emissions associated with blue H2 production.
The change in H2 emissions can be estimated from the amount of hydrogen produced to substitute fossil fuels and the HEI, namely the percentage of H2 produced that is lost to the atmosphere. Losses can occur due to venting, purging, incomplete combustion and leaks across the hydrogen value chain. The HEI of the future global H2 value chain is very uncertain. Literature values range from 1 to 12%4,9,23, but the upper bound is unlikely to occur at large scales because it would be both unsafe and too expensive. Recent empirical estimates for specific H2 infrastructures suggest HEI’s ranging from 0.1 to 6.9%, critically depending on the pathway of hydrogen production and transport6. To account for these uncertainties and to explore a broad spectrum of possible scenarios, here we vary HEI from 0 to 10% of the total hydrogen produced (Fig. 2a). The lower and upper bounds of this range represent a perfectly sealed and a highly leaking global H2 value chain, respectively. With a perfectly sealed hydrogen value chain, H2 emissions would only decrease due to the lower fossil fuel use. On the contrary, a highly leaking H2 value chain, coupled with an envisioned penetration of H2 in the energy market, could increase hydrogen emissions up to several times the total current sources, which are around 80 Tg H2 yr−1.
a Changes in H2 sources (\({{\Delta }}{S}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\)) as a function of fossil fuel replacement for different hydrogen emission intensity (HEI). b Changes in CH4 sources (\({{\Delta }}{S}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\)) as a function of fossil fuel replacement for different H2 production pathways. Methane leak rates associated with blue H2 production are 0.2, 1, and 2%. Bands for \({{\Delta }}{S}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\) account for different amounts of blue H2 produced and lost. c Response of the tropospheric concentrations of H2 and CH4 for the emission scenarios of the previous panels. Symbols mark the different percentages of fossil fuel displacement. Only symbols for 100% fossil fuel replacement are reported for blue H2 with 1% CH4 leakage. Also reported is the difference in CO2 concentration (Δ[CO2e]) that would produce equivalent radiative forcing to the change in equilibrium CH4 (upper axis).
The variation in CH4 emissions depends not only on the percentage of fossil fuel energy that is displaced by hydrogen, but also on the hydrogen production pathway. For green H2, i.e., hydrogen obtained from renewable sources, we scale CH4 emissions based on the reduced consumption of fossil fuels resulting from hydrogen usage (Fig. 2b). Estimates of current methane emissions associated with fossil fuel extraction and distribution are in the range 80–160 Tg CH4 yr−118,24,25 and relatively equally distributed among coal, oil, and gas sectors26. Here we use the top-down estimate of 111 Tg/year18.
For blue H2, which is derived from steam methane reforming (SMR), the variation in CH4 sources not only accounts for the reduced consumption of fossil fuels but also for the methane emissions (venting, incomplete combustion, fugitive) associated with blue hydrogen production. These emissions depend on the amount of CH4 needed to produce H2, i.e., feedstock and energy requirements of the SMR process (“Methods”), and the CH4 leak rate. The precise average leak rate of the global natural gas supply chain remains uncertain. One of the reasons is that national inventories generally underestimate real emissions27,28,29,30. More detailed studies relying on field measurements in the United States and Canada estimate average leak rates around 2%28,29,30, with large spatial heterogeneity between different operators31. Although national inventories suggest that some countries, like Venezuela and Turkmenistan, have higher leak rates26, here we adopt 2% as the maximum global CH4 leak rate for our scenarios, because methane-mitigation efforts are likely to decrease future global leak rates21 and, more importantly, because not all hydrogen produced will be blue H2. In this regard, the scenario of blue H2 with a 2% CH4 leak rate can also be interpreted as a combination of equal production of green H2 and blue H2 with 4% CH4 leak rate. We use 0.2% as a lower bound for the CH4 leak rate, since this has been declared as the target of several energy companies for 202532. 1% represents an intermediate scenario of blue H2 production.
Figure 2b shows the resulting CH4 emissions associated with green and blue H2 production with methane leak rates of 0.2, 1, and 2%. The different leak rates have a great impact on the methane emissions. Compared to the fossil fuel energy system, CH4 emissions are reduced in the blue H2 scenario with 0.2% methane losses, but largely increased in the blue H2 scenario with 2% methane losses. The fossil fuel displacement by blue H2 with 1% methane losses shows basically no net effect on the CH4 emissions.
As a specific case, we also investigate the H2 and CH4 emission changes associated with estimates of future hydrogen production in a set of net-zero scenarios. H2 production is expected to increase from current 90 Tg/year to 530–660 Tg/year in 20502,33,34. We thus consider a 500 Tg/year rise in the global H2 production, which is energetically equivalent to about 15% of current fossil fuel energy. Figure 3a shows how, depending on the H2 production pathway and the different hydrogen and methane leak rates, the emission changes of these two gases can vary substantially.
a Changes in H2 and CH4 sources (ΔS) due to green and blue H2 production (≈500 Tg yr−1). HEI is the H2 emission intensity. Gray lines mark the case for HEI = 0%. Blue bars for \({{\Delta }}{S}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\) are obtained with HEI = 10%. b Response of CH4 atmospheric concentration. The right axis shows the Δ[CO2e] that would produce equivalent radiative forcing to the change in equilibrium CH4.
Tropospheric response
For the previous emission scenarios, we evaluate the changes in the equilibrium concentrations of tropospheric hydrogen and methane, namely Δ[H2] and Δ[CH4]. The timescales to equilibrium are dictated by the gas average lifetimes (“Methods”). The corresponding variations in steady state concentration of OH are reported in Supplementary Figs. 1 and 2.
The H2 economy causes a rise in tropospheric H2 as a result of the additional emissions (Fig. 2c). The intensity of this increase varies considerably as a function of the emissions of the hydrogen value chain. The concentration variation could go from less than 100 ppbv to more than 2000 ppb in envisioned scenarios of the H2 economy, namely a +300% from the current H2 tropospheric level.
The response of atmospheric CH4 results from the combination of the methane emission change and the methane sink weakening due to the higher hydrogen emissions. To discriminate between the two mechanisms, it is useful to focus on the scenarios of fossil fuel displacement by green H2. In the case of a perfectly sealed green H2 value chain (HEI = 0%), [CH4] and [H2] both decrease due to reduction in fossil fuel emissions. As H2 emissions increase (HEI > 0), Δ[CH4] increases too. Up to the point that when HEI overcomes a critical threshold, there is an increase in atmospheric methane, i.e., Δ[CH4] > 0, even though methane emissions are lower. This critical HEI is in the range 8–10% for green H2 as it has a weak nonlinear dependence on the percentage of fossil fuel energy that is replaced by H2 (see also Supplementary Fig. 3).
The scenarios of blue H2 with 0.2% CH4 leak rates are not very different from the green H2 scenarios, with the critical HEI being in the range 7–8%. Regarding the scenarios of blue H2 with 1% CH4 leak rates, since there is basically no change in the methane emissions (Fig. 2b), the methane response is only associated with the reduction in OH availability due to the higher H2 concentration. The critical HEI is not defined for this blue H2 as the methane burden increases in all cases. The worst scenarios of blue H2 with 2% CH4 leak rates show drastic differences in the tropospheric concentrations of the two gases, which increase considerably, with a weakly nonlinear effect due to the drop in atmospheric OH.
The atmospheric methane response to future H2 production2,33,34 shows qualitatively similar results as a function of the H2 production pathway and the percentage of H2 lost to the atmosphere (Fig. 3b). Positive effects in terms of methane mitigation are observed only for green and blue H2 with low methane losses, if the H2 emission intensity is well below 10%. Otherwise, the tropospheric methane burden is enhanced.
We also evaluated the change in CO2 concentration (Δ[CO2e]) that would produce equivalent radiative forcing to the change in the equilibrium concentration of CH4 (Figs. 2c and 3b). We used the radiative efficiency of CH4 that includes indirect effects on O3 and stratospheric H2O35. Under the worst scenario of blue H2 production with 2% CH4 losses and 10% H2 losses, the rise in equilibrium CH4 due to future H2 production would be like adding 9 ppm of CO2 to the atmosphere (Fig. 3b). For the same blue H2, the rise in CH4 following the entire displacement of fossil fuels would be like adding around 70 ppm of CO2 (Fig. 2c). This is equivalent to around 50% of the CO2 increase from preindustrial times (278 ppm) to current days (417 ppm). Since the goal of keeping the global average temperature rise below 1.5 ∘C requires a mid-century maximum of CO2 close to 450 ppm, these results support previous concerns about the sustainability of blue H236 unless fugitive emissions can be kept sufficiently low.
Critical HEI for methane mitigation
The quantification of the critical hydrogen emission intensity (HEIcr) for methane mitigation is key to assess whether displacing fossil fuels with hydrogen would mitigate or enhance the tropospheric burden of CH4. Here we investigate how the HEIcr is affected by the hydrogen production pathway and by two of the most uncertain terms in the CH4-H2-OH balance: (i) the partitioning of the OH sink among the tropospheric gases; (ii) the rate of H2 uptake by soil bacteria. The derivation of an analytical solution for the HEIcr is reported in the “Methods”.
The very short lifetime of OH makes the quantification of its atmospheric dynamics extremely challenging. Indirect methods are typically used to estimate OH concentrations, sources, and sink partitioning37,38,39. Using a range of OH partition estimates38,40, we investigate the dependence of the HEIcr to different values of OH excess (EOH), EOH being the excess of OH that is consumed by other tropospheric gases besides hydrogen, methane, and carbon monoxide. Figure 4 shows the quasi-linear response of the HEIcr to EOH. We stress that a variation in EOH is equivalent to a variation in the OH sources since we preserve the current average OH concentration, which is relatively well constrained by inverse modeling37,41.
Critical HEI as a function of OH excess (EOH) and hydrogen production method (green and blue H2 with 0.2, 0.5, 1% CH4 leak rates, respectively). Dashed (dotted) lines are obtained for a 20% increase (decrease) in the H2 uptake rate by soil bacteria (kd). Triangles mark the critical HEI for the best estimate of EOH.
The HEIcr is much lower for blue H2 than for green H2 because of the methane emissions associated with blue H2 production. For the current tropospheric conditions, we find that HEIcr is around 9% for green H2, around 7% for blue H2 with 0.2% methane leak rates, and 4.5% for blue H2 with 0.5% methane leak rates. Blue H2 with 1% methane leak rate has a HEIcr that is close to zero, as displacement of fossil fuel with this hydrogen does not reduce methane emissions (Fig. 3b). For even higher methane leak rates, the methane burden would increase regardless of the H2 emissions, so that the HEIcr is negative.
The H2 uptake by soil bacteria is another crucial process in the evaluation of HEIcr and in the overall CH4–H2–OH dynamics, since it accounts for 70–80% of H2 tropospheric removal11. Despite recent research on uptake modeling42,43 and the microbial characterization of the H2-oxidizing bacteria44, the spatial heterogeneity of the uptake as driven by local hydro-climatic and biotic conditions hinders bottom-up estimates of the global average uptake rate. In atmospheric studies, the average uptake rate is usually adjusted in order to obtain a reasonable simulation of observed surface hydrogen concentrations14,45. To account for these potential sources of uncertainties, we show how a ±20% variation in the uptake rate influences the critical HEI (bands in Fig. 4). A stronger biotic sink (dashed lines) reduces the consumption of OH by H2 and, consequently, increases the HEIcr. A weaker biotic sink (dotted line) has the opposite effect.
Regarding the impact of climate change on the H2 soil sink, recent studies indicate that increasing temperatures are expected to slightly favor the uptake on a global scale14, while shifts in rainfall regimes will be the significant drivers of H2 uptake changes at the local scale43. From a biotic perspective, the adaptability of H2-oxidizing bacteria to extreme environments46 suggests that their presence will remain widespread in the future, but their spatial heterogeneity may change as a result of climate and anthropogenic pressures.
Another source of uncertainties in the evaluation of HEIcr is related to the estimate of CH4 emissions associated with fossil fuel use. Since there is a quasi linear relationship between these emissions and the HEIcr (Eq. (16) in “Methods”), the same relative uncertainty of fossil fuel methane emissions (Fig. 1) applies to the HEIcr.
Discussion
The success of the global net-zero transition hinges on hydrogen as a scalable low-carbon energy carrier that can replace fossil fuels in several hard-to-electrify energy and transport sectors. More than 20 governments and many companies have already announced strategies for hydrogen production, and the numbers are likely to increase as policy frameworks that facilitate hydrogen adoption are promoted1,2. Considerable investments are still needed to achieve such a transition, as the current hydrogen momentum falls short compared to net-zero goals. The Hydrogen Council2 estimates that there is a USD 540 billion gap between the investments of announced projects (USD 160 billion) on hydrogen production and the investments required by 2030 to be on a net-zero pathway (USD 700 billion).
While the positive effects of a more hydrogen-based economy are relatively established (e.g., lower CO2 emissions, decreased urban pollution, etc.), considerable uncertainty still surrounds the consequences of hydrogen emissions to the atmosphere, because of potential indirect GHG effects14,19. Here we have focused on the impact of a more hydrogen-based energy system on tropospheric methane, the second most important greenhouse gas.
We have shown how the replacement of fossil fuel energy with green or blue hydrogen could have very different consequences for tropospheric CH4, depending on the amount of hydrogen lost to the atmosphere and the methane emissions associated with hydrogen production (Figs. 2 and 3). Specifically, tropospheric CH4 would decrease due to the fossil fuel displacement only if the rate of H2 losses is kept below the critical HEI.
This is around 9 ± 3% for green H2 (Fig. 4). The same critical value would apply to other H2 colors that do not entail the use of fossil fuels, like white or orange H2 extracted from underground deposits12,47. The critical HEI for blue H2 is much lower due to the CH4 emission associated with blue H2 production. We have found that the methane emissions in a blue H2 economy could be higher than in a fossil fuel economy if the methane supply chain had an average leak rate above 1%. Furthermore, the superimposition of CH4 and H2 emissions may have undesired consequences for the tropospheric burden of CH4. This may be a potential problem in the near term, given that steam methane reforming will be used to bridge the gap between increasing H2 demand and limited green H2 production capacities2. Our results suggest that including hydrogen emissions would aggravate the greenhouse gas footprint of blue H236.
In addition to the CH4 feedback, H2 emissions are also expected to impact ozone (O3) and stratospheric water vapor (H2O), with negative consequences for both air quality and radiative forcing. Accounting for these effects, we can provide a comparison between the radiative forcing of hydrogen-based and fossil fuel-based energy systems. Because both H2 and CH4 are short-lived gas compared to CO2, the time horizon for this comparison is crucial48. Here we consider 20-year and 100-year time horizons. The GWP of H2 is estimated at 11 ± 5 (100-year) and 33\({}_{-13}^{+11}\) (20-year)16. The GWP of CH4 is estimated at 28 (100-year) and 80 (20-year)35. In an envisioned hydrogen economy that replaces the current fossil fuel industry, the H2 emissions could be in the range 23 to 370 Tg H2 yr−1, for a H2 emission intensity going from 1 to 10% (Fig. 2a). These emissions would have a radiative forcing impact of 0.7–12% (100-year) and 2–35% (20-year) of the current CO2 emissions from fossil fuels (≈35 Pg CO2 yr−1). If the global H2 economy relied on blue H2 with a 2% methane leakage rate, methane emissions would cause an additional radiative forcing impact that is around 10% (100-year) and 27% (20-year) of the current CO2 emissions from fossil fuels. Hence, in the worst scenario, up to 22% of the climate benefits of the hydrogen economy could be offset by gas losses over a 100-year horizon. The percentage could be as large as 65% over a 20-year horizon. These values could be higher on a regional scale if the leak rate of the natural gas supply chain is above 2%.
To maximize the climate benefit of hydrogen adoption, minimizing both H2 and CH4 losses across the supply chain of hydrogen production will need to be a priority. On the methane side, some governments and companies have already committed to reducing the leaks from the oil and gas sector, because this could be the most cost-effective and impactful action for near-term climate mitigation21. The International Energy Agency (IEA) estimates that, with the recent rise in natural gas prices, the abatement of methane emissions from the global gas and oil sector could be implemented at no net cost49. Hence, the accomplishment of this mitigation is only a matter of political will for the limited number of companies involved.
On the hydrogen side, the global value chain still has to be built. This offers the advantage of tackling the hydrogen emission problem ahead of time. On the one hand, energy companies will have a great interest in minimizing economic loss and safety risks due to hydrogen leaks. On the other hand, however, many technological challenges still need to be addressed. First, H2 containment may remain an issue even as technologies progress. The high diffusivity of the small H2 molecule has already challenged the scientific community’s ability to measure the H2 concentration in the atmosphere50 and in the firn air of ice sheets51. Second, while more field-based estimates of H2 losses are needed, there is currently no commercially available sensing technology able to detect small H2 leaks at the ppb level48. Third, global-space monitoring, which is bringing a much-needed transparency to the quantification of real methane emissions27,31, will also require new technology since H2, unlike CH4 or CO2, does not absorb infrared radiation. For all these reasons, the uncertainty about future emissions from the H2 value chain remains large.
Our versatile atmospheric model allowed a broad exploration of scenarios in a hydrogen-based energy system. Simulations with high resolution three-dimensional atmospheric chemistry models, which are more comprehensive but more computationally demanding, could refine our results for specific scenarios. In particular, a more detailed model could improve the assessment of H2 displacement of fossil fuels by accounting for the emission changes of other chemical species, like CO and NOx, which impact the CH4–H2–OH dynamics. Further analyses could also refine the potential changes in emission inventories due to H2 displacement of different fossil fuels.
Methods
The Model
With the increasing anthropogenic alteration of atmospheric chemistry, detailed three-dimensional atmospheric chemistry models have become critical to evaluate the atmospheric interactions with the climate forcing52,53. Nonetheless, thanks to their versatility, simplified models of atmospheric chemistry have also proven very useful to investigate the fundamental processes governing the coupling between atmospheric gases and the consequences of their possible perturbations (e.g., refs. 54,55,56,57,58,59). The insights obtained with the CH4–CO–OH model by Prather et al.54, in particular, led to a +40% revision of the IPCC’s GWP for CH460. Here we extend Prather’s seminal model by adding the mass balance equation for atmospheric H2. The purpose is to identify the key components that control the H2 feedback on the tropospheric dynamics of CH4 (Fig. 1).
The chemical reactions considered are
with R representing the rates of reactions, [ ⋅ ] the concentrations, and ki the rate coefficients. We indicated only the products with which we are concerned, the CO and H2 produced by oxidation of CH4(1). H2 production through CH4 oxidation has yield α ≈ 0.3713. X encompasses all the other species, besides CH4, CO, and H2, that consume OH. Based on the above reactions, the balance equations for the CH4–H2–CO–OH system are
where Rd = kd[H2] is the H2 uptake by soil bacteria, which plays a crucial role in the global balance of H2 since it accounts for around 70–80% of tropospheric removal11,43,61; Rs = ks[CH4] accounts for the smaller sinks of CH4, namely soil uptake, stratospheric loss and reactions with chlorine radicals62. For simplicity, we neglect the smaller sinks of H2, i.e., stratospheric loss (≈1% of removal63), and CO, i.e., soil uptake and stratospheric loss (<10% of removal64).
The solution at quasi steady state (i.e., d[ ⋅ ]/dt = 0) provides the sources for fixed tropospheric concentrations. Positive solutions for OH occurs if \({S}_{{{{{{{{\rm{OH}}}}}}}}} > (2+\alpha )({S}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}-{R}_{s})+{S}_{{{{{{{{\rm{CO}}}}}}}}}+{S}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}-{R}_{d}\), i.e., when there is enough OH to oxidize all CO sources, the part of CH4 sources that is not balanced by smaller sinks, and the part of H2 sources that is not balanced by the soil uptake. The excess of OH consumed by other gases, besides CH4, CO, and H2, can be defined as \({E}_{{{{{{{{\rm{OH}}}}}}}}}={R}_{{{{{{{{\rm{X}}}}}}}}}/({R}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}+{R}_{{{{{{{{\rm{CO}}}}}}}}}+{R}_{{{{{{{{{\rm{H}}}}}}}}}_{2}})\). The values representing average tropospheric conditions are summarized in Table 1. The values of SOH and SCO are kept constant in all scenarios.
Linear stability and transient dynamics
We investigate the effects of an emission pulse of H2 on the tropospheric system (5)–(8). The timescales and modes of the atmospheric response to chemical perturbations are defined by the eigenvalues and eigenvectors of the system54,55. Indicating with c(t) the solution vector of the system (5)–(8), the temporal dynamics of a small perturbation \(\hat{{{{{{{{\bf{c}}}}}}}}}\) around c evolves as
where J is the Jacobian of the system evaluated in c. For the equilibrium solution c0 representing the current tropospheric concentrations, the eigenvalues and eigenvectors, or modes, of the linearized system (9) are reported in Table 1. Since all eigenvalues are real and negative (λi < 0), the equilibrium solution c0 is a stable node. As a result, any small perturbation asymptotically decays in time with a timescale defined by the negative reciprocal of the eigenvalue.
Because the system equations are coupled, the decay timescale (\(-{\lambda }_{i}^{-1}\)) of a gas perturbation does not necessarily correspond to the gas steady state average lifetime (τi). The CH4 perturbation, in particular, decays with a timescale that is much larger than what predicted by its steady state lifetime, i.e., \(R\,=\,-{\lambda }_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}^{-1}/{\tau }_{{{{{{{{{\rm{CH}}}}}}}}}_{4}} > 1\). This mechanism, known as the CH4 feedback effect55,65, has a crucial role in increasing the GWP and the environmental impact of CH4 emissions. Detailed models of atmospheric chemistry usually provide R around 1.3–1.465. We find a marginally higher feedback factor, namely R ≈ 1.5, in agreement with previous findings using Prather’s box model54,55,57. The decay timescale of the H2 perturbation instead corresponds to the H2 average lifetime, namely \(-{\lambda }_{{{{{{{{{\rm{H}}}}}}}}}_{2}}^{-1}\approx {\tau }_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\), in agreement with results from detailed atmospheric chemistry models19.
While the modal eigenvalue analysis correctly captures the asymptotic stability of the solution c0, it does not describe the perturbation dynamics at finite times, i.e., before the asymptotic decay. Still within the domain of the linearized system (9), a more complete picture can be obtained by analyzing the temporal evolution of the solutions with specific attention to the emergence of transient growth phenomena, which are known to occur in systems where the modes are non-orthogonal, as in the present case. When large enough, a transient growth can even trigger nonlinearities that destabilize the equilibrium solution66.
Figure 5 shows the transient growth phase of tropospheric CH4 and CO that follows a 10% perturbation of H2 concentration. Specifically, the pulse of H2 causes a drop in OH and a build-up of CH4 that lasts a few years, while the H2 perturbation decays with the timescale \({\tau }_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\). The CH4 build-up then decays in the same manner as would a direct pulse of CH4 with a timescale defined by the CH4 feedback effect. In analytical terms, the perturbation of tropospheric CH4 mainly due to the excitation of H2 and CH4 modes is given by \(\delta [{{{{{{{{\rm{CH}}}}}}}}}_{4}]\approx 2.76{{{{{\rm{e}}}}}}^{{\lambda }_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}t}-2.82{{{{{\rm{e}}}}}}^{{\lambda }_{{{{{{{{{\rm{H}}}}}}}}}_{2}}t}+0.06{{{{{\rm{e}}}}}}^{{\lambda }_{{{{{{{{\rm{CO}}}}}}}}}t}\).
Using this result in traditional GWP formulas35 yields a GWP for H2 due to direct CH4 perturbation around 7.8 with the 100-year time-horizon and 22 with the 20-year time horizon. It is estimated that around half of the H2 indirect radiative forcing is due to the direct CH4 perturbation, and the other half to the O3 and stratospheric H2O impacts caused by both H2 and H2-induced CH4 perturbations14. Taking this into account yields a total GWP for H2 of 15.6 with the 100-year time-horizon and 44 with the 20-year time-horizon. These values are in the upper range of the recent estimates of 11 ± 5 for GWP100 and 33\({}_{-13}^{+11}\) for GWP20 obtained with a detailed model of atmospheric chemistry16. Notably, the consequences of the H2 pulse on CH4 are relatively small in magnitude because most of the additional H2 is oxidized by soil bacteria and not by OH. The stability of this biotic sink as affected by climate change and anthropic pressure is hence a crucial aspect for the impact of future H2 emissions, as further discussed in the main text.
Critical hydrogen emission intensity
We here derive an explicit expression for the critical H2 emission intensity (HEIcr) for methane mitigation, defined as the emission rate that offsets the H2 replacement of fossil fuels. The expression is derived for an infinitesimal replacement of fossil fuel energy with H2 (dE in ExJ/yr), but well approximates the critical HEI for finite replacement of fossil fuel energy (see Supplementary Fig. 3). As a first step, we differentiate the system (5)–(8) at equilibrium (d[⋅]/dt = 0) with respect to E. This yields
where subscript E indicates d ⋅ /dE. \({[{{{{{{{{\rm{CH}}}}}}}}}_{4}]}_{E}\,=\,0\) because of the definition of the critical H2 emission intensity, which leaves the methane concentration unaltered. We consider that only H2 and CH4 sources vary with E, while SOH,E = SCO,E = 0. These variations can be estimated as
where HEI and MEI are the hydrogen and methane emission intensities, respectively (MEI = 0 for green H2); \({\eta }_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\) is H2 higher heating value; r is the amount of CH4 needed to produce a unit of blue H2; \({{{{{{{{\rm{ff}}}}}}}}}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\) and \({{{{{{{{\rm{ff}}}}}}}}}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\) are the average amounts of CH4 and H2 emitted per ExJ of fossil fuel energy; \({a}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\) and \({a}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\) are conversion factors.
Substituting Eqs. (14), (15) into the system (10)–(13) and after some algebra, one obtains the critical H2 emission intensity
where the dependence to the atmospheric composition is embedded in \(A={k}_{d}({k}_{4}[{{{{{{{\rm{X}}}}}}}}]+{k}_{2}[{{{{{{{{\rm{H}}}}}}}}}_{2}]+2{k}_{1}[{{{{{{{{\rm{CH}}}}}}}}}_{4}])+{k}_{2}[{{{{{{{\rm{OH}}}}}}}}]\left((\alpha+2){k}_{1}[{{{{{{{{\rm{CH}}}}}}}}}_{4}]+{k}_{4}[{{{{{{{\rm{X}}}}}}}}]\right)\) and B = 8k1k2[CH4][OH]. Parameters have been defined as follows: \({\eta }_{{{{{{{{{\rm{H}}}}}}}}}_{2}}=0.143\) ExJ/Tg\({}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\), r = 3.2 kg\({}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\)/kg\({}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\), \({{{{{{{{\rm{ff}}}}}}}}}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}=0.225\) Tg\({}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\)/ExJ, \({{{{{{{{\rm{ff}}}}}}}}}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}=0.0225\) Tg\({}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\)/ExJ, \({a}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\,=\,0.43\) ppb/Tg, \({a}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\,=\,8{a}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\). To obtain the value of r, we used the estimate of 3.7 kg of natural gas for kg of H267, which includes feedstock and energy requirements, and we assumed that 85% of natural gas by weight is composed by methane. \({{{{{{{{\rm{ff}}}}}}}}}_{{{{{{{{{\rm{CH}}}}}}}}}_{4}}\) and \({{{{{{{{\rm{ff}}}}}}}}}_{{{{{{{{{\rm{H}}}}}}}}}_{2}}\) are obtained as the ratio between the global CH4 and H2 emissions due to fossil fuel use and the global fossil fuel energy.
Data availability
All data generated during this study are provided in the supplementary dataset file.
Code availability
The code used to generate the results is provided in the supplementary dataset file.
References
IEA. Global Hydrogen Review. Technical Report (International Energy Agency, 2021).
Hydrogen Council. Hydrogen for Net-Zero: A Critical Cost-Competitive Energy Vector. Technical Report (European Union, 2021).
Wang, D. et al. Impact of a future H2-based road transportation sector on the composition and chemistry of the atmosphere–part 1: tropospheric composition and air quality. Atmos. Chem. Phys. 13, 6117–6137 (2013).
van Ruijven, B., Lamarque, J.-F., van Vuuren, D. P., Kram, T. & Eerens, H. Emission scenarios for a global hydrogen economy and the consequences for global air pollution. Glob. Environ. Change 21, 983–994 (2011).
Frazer-Nash Consultancy. Fugitive Hydrogen Emissions in a Future Hydrogen Economy. Technical Report (Frazer-Nash Consultancy, 2022).
Cooper, J., Dubey, L., Bakkaloglu, S. & Hawkes, A. Hydrogen emissions from the hydrogen value chain-emissions profile and impact to global warming. Sci. Total Environ. 830, 154624 (2022).
Derwent, R. G., Collins, W. J., Johnson, C. & Stevenson, D. Transient behaviour of tropospheric ozone precursors in a global 3-D CTM and their indirect greenhouse effects. Climatic Change 49, 463–487 (2001).
Tromp, T. K., Shia, R.-L., Allen, M., Eiler, J. M. & Yung, Y. L. Potential environmental impact of a hydrogen economy on the stratosphere. Science 300, 1740–1742 (2003).
Schultz, M. G., Diehl, T., Brasseur, G. P. & Zittel, W. Air pollution and climate-forcing impacts of a global hydrogen economy. Science 302, 624–627 (2003).
Warwick, N., Bekki, S., Nisbet, E. & Pyle, J. Impact of a hydrogen economy on the stratosphere and troposphere studied in a 2-d model. Geophys. Res. Lett. 31, p.L05107 (2004).
Ehhalt, D. H. & Rohrer, F. The tropospheric cycle of H2: a critical review. Tellus B: Chem. Phys. Meteorol. 61, 500–535 (2009).
Zgonnik, V. The occurrence and geoscience of natural hydrogen: a comprehensive review. Earth-Sci. Rev. 203, 103140 (2020).
Novelli, P. C. et al. Molecular hydrogen in the troposphere: global distribution and budget. J. Geophys. Res.: Atmospheres 104, 30427–30444 (1999).
Paulot, F. et al. Global modeling of hydrogen using GFDL-AM4.1: sensitivity of soil removal and radiative forcing. Int. J. Hydrog. Energy 46, 13446–13460 (2021).
Vogel, B., Feck, T., Grooß, J.-U. & Riese, M. Impact of a possible future global hydrogen economy on Arctic stratospheric ozone loss. Energy Environ. Sci. 5, 6445 (2012).
Warwick, N. et al. Atmospheric Implications of Increased Hydrogen Use. Technical Report (Department for Business, Energy & Industrial Strategy Policy Paper, 2022).
Kirschke, S. et al. Three decades of global methane sources and sinks. Nat. Geosci. 6, 813–823 (2013).
Saunois, M. et al. The global methane budget 2000–2017. Earth Syst. Sci. Data 12, 1561–1623 (2020).
Derwent, R. G. et al. Global modelling studies of hydrogen and its isotopomers using STOCHEM-CRI: Likely radiative forcing consequences of a future hydrogen economy. Int. J. Hydrog. Energy 45, 9211–9221 (2020).
Patterson, J. D. et al. Atmospheric history of H2 over the past century reconstructed from south pole firn air. Geophys. Res. Lett. 47, e2020GL087787 (2020).
European Commission. Launch by the United States, the European Union, and Partners of the Global Methane Pledge to Keep 1.5C Within Reach. https://ec.europa.eu/commission/presscorner/detail/en/statement_21_5766. Accessed 2021-11-30 (2021).
BP. Statistical Review of World Energy. Technical Report (British Petroleum, 2021).
Bond, S., Gül, T., Reimann, S., Buchmann, B. & Wokaun, A. Emissions of anthropogenic hydrogen to the atmosphere during the potential transition to an increasingly H2-intensive economy. Int. J. Hydrog. Energy 36, 1122–1135 (2011).
Schwietzke, S. et al. Upward revision of global fossil fuel methane emissions based on isotope database. Nature 538, 88–91 (2016).
Jackson, R. B. et al. Increasing anthropogenic methane emissions arise equally from agricultural and fossil fuel sources. Environ. Res. Lett. 15, 071002 (2020).
IEA. Global Methane Tracker 2022. Technical Report (International Energy Agency, 2022).
Zhang, Y. et al. Quantifying methane emissions from the largest oil-producing basin in the united states from space. Sci. Adv. 6, 5120 (2020).
Alvarez, R. A. et al. Assessment of methane emissions from the U.S. oil and gas supply chain. Science 361, 186–188 (2018).
Shen, L. et al. Satellite quantification of oil and natural gas methane emissions in the U.S. and Canada including contributions from individual basins. Atmos. Chem. Phys. Discuss. 22, 11203–11215 (2022).
MacKay, K. et al. Methane emissions from upstream oil and gas production in Canada are underestimated. Sci. Rep. 11, 1–8 (2021).
Lauvaux, T. et al. Global assessment of oil and gas methane ultra-emitters. Science 375, 557–561 (2022).
UNEP. An Eye on Methane: International Methane Emissions Observatory. Technical Report (United Nations Environment Program, 2021).
IEA. Net Zero by 2050 - A Roadmap for the Global Energy Sector. Technical Report (International Energy Agency, 2021).
IRENA. World Energy Transitions Outlook: 1.5∘ C Pathway. Technical Report (International Renewable Energy Agency, 2021).
Forster, P. et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Chap. 7 (Cambridge University Press, 2021).
Howarth, R. W. & Jacobson, M. Z. How green is blue hydrogen? Energy Sci. Eng. 9, 1676–1687 (2021).
Naik, V. et al. Preindustrial to present-day changes in tropospheric hydroxyl radical and methane lifetime from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmos. Chem. Phys. 13, 5277–5298 (2013).
Lelieveld, J., Gromov, S., Pozzer, A. & Taraborrelli, D. Global tropospheric hydroxyl distribution, budget, and reactivity. Atmos. Chem. Phys. 16, 12477–12493 (2016).
Murray, L. T., Fiore, A. M., Shindell, D. T., Naik, V. & Horowitz, L. W. Large uncertainties in global hydroxyl projections tied to fate of reactive nitrogen and carbon. Proc. Natl Acad. Sci. USA 118, e2115204118 (2021).
Warneck, P. Chemistry of the Natural Atmosphere (Elsevier, 1999).
Montzka, S. A. et al. Small interannual variability of global atmospheric hydroxyl. Science 331, 67–69 (2011).
Ehhalt, D. & Rohrer, F. Deposition velocity of H2: a new algorithm for its dependence on soil moisture and temperature. Tellus B: Chem. Phys. Meteorol. 65, 19904 (2013).
Bertagni, M. B., Paulot, F. & Porporato, A. Moisture fluctuations modulate abiotic and biotic limitations of soil H2 uptake. Global Biogeochem. Cycles 35, e2021GB006987 (2021)
Bay, S. K. et al. Trace gas oxidizers are widespread and active members of soil microbial communities. Nat. Microbiol. 6, 246–256 (2021).
Yashiro, H., Sudo, K., Yonemura, S. & Takigawa, M. The impact of soil uptake on the global distribution of molecular hydrogen: chemical transport model simulation. Atmos. Chem. Phys. 11, 6701–6719 (2011).
Ji, M. et al. Atmospheric trace gases support primary production in Antarctic desert surface soil. Nature 552, 400–403 (2017).
Osselin, F. et al. Orange hydrogen is the new green. Nat. Geosci. 15, 1–5 (2022).
Ocko, I. B. & Hamburg, S. P. Climate consequences of hydrogen emissions. Atmos. Chem. Phys. 22, 9349–9368 (2022).
IEA. Driving Down Methane Leaks from the Oil and Gas Industry. Technical Report (International Energy Agency, 2021).
Jordan, A. & Steinberg, B. Calibration of atmospheric hydrogen measurements. Atmos. Meas. Tech. 4, 509–521 (2011).
Patterson, J. D. & Saltzman, E. S. Diffusivity and solubility of H2 in ice ih: implications for the behavior of H2 in polar ice. J. Geophys. Res.: Atmos. 126, 2020–033840 (2021).
Lamarque, J.-F. et al. The atmospheric chemistry and climate model intercomparison project (ACCMIP): overview and description of models, simulations, and climate diagnostics. Geosci. Model Dev. 6, 179–206 (2013).
Shindell, D. T. et al. Radiative forcing in the ACCMIP historical and future climate simulations. Atmos. Chem. Phys. 13, 2939–2974 (2013).
Prather, M. J. Lifetimes and eigenstates in atmospheric chemistry. Geophys. Res. Lett. 21, 801–804 (1994).
Prather, M. J. Time scales in atmospheric chemistry: theory, GWPs for CH4 and CO, and runaway growth. Geophys. Res. Lett. 23, 2597–2600 (1996).
Manning, M. R. Characteristic modes of isotopic variations in atmospheric chemistry. Geophys. Res. Lett. 26, 1263–1266 (1999).
Prather, M. J. Lifetimes and time scales in atmospheric chemistry. Philos. Trans. R. Soc. A: Math., Phys. Eng. Sci. 365, 1705–1726 (2007).
Gaubert, B. et al. Chemical feedback from decreasing carbon monoxide emissions. Geophys. Res. Lett. 44, 9985–9995 (2017).
Heimann, I. et al. Methane emissions in a chemistry-climate model: Feedbacks and climate response. J. Adv. Modeling Earth Syst. 12, 2019–002019 (2020).
Houghton, J. T. et al. Climate Change 2001: The Scientific Basis (The Press Syndicate of the University of Cambridge, 2001).
Rhee, T. S., Brenninkmeijer, C. A. M. & Rockmann, T. The overwhelming role of soils in the global atmospheric hydrogen cycle. Atmos. Chem. Phys. 6, 1611–1625 (2006).
Prather, M. J., Holmes, C. D. & Hsu, J. Reactive greenhouse gas scenarios: Systematic exploration of uncertainties and the role of atmospheric chemistry. Geophys. Res. Lett. 39, L09803 (2012).
Xiao, X. et al. Optimal estimation of the soil uptake rate of molecular hydrogen from the Advanced Global Atmospheric Gases Experiment and other measurements. J. Geophys. Res.: Atmos. https://doi.org/10.1029/2006JD007241 (2007).
Zheng, B. et al. Global atmospheric carbon monoxide budget 2000–2017 inferred from multi-species atmospheric inversions. Earth Syst. Sci. Data 11, 1411–1436 (2019).
Holmes, C. D. Methane feedback on atmospheric chemistry: methods, models, and mechanisms. J. Adv. Modeling Earth Syst. 10, 1087–1099 (2018).
Schmid, P. J. Nonmodal stability theory. Annu. Rev. Fluid Mech. 39, 129–162 (2007).
Collodi, G., Azzaro, G., Ferrari, N. & Santos, S. Techno-economic evaluation of deploying ccs in SMR based merchant H2 production with NG as feedstock and fuel. Energy Proc. 114, 2690–2712 (2017).
Trenberth, K. E. & Smith, L. The mass of the atmosphere: a constraint on global analyses. J. Clim. 18, 864–875 (2005).
Acknowledgements
We acknowledge support from the US National Science Foundation (NSF) grant nos. EAR1331846 and EAR-1338694, the BP through the Carbon Mitigation Initiative (CMI) at Princeton University, and the Moore Foundation. We thank Larry Horowitz for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
M.B.B., S.W.P., and A.P. conceptualized the work. M.B.B. developed the analytical model with contributions from F.P., analyzed the results and prepared the manuscript. A.P., F.P., and S.W.P. supervised the work and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jasmin Cooper and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bertagni, M.B., Pacala, S.W., Paulot, F. et al. Risk of the hydrogen economy for atmospheric methane. Nat Commun 13, 7706 (2022). https://doi.org/10.1038/s41467-022-35419-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-35419-7
This article is cited by
-
Two-Stage and One-Stage Anaerobic Co-digestion of Vinasse and Spent Brewer Yeast Cells for Biohydrogen and Methane Production
Molecular Biotechnology (2024)
-
Hydrogen in the energy transition: some roles, issues, and questions
Clean Technologies and Environmental Policy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.