Abstract
The selective cleavage of C-C bonds is of fundamental interest because it provides an alternative approach to traditional chemical synthesis, which is focused primarily on building up molecular complexity. However, current C-C cleavage methods provide only limited opportunities. For example, selective C(sp3)-C(sp3) bond cleavage generally relies on the use of transition-metal to open strained ring systems or iminyl and alkoxy radicals to induce β-fragmentation. Here we show that by merging photoredox catalysis with copper catalysis, we are able to employ α-trisubstituted carboxylic acids as substrates and achieve consecutive C-C bond cleavage, resulting in the scission of the inert β-CH2 group. The key transformation relies on the decarboxylative oxidation process, which could selectively generate in-situ formed alkoxy radicals and trigger consecutive C-C bond cleavage. This complicated yet interesting reaction might help the development of other methods for inert C(sp3)-C(sp3) bond cleavage.
Similar content being viewed by others
Introduction
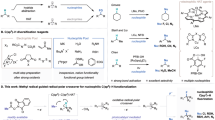
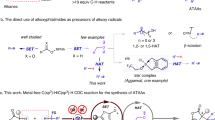
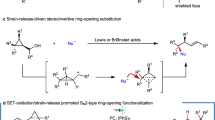
The art of making and breaking bonds has been the driving forces of innovation for chemists1,2,3. It is also the basis of metabolism, enzymology, and biochemistry as a whole4. In this context, the cleavage of C-C bonds is of fundamental interest for chemists because it provides an alternative approach to traditional chemical synthesis, which is focused primarily on building up molecular complexity5,6. The oxidative cleavage of alkyl aromatics could also lead to the selective degradation of polystyrene related plastics and generate high-value chemicals from abundant polystyrene wastes7,8,9. Despite many impressive advances in this field, the cleavage of inert C(sp3)-C(sp3) bonds and their subsequent functionalization is still one of the most sought-after challenges in chemistry10. Generally, the known strategies for C(sp3)-C(sp3) bond cleavage could be divided into two classes. One focuses on the strained ring systems employing transition-metal-catalyzed processes that are triggered by C-C bond activation and β-carbon elimination11,12. Another process exploits the chemistry of iminyl and alkoxy radicals because of their abilities to break into an alkyl radical species and an unsaturated fragment through β-fragmentation13,14,15. Recently, the group of Sarpong developed the first homolytic C-C bond cleavage method for the deconstructive diversification of cyclic amines mediated by a silver salt by breaking one C(sp3)-C(sp3) bond through an in-situ installed hydroxyl group (Fig. 1)16,17. Acids are inexpensive, highly stable, and readily available compounds. Decarboxylation enabled C(sp2)-C(sp3) and C(sp2)-C(sp2) bond cleavage has been employed for many useful transformations18,19,20,21,22,23,24. However, little studies have been performed using acids as potential substrates for C(sp3)-C(sp3) bond cleavage.
We propose to generate peroxyl radicals through the decarboxylative oxidation process25,26,27,28,29. We anticipate the photoredox decarboxylation of α-trisubstituted acid I would produce tertiary radical II which could trap oxygen to create the in-situ formed peroxyl radical III. This peroxyl radical then collapses to deliver the radical species IV through fragmentation. A few more oxidative transformations from IV will provide the final product VI.
Here we show, by merging photoredox catalysis with copper catalysis, we are able to use α-trisubstituted acids as substrates and employ a decarboxylative oxidation process to achieve consecutive C-C bond cleavage, resulting in the complete scission of the inert β-CH2 group.
Results
We began our investigation by using acid 1a as the substrate and examined different conditions, as shown in Table 1. First we tried Ir-1 as the photocatalyst and there was little product observed (Table 1, Entry 1). When Cu(OAc)2 was added, we were able to isolate the desired product 1b with 26% yield (Table 1, Entry 2). The yield was further improved to 48% when ligand L1 was used (Table 1, Entry 3). A further screening of other ligands revealed L4 as a better choice (Table 1, Entries 3–8). We then evaluated different copper sources (Table 1, Entries 9–11). When the reaction was carried out at 30 °C, the yield jumped to 81% after 40 h (Table 1, Entry 12). Given the fact the whole transformation is composed of a few steps, the average yield for each step is impressingly high. At last, we tested different photocatalysts and Ir-1 proved to be the best (Table 1, Entries 12–15). Further control experiments revealed no reactions occurred in the absence of photocatalyst, Cs2CO3, or blue LEDs (for a detailed account of the optimization studies, see Supplementary Figs. 12–19).
With the optimized conditions in hand, we proceeded to investigate the scope of this transformation. We first evaluated this method with different aromatic substituted piperidine-4-carboxylic acids (Fig. 2). N-substituted piperidine derivatives bearing tert-butoxycarbonyl and benzoyl groups were well tolerated (Fig. 2, substrates 1a-2a). A diverse range of electron-withdrawing and electron-donating functional groups were entirely compatible and delivered the products smoothly (Fig. 2, substrates 3a-8a). F, Cl, t-Bu, and Ph groups furnished the products in good yields (Fig. 2, substrates 3a, 4a, 7a, 8a). However, strong electron-withdrawing group such as CF3 and strong electron-donating group such as OMe only gave the product in moderate yields (Fig. 2, substrates 5a and 6a). Other aromatic group such as thiophene also worked well (Fig. 2, substrate 9a). For the indole substrate, the cleavage happened between indole and piperidine (Fig. 2, substrate 10a). Thus, indoline-2,3-dione (10b) and piperidin-4-one (10c) were isolated instead.
We further applied this method to all carbon cyclic acids (Fig. 2, substrates 11a-14a). For cyclobutanecarboxylic acid substrates, 1,4-dicarbonyl compounds were isolated in good yields (Fig. 2, substrates 11a-13a). When cyclopentanecarboxylic acid was used, 1,5-dicarbonyl compound 14b was obtained in 65% yield (Fig. 2, substrates 14a), along with small amount of 11b, which was the over-oxidized product.
We then examined aliphatic substituted piperidine-4-carboxylic acids (Fig. 3). For these compounds, Ir-2 turned out to be the better catalyst. Methyl and ethyl groups provided the products in moderate yields (Fig. 3, substrates 15a and 16a). However, for allyl, benzyl and isopropyl substituted substrates, oxidation of these functionalities happened and the main isolated products were 4-piperidinone (Fig. 3, substrates 17a-19a). Four-membered azetidinone substrate gave α-amino ketone product in 58% yield (Fig. 3, substrate 20a). For five-membered pyrrolidine-3-carboxylic acid substrate 21a, β-amino ketone product was isolated in 64% yield. As expected, piperidine-3-carboxylic acids furnished γ-amino ketone products in good yields (Fig. 3, substrate 22a, 23a, 25a). However, due to the oxidation of the benzyl group, the yield for substrate 24a was low.
We were pleased to find out that this method worked with acyclic acids (Fig. 4). For 2,2,2-triphenylacetic acid and 2,2-diphenylpentanoic acid, benzophenone was isolated as the main product in moderate yields (Fig. 4, substrates 26a-27a). However, for 2,2-diphenylpropanoic acid, the main product was acetophenone (Fig. 4, substrates 28a). Different substituted 2-methyl-2-phenylpropanoic acids also furnished acetophenone type products (Fig. 4, substrates 29a-32a). Accordingly, substrate 33a provided propiophenone as the main product in good yield. It’s noteworthy that 3-phenylpropanoic acids could also be compatible and benzaldehyde type products were isolated (Fig. 4, substrates 34a-36a). For 3-phenylbutanoic acid, acetophenone was isolated in 66% yield (Fig. 4, substrate 37a).
We also evaluated this method with β-hydroxy acids (Fig. 5). Interestingly, the C-C cleavage tended to happen at the α-β position, probably because the radical intermediates were stabilized by the β-hydroxy group. Thus, diketones were usually provided as the main products. For substrates which contain tertiary β-hydroxy groups (Fig. 5, substrates 38a-40a), the products were isolated in good yields. However, substrates with secondary β-hydroxy groups delivered the products only in moderate yields (Fig. 5, substrates 41a-42a). For substrate 43a, the C-C cleavage happened at both sides with about 3:1 ratio.
Based on our proposed mechanism (Fig. 1), radical intermediate IV was formed during the process. To capture this intermediate, we tried to add additives. Eventually, we found the addition of Selectfluor successfully delivered ketone-alcohol as the main product. Thus, under the optimized condition, cyclobutanecarboxylic acid substrates provided 4-hydroxybutyrophenones in good yields (Fig. 6, substrates 44a-46a). When cyclopentanecarboxylic acid was used, 5-hydroxybutyrophenone was provided instead (Fig. 6, substrates 47a). For cyclohexanecarboxylic acid, 6-hydroxyhexaphenone was isolated in 75% yield (Fig. 6, substrates 48a). We also tested other six-member cyclic acids, all of them worked smoothly and delivered the products in moderate to good yields (Fig. 6, substrates 49a-53a).
This reaction provides a direct method to construct different diketones, which are versatile building blocks in the synthesis of natural products and bioactive compounds. Thus, we performed the synthesis of Primaperone, Melperone and Haloperidol (Fig. 7). These drugs could be accessed in one step from product 12b via reductive amination in good yields. We also did late-stage modification of commercial drug and complex natural products. For Sertraline derivative 54a, the reaction worked smoothly and the product was isolated in 83% yield. For steroids 55a and 56a, the regioselectivities were good and we only isolated one product. However, the reactions were sluggish and much of the starting materials were recovered.
To better understand the mechanism, we did control experiments (Fig. 8). First, when substrate 15a was evaluated under the reaction condition, compound 15c was isolated as a byproduct in 31% yield. However, when 15c was submitted to the reaction condition, no 15b was formed, which clearly indicates 15c is a by-product, not a reacting intermediate. We did Stern-Volmer experiments and found out the acid 1a could quench the photocatalyst under basic condition. We also found that no reaction happened when compound 57 was treated with the standard condition. These observations clearly demonstrated that photocatalytic decarboxylation was the initial step and the key to induce the consecutive C-C bond cleavage. We also synthesized aldehyde 58a and 59a. When aldehyde 58a was reacted under standard condition, product 58b was formed and we observed the formation of formaldehyde33. When 59a were treated with copper, base and O2, we were able to isolate one carbon shorter products 59b30.
Based on the above experiments and literatures29,30,31,32,33,34, especially the recent publication by Xia31, we propose a plausible mechanism as shown in Fig. 9. Photocatalyzed decarboxylation of substrate 15a provides radical A, which is captured by oxygen to generate peroxyl radicals B. Intermediate B might form the dioxetane intermediate C through hydrogen atom transfer (HAT). Upon thermal cleavage, it delivers dicarbonyl compound D. The oxidative dehomologation of intermediate D renders 15b as the final product. In another possible way, intermediate E might form. The extrusion of formaldehyde through β-scission delivers intermediate F33, which is further oxidized by oxygen to provide 15b as the final product29.
Discussion
In conclusion, we have successfully developed consecutive C-C bond cleavage by taking the advantages of photoredox catalysis along with copper catalysis. This complicated process exploits the use of stable α-trisubstituted acids as substrates and efficiently breaks three C-C bonds at the same time. The key transformation features a decarboxylative oxidation process to generate the in-situ formed alkoxy radical which could trigger fragmentation and the following oxidation process. We believe this finding might shed light on the development of other methods for inert C(sp3)-C(sp3) bond cleavage.
Methods
General procedure for consecutive C-C bond cleavage products
To a 50 ml round bottomed flask equipped with a magnetic stirrer bar were added acid (0.5 mmol, 1.0 equiv.), Ir-1 or Ir-2 (0.015 mmol, 0.03 equiv.), Cu(OAc)2 (18.1 mg, 0.1 mmol, 0.2 equiv.), 2,2′-bipyridine (19.5 mg, 0.125 mmol, 0.25 equiv.), Cs2CO3 (245 mg, 0.75 mmol, 1.5 equiv.) and DCM (10 mL). The flask was quickly degassed three times and flushed with oxygen through balloon, and then the mixture was heated to 30 °C in an oil bath and irradiated with three 45 W blue LEDs (5 cm away) for 40 h or 72 h. The reaction mixture was filtered and concentrated. The residue was purified by column chromatography on silica with petroleum ether/ethyl acetate mixture as the eluent.
General procedure for ketone-alcohol products
To a 50 ml round bottomed flask equipped with a magnetic stirrer bar were added acid (0.5 mmol, 1.0 equiv.), Ir-1 (22.3 mg, 0.015 mmol, 0.03 equiv.), Cu(OAc)2 (18.1 mg, 0.1 mmol, 0.2 equiv.), 2,2′-bipyridine (19.5 mg, 0.125 mmol, 0.25 equiv.), Cs2CO3 (245 mg, 0.75 mmol, 1.5 equiv.), Selectfluor (265 mg, 0.75 mmol, 1.5 equiv.) and DCM (10 mL). The flask was quickly degassed three times and flushed with oxygen through balloon, and then the mixture was heated to 30 °C in an oil bath and irradiated with three 45 W blue LEDs (5 cm away) for 40 h. The reaction mixture was filtered and concentrated. The residue was purified by column chromatography on silica with petroleum ether/ethyl acetate mixture as the eluent.
Data availability
Materials and methods, optimization studies, experimental procedures, mechanistic studies, 1H NMR spectra, 13C NMR spectra and mass spectrometry data generated in this study are provided in the Supplementary Information file.
References
Jones, W. D. The fall of the C-C bond. Nature 364, 676–677 (1993).
Sattler, A. & Parkin, G. Cleaving carbon-carbon bonds by inserting tungsten into unstrained aromatic rings. Nature 463, 523–526 (2010).
Jun, C. H. Transition metal-catalyzed carbon-carbon bond activation. Chem. Soc. Rev. 33, 610–618 (2004).
Guengerich, F. P. & Yoshimoto, F. K. Formation and Cleavage of C-C Bonds by Enzymatic Oxidation−Reduction Reactions. Chem. Rev. 118, 6573–6655 (2018).
Murakami, M. & Ishida, N. Potential of metal-catalyzed C-C single bond cleavage for organic synthesis. J. Am. Chem. Soc. 138, 13759–13769 (2016).
Drahl, M. A., Manpadi, M. & Williams, L. J. C-C fragmentation: origins and recent applications. Angew. Chem. Int. Ed. 52, 11222–11251 (2013).
Zhang, G., Zhang, Z. & Zeng, R. Photoinduced FeCl3-catalyzed alkyl aromatics oxidation toward degradation of polystyrene at room temperature. Chin., J. Chem. 39, 3225–3230 (2021).
Wang, C. et al. Jie. Photo-induced deep aerobic oxidation of alkyl aromatics. Sci. China Chem. 64, 1487–1492 (2021).
Wang, M., Wen, J., Huang, Y. & Hu, P. Selective Degradation of styrene-related plastics catalyzed by iron under visible light. ChemSusChem 14, 5049–5056 (2021).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Fumagalli, G., Stanton, S. & Bower, J. F. Recent methodologies that exploit C-C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 117, 9404–9432 (2017).
Marek, I., Masarwa, A., Delaye, P. O. & Leibeling, M. Selective carbon-carbon bond cleavage for the stereoselective synthesis of acyclic systems. Angew. Chem. Int. Ed. 54, 414–429 (2015).
Sivaguru, P., Wang, Z., Zanoni, G. & Bi, X. Cleavage of carbon-carbon bonds by radical reactions. Chem. Soc. Rev. 48, 2615–2656 (2019).
Yu, X. Y., Chen, J. R. & Xiao, W. J. Visible light-driven radical-mediated C-C bond cleavage/functionalization in organic synthesis. Chem. Rev. 121, 506–561 (2021).
Morcillo, S. P. Radical-promoted C-C bond cleavage: A deconstructive approach for selective functionalization. Angew. Chem. Int. Ed. 58, 14044–14054 (2019).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science 361, 171–174 (2018).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018).
Zuo, Z. et al. Merging photoredox with Nickel catalysis: coupling of α-carboxyl sp3 -carbons with aryl halides. Science 345, 437–440 (2014).
Liu, J. et al. Visible-light-mediated decarboxylation/oxidative amidation of α-keto acids with amines under mild reaction conditions using O2. Angew. Chem., Int. Ed. 53, 502–506 (2014).
Huang, H., Zhang, G. & Chen, Y. Dual hypervalent iodine(III) reagents and photoredox catalysis enable decarboxylative ynonylation under mild conditions. Angew. Chem., Int. Ed. 54, 7872–7876 (2015).
Chu, L., Lipshultz, J. M. & MacMillan, D. W. C. Merging photoredox and nickel catalysis: the direct synthesis of ketones by the decarboxylative arylation of α-oxo acids. Angew. Chem., Int. Ed. 54, 7929–7933 (2015).
Tan, H., Li, H., Ji, W. & Wang, L. Sunlight-driven decarboxylative alkynylation of α-keto acids with bromoacetylenes by hypervalent iodine reagent catalysis: a facile approach to ynones. Angew. Chem., Int. Ed. 54, 8374–8377 (2015).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Sun, X., Chen, J. & Ritter, T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nat. Chem. 10, 1229–1233 (2018).
Marchaj, A., Kelley, D. G., Bakac, A. & Espenson, J. H. Kinetics of the reactions between alkyl radicals and molecular oxygen in aqueous solution. J. Phys. Chem. 95, 4440–4441 (1991).
Taatjes, C. A. Uncovering the fundamental chemistry of alkyl + O2 reactions via measurements of product formation. J. Phys. Chem. 110, 4299–4312 (2006).
Song, H. et al. Photocatalytic decarboxylative hydroxylation of carboxylic acids driven by visible light and using molecular oxygen. J. Org. Chem. 81, 7250–7255 (2016).
Khan, S. N., Zaman, M. K., Li, R. & Sun, Z. A general method for photocatalytic decarboxylative hydroxylation of carboxylic acids. J. Org. Chem. 85, 5019–5026 (2020).
Faraggi, T. M., Li, W. & MacMillan, D. W. C. Decarboxylative oxygenation via photoredox catalysis. Isr. J. Chem. 60, 410–415 (2020).
Kim, S. M., Shin, H. Y., Kim, D. W. & Yang, J. W. Metal-Free Chemoselective Oxidative Dehomologation or Direct Oxidation of Alcohols: Implication for Biomass Conversion. ChemSusChem. 9, 241–245 (2016).
Tu, J. L. et al. Iron-catalyzed ring-opening of cyclic carboxylic acids enabled by photoinduced ligand-to-metal charge transfer. Green. Chem. 24, 5553–5558 (2022).
Zhao, J. et al. Site-specific oxidation of (sp3)C–C(sp3)/H bonds by NaNO2/HCl. Org. Lett. 23, 4057–4061 (2021).
Zhang, K., Chang, L., An, Q., Wang, X. & Zuo, Z. Dehydroxymethylation of alcohols enabled by Cerium photocatalysis. J. Am. Chem. Soc. 141, 10556–10564 (2019).
Ryland, B. L. & Stahl, S. S. Practical aerobic oxidations of alcohols and amines with homogeneous Copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. 53, 8824–8838 (2014).
Acknowledgements
We thank Shanghai Jiao Tong University for financial support (WF220417003 to Z.S.; AF1700038 to R.L.).
Author information
Authors and Affiliations
Contributions
R.L. and Y.D. contributed equally to this paper. Z.S. conceived the project. R.L., Y.D., S.N.K., M.K.Z., J.Z., P.M. and L.H. performed all experiments. All authors analysed the results. Z.S. and R.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, R., Dong, Y., Khan, S.N. et al. Decarboxylative oxidation-enabled consecutive C-C bond cleavage. Nat Commun 13, 7061 (2022). https://doi.org/10.1038/s41467-022-34829-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34829-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.