Abstract
Metal oxides are promising for photoelectrochemical (PEC) water splitting due to their robustness and low cost. However, poor charge carrier transport impedes their activity, particularly at low-bias voltage. Here we demonstrate the unusual effectiveness of phosphorus doping into bismuth vanadate (BiVO4) photoanode for efficient low-bias PEC water splitting. The resulting BiVO4 photoanode shows a separation efficiency of 80% and 99% at potentials as low as 0.6 and 1.0 VRHE, respectively. Theoretical simulation and experimental analysis collectively verify that the record performance originates from the unique phosphorus-doped BiVO4 configuration with concurrently mediated carrier density, trap states, and small polaron hopping. With NiFeOx cocatalyst, the BiVO4 photoanode achieves an applied bias photon-to-current efficiency of 2.21% at 0.6 VRHE. The mechanistic understanding of the enhancement of BiVO4 properties provides key insights in trap state passivation and polaron hopping for most photoactive metal oxides.
Similar content being viewed by others
Introduction
Hydrogen production via photoelectrochemical (PEC) water splitting is an underpinning technology to converting intermittent solar energy into storable and transportable chemical fuels1. Developing efficient photoelectrode materials has proved to be highly demanding, especially for photoanodes, due to sluggish water oxidation reaction kinetics and anodic (photo)corrosion2. Monoclinic bismuth vanadate (BiVO4) is particularly interesting; it was first used as a powder photocatalyst in suspension system3 and further developed to be a top-performing metal oxide photoanode to photoelectrochemically split water4. However, its poor charge carrier transport leads to severe bulk and surface charge recombination, preventing BiVO4 photoanodes from achieving the theoretical maximum activity5, particularly at low-bias voltage. The poor charge transport is mainly due to trap-limited and polaron-bounded electron transport6,7.
Most studies of modified BiVO4 photoanodes achieved relatively high performance only with the assistance of relatively high bias voltages, typically up to 1.23 V vs. reversible hydrogen electrode potential (VRHE)8,9,10,11,12. When BiVO4 photoanodes operate at lower bias voltages, a large fraction of the photoexcited charge carriers cannot be extracted efficiently, resulting in recombination losses by potential “traps” along the transport pathway. As a result, the reported charge separation efficiencies at potentials ≤0.6 VRHE remain lower than 70%13,14,15,16.
Recent studies have uncovered that native defects17,18 may introduce deep and/or mid-bandgap trap states, stimulating photoinduced carrier trapping and nonradiative recombination19. However, even when intrinsic defects have been alleviated, the low electron mobility and poor charge carrier transport are not fully overcome. This has recently been attributed to small polaron formation20,21,22,23, which ultimately determines the excited state carrier dynamics of BiVO4. The movement of small polarons occurs via hopping to overcome the energy barriers induced by the local polarization of the lattice and is much slower than band transport. Like deep or mid-bandgap trap states, small polaron formation can undermine the performance of BiVO4 mainly in two ways, i.e., by limiting the photoinduced Fermi-level splitting (photovoltage)24,25 and by increasing the recombination losses26.
Herein, we combine climbing nudged elastic band (cNEB) simulation and temperature-dependent conductance measurements and provide evidence for the decrease of polaron hopping activation barriers of BiVO4 photoanodes upon incorporating phosphorus. Transient open circuit potential (OCP) measurements confirm that phosphorus doping also passivates the shallow and deep trap states that are intrinsically formed on the BiVO4 surface, thereby increasing the open-circuit photovoltage. Time-resolved microwave conductivity measurements (TRMC) reveal that the phosphorus-doped BiVO4 photoanode has ~1.7 times longer lifetime, ~2.8 times higher mobility, and ~2.2 times longer effective diffusion length of charge carriers compared to the pristine sample. The modulated charge transport in phosphorus-doped BiVO4 by concurrently mediating the carrier density, polaron hopping, and trap state introduces efficient low-bias PEC water splitting.
Results
Characterization of phosphorus-doped BiVO4 samples
The synthesis recipe for BiVO4 thin films was adopted from the literature4. Subsequently, phosphorus doping in BiVO4 was achieved by exposure to locally generated phosphine gas and water vapor in a tube furnace (Supplementary Fig. 1). This approach enables precise control over the doping degree of the samples (details in Methods), producing three samples labeled P-BiVO4−0.5 mg, P-BiVO4−1 mg, and P-BiVO4−5 mg based on the phosphorus precursor amount of 0.5 mg, 1 mg, and 5 mg, respectively.
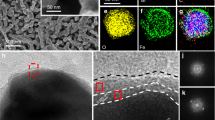
The scanning electron microscopy (SEM) images and X-ray diffraction (XRD) patterns of phosphorus-doped BiVO4 samples show negligible variations compared to the pristine sample (Fig. 1a–c and Supplementary Fig. 2a). A more detailed discussion regarding their morphological and crystallographic properties is provided in Supplementary Information. The high-angle annular dark-field (HAADF) image of the phosphorus-doped BiVO4 sample is shown in Fig. 1e. The energy-dispersive X-ray spectroscopy (HAADF-EDX) elemental maps illustrate that P, Bi, V, and O are homogeneously distributed within the phosphorus-doped BiVO4 sample (Fig. 1f–i), indicating that the phosphorus has been doped into the BiVO4 lattice without inducing segregated phase. The incorporation of phosphorus into the BiVO4 lattice is further confirmed from the X-ray photoelectron spectra (XPS), as shown in Fig. 2a–d and Supplementary Fig. 3a–d. The emergence of the P 2p peak indicates the presence of phosphorus in the BiVO4 lattice. The location of the P 2p peak (132.9 eV) agrees well with the P5+ ions in PO43- anions27, suggesting that the phosphorus ions reside at the V sites in the BiVO4 lattice. The Bi 4 f and V 2p peaks of phosphorus-doped BiVO4 samples are slightly shifted to higher binding energies than those of the pristine BiVO4. The shifts indicate a change in the local coordination of Bi and V ions. In previous studies on N-doped BiVO4, a shift to lower binding energies was observed and attributed to an increase in the concentration of oxygen vacancies13,28. Based on this, we tentatively assign the shift to higher binding energies after phosphorus-doping to a decrease in the concentration of oxygen vacancies. Note that oxygen vacancies are ubiquitous in the BiVO4 synthesized by the adopted strategy and in commercial samples17,19.
Scanning electron microscopy (SEM) image of a the pristine BiVO4 and b the phosphorus-doped BiVO4 sample (P-BiVO4-1 mg). c X-ray diffraction patterns and d light harvesting efficiency (LHE) derived from the UV-vis absorption spectra of the BiVO4 and phosphorus-doped BiVO4 samples. The asterisk (*) remark in c denotes the diffraction peak of the FTO substrate. e High-angle annular dark-field (HAADF) image and the corresponding energy-dispersive X-ray (EDX) mapping images of f Bi, g V, h O, and i P of the phosphorus-doped BiVO4 photoanode.
The O 1 s spectra of phosphorus-doped BiVO4 samples can be fitted using three main peaks (Fig. 2d). The lowest binding energy peak (529.5 eV) is assigned to the lattice oxygen of BiVO4. The other peaks at binding energies of 530.8 eV and 532.6 eV correspond to oxygen atoms in the P=O bond and the P-O-P bond of PO43- anions, respectively27. Indeed, with an increasing amount of phosphorus precursor, the ratio of the peak area between that of oxygen atoms in the P=O bond and the lattice oxygen of BiVO4 gradually increases, suggesting a reasonable control of the doping degree enabled by the gas-phase doping method (Supplementary Fig. 3d)27. The intensity of the peak at 531.6 eV, which is also present in the pristine BiVO4 and has been frequently assigned to surface-bounded hydroxyl radicals due to the oxygen vacancies of BiVO429, is reduced.
The wavelength-dependent light-harvesting efficiencies (LHEs) were calculated from the measured transmission (T) and reflection (R) as LHE\(=\)1-T-R. The phosphorus-doped BiVO4 samples show a slightly shifted band-to-band absorption onset to longer wavelengths than the pristine sample (515 nm), as shown in Fig. 1d. Moreover, the phosphorus-doped samples show a stronger light absorption in the 380–600 nm wavelength range (Supplementary Fig. 2b). The improvement of the LHE at longer wavelengths is tentatively ascribed to phosphorus dopants with strong electron-donating ability, which modifies the atomic arrangement of BiVO4 with less strongly localized electrons and increases free-electron absorption.
Photon-to-current conversion efficiencies
Initial photoelectrochemical measurements of the phosphorus-doped and pristine BiVO4 samples in a 0.1 M phosphate buffer (KPi) solution were done in the presence of a hole scavenger (SO32-). The amount of precursor used for phosphorus-doping was optimized based on the incident photon-to-current conversion efficiency (IPCE) and photocurrent density results (Supplementary Fig. 4a, b), and the optimum amount of 1 mg was chosen for further PEC sulfite oxidation studies.
In Fig. 3a, the optimized phosphorus-doped BiVO4 sample shows enhanced IPCE values under monochromatic irradiance measured at 0.6 VRHE, and the IPCE onset is red-shifted by ~10 nm. The phosphorus-doped BiVO4 sample shows an IPCE value of 75% at 410 nm and an onset at ~530 nm. In comparison, the IPCE of the pristine BiVO4 is only 48% at 410 nm, and the onset is between 510 and 520 nm, which matches the measured LHE and the literature13. As the LHE of BiVO4 is slightly changed upon phosphorus doping, the absorbed photon-to-current conversion efficiency (APCE) offers a more precise evaluation of the internal photon-to-current conversion efficiency by BiVO4 samples. The calculated APCE values at 0.6 VRHE of the phosphorus-doped BiVO4 are higher than those of the pristine sample (e.g., 87% vs. 56% at 410 nm, see Fig. 3b). The enhanced APCE indicates that the charge separation in BiVO4 was also improved in addition to the increased light absorption by incorporating phosphorus4.
a IPCE curves and b APCE curves of the BiVO4 and phosphorus-doped BiVO4 samples measured at 0.6 VRHE. c Current density-voltage curves of the BiVO4 and phosphorus-doped BiVO4 samples measured under rear side illumination with AM 1.5 G simulated light. d ηsep calculated from the current density-voltage curves for the BiVO4 and phosphorus-doped BiVO4 samples.
As shown in the current density-voltage (J-V) plots measured under rear side illumination with AM 1.5 G simulated sunlight (Fig. 3c), the phosphorus-doped BiVO4 sample exhibits a photocurrent density for sulfite oxidation (Jsulfite) of 4.08 (\(\pm\)0.2) mA cm−2 at 0.6 VRHE. In contrast, the pristine BiVO4 sample only produces a Jsulfite of 2.62 (\(\pm\)0.3) mA cm−2 under identical conditions. The phosphorus-doped BiVO4 sample shows a slightly negative shift of the onset potential (~50 mV) and a faster increase of photocurrent (i.e., higher slope) between 0.2 to 0.4 VRHE compared with the pristine BiVO4. This indicates an improved fill factor, which likely arose from the work function tuning upon phosphorus incorporation14.
Integrating the product of the LHE of the phosphorus-doped and pristine BiVO4 samples with the solar photon flux gives the theoretical maximum absorbed photon-to-current density (Jabs) of 5.10 mA cm−2 and 4.68 mA cm−2, respectively14. The charge separation efficiency (ηsep) can then be calculated using the following relationship: ηsep \(\approx\) Jsulfite/Jabs. The J-V curves measured at the potential between 0 to 1.23 VRHE for the phosphorus-doped BiVO4 photoanode show the steeper rise of current densities above 1.0 VRHE both under light irradiation and in the dark (Supplementary Fig. 5a), which indicates the arising of the electrochemical oxidation process of SO32−. To avoid the contribution of current density from electrochemical SO32− oxidation, ηsep was calculated at the applied potential up to 1.0 VRHE. The ηsep of the phosphorus-doped BiVO4 measured at 0.6 VRHE is 80%, which is ~1.43 times higher than that of the pristine sample (Fig. 3d). Remarkably, the doped BiVO4 achieves a record high ηsep of 99% at 1.0 VRHE30,31,32. The ηsep calculated from the net photocurrent density between 0 to 1.23 VRHE for the phosphorus-doped BiVO4 photoanode was also provided (Supplementary Fig. 5b). We note that the excellent sulfite oxidation performances of the phosphorus-doped BiVO4 samples at low applied potentials (≤1.0 VRHE) are not only better than those of the state-of-the-art BiVO4 photoanodes (Supplementary Table 1) but also superior to those of other metal oxide photoanodes to date33,34.
Insights into the charge transport mechanism
TRMC measurements further evaluated the charge carrier transport properties of the prepared samples. We are interested in how phosphorus incorporation affects the carrier mobility, lifetime, and diffusion length of BiVO4. Figure 4 shows the TRMC signals as a function of time for the pristine and phosphorus-doped BiVO4, measured under pulsed laser excitation of 470 nm with an intensity of 2.5 × 1014 photons cm−2 pulse−1. Based on the peak amplitude of TRMC signals (\(\phi \sum {{{{{\rm{\mu }}}}}}\)), the carrier mobility of BiVO4 increases by ~2.8 times upon phosphorus doping. The carrier lifetime, obtained from the exponential fit of the \(\phi \sum {{{{{\rm{\mu }}}}}}\) signal transients, increases from 47.5 to 80.6 ns upon phosphorus doping. We also found that the effective diffusion length of the BiVO4 sample increases by ~2.2 times after phosphorus doping (details in Methods). TRMC measurements at other wavelengths of 410 nm and 450 nm were also performed (Supplementary Fig. 6a, b), showing comparable mobility increments of ~2.0 and ~2.5 times.
Recent reports suggested that the modest mobility of BiVO4 is due to a small polaron hopping mechanism20,21,22,23. The small polarons, which are electrons that are self-trapped in a local lattice distortion induced by the electron’s own charge, require thermal vibration energy to hop from one site to the next. The energy required for this process is known as the polaron hopping activation energy (Ea). Specifically, the Ea of BiVO4 samples can be derived from the equation \(\sigma (T)=A{T}^{-1}{\exp }\left(-{E}_{a}/{kT}\right)\), where σ(T) is the conductance at a particular temperature (T), A is a constant, and k is the Boltzmann constant. We determined the conductivity of BiVO4 electrodes at different temperatures (15 oC–60 oC) from dark EIS analysis with an applied bias of 0.6 VRHE. The real part of conductivity (\({\sigma }^{{\prime} }\left(\omega \right)\)) as a function of frequency (ω) for both BiVO4 electrodes with and without phosphorous doping at different temperatures is shown in Figs. 5a and 5b, respectively. The d.c. conductivity (\({\sigma }_{d.c.}\)) was determined by fitting the (\({\sigma }^{{\prime} }\left(\omega \right)\) to the relation \({\sigma }^{{\prime} }\left(\omega \right)={\sigma }_{d.c.}+A\left(T\right){\omega }^{n}\), where the \({A}\left(T\right)\) is the temperature dependent frequency pre-exponential factor; and the exponent, n, generally varies between 0 and 135,36. There is close agreement between the experimental and fitted data, as shown in the inset of Fig. 5a, typically for the BiVO4 sample at 25 oC. Their conductivities were increased with raising the temperature, and the n values ranged from 0.6 to 0.8, suggesting the small polaron transport behavior37,38. Moreover, as further determined from the \({ln}\left[\sigma \left(T\right)\times T\right]-1/T\) plots (Fig. 5c), the Ea of BiVO4 decreases from 434 meV to 273 meV after incorporating phosphorus. It is worthy note that the experimental value of Ea for pristine BiVO4 is very close to the reported value of a recent work by Tang et al.39 The smaller Ea in the phosphorus-doped BiVO4 means that electrons require less energy to hop from one BiVO4 lattice site to the next, which likely arises from the changes of coordination environment induced by incorporating the phosphorus dopant. Such temperature-dependent conductance can also be attributed to charge transfer processes at the surface; however, this is unlikely be the only contributing factor considering the relatively high value of the activation energy that a facile hole scavenger was used in the electrolyte. The decrease of Ea also agrees well with the higher carrier mobility measured using TRMC. Further investigation on the variation of small polaron hopping behavior upon phosphorus doping is uncovered by computational studies, which will be discussed in the following section of theoretical calculations.
Real part of conductance plots measured at different temperatures in the dark with a bias voltage of 0.6VRHE for a pristine BiVO4 and b phosphorus-doped BiVO4 in the electrolyte of 0.1 M KPi buffer solution containing Na2SO3. Inset: fitted ac conductivity plot at 25oC for the BiVO4 sample. c DC Conductance fitting based on the small polaron transport derived from temperature-dependent conductance measured at 0.6 VRHE.
After investigating the charge transport, we are now in a position to understand the surface charge collection behavior of BiVO4 upon phosphorus doping. Previous studies have suggested that measuring the ND is an effective method to compare the variations of depletion width (WD), thus investigating the band bending for hole collection at the semiconductor/electrolyte interface (SEI)33. As observed from the Mott-Schottky results (Supplementary Fig. 7), the phosphorus-doped BiVO4 has a positive shift of EFB by 0.05 V and an increase of ND by 1.5 times. We thereby found that the WD of the BiVO4 sample decreases more than 18% upon phosphorus doping (details in Methods), suggesting a steeper band bending formed at SEI. The steeper band bending might enhance hole extraction and suppress surface recombination in the depletion region.
Moreover, the OCP profiles show that the phosphorus-doped BiVO4 has a higher photovoltage (Vph, 0.49 V) relative to the pristine BiVO4 sample (0.37 V) suggesting a more favorable thermodynamic driving force for the surface redox reaction (Supplementary Fig. 8). The pristine BiVO4 shows a more positive OCPdark (0.48 VRHE) and a moderate OCPlight (0.11 VRHE), which is possibly due to Fermi-level pinning by surface states (trapping electrons at V4+ sites)40. On the other hand, the phosphorus-doped sample achieved an enlarged Vph with an OCPdark of 0.55 VRHE and an OCPlight of 0.06 VRHE, attributed to the passivation of the surface states and the absence of Fermi-level pinning14. The resulting higher Vph upon phosphorus doping is in good agreement with the negatively shifted onset potential shown in the J–V curves (Fig. 2c). The OCP decay transients further support the passivation of surface states in the BiVO4 sample upon phosphorus doping. Upon turning off the illumination, the transient OCP profile of the phosphorus-doped sample shows a faster decay than that of the pristine BiVO4. This suggests that the trap-limited recombination process at the surface states is mediated after incorporating phosphorus. More interestingly, the OCP response of the pristine BiVO4 is less reversible than that of the phosphorus-doped BiVO4, which indicates that more charge carriers are trapped in deep trap states of the pristine BiVO4 relative to the phosphorus-doped sample, likely at the BiVO4 surface41.
Theoretical calculations
To offer insights into the superior charge separation performance of phosphorus-doped BiVO4 sample, the hopping processes of small polaron in BiVO4 with and without phosphorus doping were compared using the cNEB method. The position of phosphorus atoms in the lattice with charged states was again confirmed by theoretical calculations (details in Methods). Under the O-poor condition (Supplementary Fig. 9a), the interstitial phosphorus with a charge state of +5 can be the most stable defect only when the Fermi energy is lower than 0.33 eV, while in a wider range of Fermi energy from 0.33 to 2.1 eV, the substitution of V by phosphorus still possesses the lowest formation energy among the other possibilities (i.e., interstitial, Bi-substitution, and O-substitution). Under the O-rich condition (Supplementary Fig. 9b), the substitution of V by phosphorus is the most stable state for the whole range of Fermi energy within the bandgap of BiVO4. The results suggest that phosphorus likely replaces V and sits at its lattice sites in BiVO4, consistent with the experimental XPS observations.
The pristine BiVO4 sample was modeled by a \(2\times 1\times 2\) supercell, while 3 V atoms were replaced with phosphorus atoms to simulate the doped sample according to the P to P+V ratio of 18% observed from XPS. Several substitution configurations were tested, and the one with the lowest energy was adopted (Fig. 6a). An extra electron was added to the simulated cell, which distorts one of the V atoms to form a small polaron. In pristine BiVO4, the polaron hopping in all directions is identical, and the barrier was calculated to be 268 meV (the dashed line shown in Fig. 6b). However, in phosphorus-doped BiVO4, the existence of phosphorus atoms breaks the symmetry of BiVO4, and the hopping path becomes anisotropic. Seven hopping paths between two adjacent V sites along different orientations were selected to investigate the polaron migration barriers in phosphorus-doped BiVO4 (Fig. 6a). The results show that the hopping barriers for most selected paths are smaller than that in pristine BiVO4, with the lowest barrier of 239 meV for the hopping path 5 (Fig. 6b and the inset table). As conductance is proportional to charge carrier mobility, the same exponential dependence on the migration barrier is expected: \(\mu \propto {\exp }\left({-E}_{a}/{kT}\right)\). Based on this relationship, a decrease of Ea by 29 meV (path 5) results in a ~3.1 times increase of charge carrier mobility at room temperature, which is in a similar magnitude with the carrier mobility increment observed by TRMC. These results suggest that phosphorus dopants, with their strong electron-donating ability, are electrostatically repulsive to the small polaron V4+O4 unit42, offering a lower barrier to hop away from the adjacent V sites (Fig. 6c, d)26,43. Moreover, due to the stronger interactions between phosphorus and O atom, the simulated P-O bond distance is 1.559 Å, which is 14.3% shorter than that of the V-O bond with a small polaron (1.820 Å), creating a structural distortion in the lattice of BiVO4. Such structural distortion has also been reported to accelerate polaron migration23,43.
a Schematic small polaron hopping paths in the phosphorus-doped BiVO4 sample for DFT calculations. Only V (red) and phosphorus (purple) atoms are displayed in the schematic structure model. Atoms highlighted are within the supercell and the rest are in the neighbor cells. The colored arrows are the selected hopping paths in the calculations. b Hopping energy barriers in phosphorus-doped BiVO4 sample along the reaction coordination of different reactions paths. The dashed line represents the hopping barrier in pristine BiVO4. The below table shows the hopping barriers of different paths in phosphorus-doped BiVO4. c Schematic small polaron formation: Initial excitation of BiVO4 leads to a charge transfer transition from oxygen (light grey) to V atom (blue). The initial excited carriers undergo optical-phonon scattering, during which the charge can be trapped in a small polaron, resulting the lattice expansion. Adapted and reprinted with permission from Rettie, A. J. E., Chemelewski, W. D., Emin, D. & Mullins, C. B. Unravelling small-polaron transport in metal oxide photoelectrodes. J. Phys. Chem. Lett. 7, 471–479 (2016). Copyright 2016 American Chemical Society. d Schematic small polaron hopping between two identical V atoms in VO4 units with potential energy landscape. Solid circles indicate the polaron location. The dark and red dashed curves indicate adiabatic surfaces of BiVO4 and phosphorus-doped BiVO4 samples, respectively. Ea and Ea’ represents the polaron hopping energy barriers of BiVO4 and phosphorus doped BiVO4 samples. Adapted and reprinted with permission from Rettie, A. J. E., Chemelewski, W. D., Emin, D. & Mullins, C. B. Unravelling small-polaron transport in metal oxide photoelectrodes. J. Phys. Chem. Lett. 7, 471–479 (2016). Copyright 2016 American Chemical Society. e The potential energy surface for the ground and the photon excited states with polaron formation states and the corresponding re-excitation energy barriers (EST) for BiVO4 (solid red) and phosphorus-doped BiVO4 (dashed red) samples.
We note that the calculated results of Ea are relatively smaller than the experimentally measured values of pristine BiVO422,23,38. We attribute the difference to the difficulty in experimentally quantifying the intrinsic oxygen vacancies in BiVO4. Recent studies have suggested that excessive oxygen vacancies could coulombically hinder small polaron migration13,17. Since oxygen vacancies were not quantified and considered in our modeling, the smaller simulated values relative to experimental data are expected. A similar phenomenon has been observed in a recent report on molybdenum-doped BiVO4 photoanode23. The previous discussion on the XPS data has suggested that phosphorus incorporation alleviated the native oxygen vacancies existed in the BiVO4 lattice. Therefore, apart from the substitution of V by phosphorus atoms, the suppressed oxygen vacancies could also contribute to moderating the polaron hopping barrier17.
The self-trapping energy of polaron states (the stabilization energy gained upon lattice distortion and localization, ΔEST) is calculated as the energy difference between the delocalized and polaronic states44. The ΔEST of pristine and phosphorus-doped BiVO4 samples were determined to be 0.39 and 0.34 eV (averaged over all polaronic positions considered in Fig. 6a), respectively. The calculated ΔEST values are comparable with the reported values in the literature13,45. A schematic diagram of the potential energy landscape for the band states with polaron formation is given in Fig. 6e, the small polaron state of BiVO4 becomes less stable and easier to be re-excited to a delocalized band state by photons upon the incorporation of phosphorus, which could facilitate charge transport and reduce the chance of recombination with photoinduced holes as electron transport in band state is more effective than that through localized states.
PEC water-splitting activity
The PEC water splitting performance of phosphorus-doped BiVO4 samples coated with oxidation cocatalyst was tested without a hole scavenger. A NiFeOx cocatalyst for the oxygen evolution reaction (OER) was deposited on the phosphorus-doped BiVO4 surfaces by pulsed photo-electrodeposition method (details in methods). The J–V results show that the phosphorus-doped BiVO4 sample coated with NiFeOx cocatalyst attains 3.51 (\(\pm\)0.15) mA cm−2 at 0.6 VRHE under AM 1.5 G illumination, which is much higher than that of the bare phosphorus-doped BiVO4 and 69% higher than that of the BiVO4 sample with NiFeOx cocatalyst (Fig. 7a). Moreover, the NiFeOx/phosphorus-doped BiVO4 shows a negatively shifted onset potential of ~320 mV compared to the phosphorus-doped BiVO4 sample, which is due to the effectively suppressed surface recombination46. Figure 7b shows the half-cell applied bias photon-to-current efficiencies (ABPEs) of the pristine and phosphorus-doped BiVO4 samples coated with NiFeOx cocatalyst. The ABPE is calculated to be 2.21% at 0.6 VRHE for the phosphorus-doped BiVO4, which is not only higher than the pristine counterpart (1.40% at 0.6 VRHE) but also among the highest reported for BiVO4 (Supplementary Table 2).
a Current density-voltage curves of the BiVO4 and phosphorus-doped BiVO4 samples with co-catalyst of NiFeOx measured under back illumination with AM 1.5 G simulated sunlight for water-splitting. b ABPEs of the BiVO4 and phosphorus-doped BiVO4 samples with NiFeOx measured in a three-electrode configuration. c Gases evolved from the phosphorus doped BiVO4 samples at 0.6 VRHE under back illumination for 3 h and the calculated amounts of gas assuming 100% Faradaic efficiency (dashed line). d Chronoamperometry curve of the phosphorus-doped BiVO4 sample with co-catalyst of NiFeOx measured at 0.6 VRHE under AM1.5 G simulated sunlight.
As demonstrated in the video (Supplementary Movie 1), the NiFeOx/phosphorus-doped BiVO4 sample exhibited a robust water oxidation process at 0.6 VRHE under AM 1.5 G irradiation. The evolved H2 and O2 gases were measured and compared with the theoretical values shown in Fig. 7c. The ratio of H2/O2 detected was ~2:1 with a calculated Faradaic efficiency of 98% for O2 production at 0.6 VRHE. The chronoamperometry result collected at 0.6 VRHE does not show obvious current decay over 10 h, which indicates that the phosphorus-doped BiVO4 sample coated with NiFeOx cocatalyst is relatively stable under the operation conditions (Fig. 7d).
Discussion
Considering that the poor charge carrier transport of BiVO4 photoanode is mainly due to the electron trapping and fast charge recombination, the reduction of polaron hopping barrier and surface trap states frees the self-trapped charge carriers and facilitates the charge transport in BiVO4. A schematic diagram of the band structures for the pristine and the phosphorus-doped BiVO4 samples is provided in Fig. 8a–f. With increased ND and positively shifted EFB, phosphorus doping steepened the band bending for charge collection at SEI (Fig. 8d–f). Moreover, the cNEB simulation results suggest that Ea and EST were reduced upon phosphorus doping, moving the polaron state of BiVO4 closer to the band edge (Fig. 8b, e). Surface states intrinsically exist in the pristine BiVO4 and elevate the Femi-level position in the dark (Fig. 8a). Together with the stronger polaron formation, the surface states reduce the maximum quasi-Fermi-level splitting that can be achieved under light illumination (Fig. 8b), thus resulting in a low Vph13,47. Benefiting from the passivated surface states, the phosphorus-doped BiVO4 sample shows a closer position of Fermi-level (OCPdark) relative to the valence band maximum (VBM) in the dark, as shown in Fig. 8d. A similar phenomenon was recently reported for the molybdenum doped BiVO414. Under AM 1.5 G irradiation, the Fermi-level position (OCPlight), CBM and VBM are all elevated, leading to the band flattening in both samples (Fig. 8b, e). At the same time, the quasi Fermi-level splitting (Vph) of BiVO4 photoanode is enlarged by phosphorus doping, which arises from the truncation of surface states and Fermi-level pinning (Fig. 8e). After turning off the light, the slow communication of the band edges with the deep surface states in the pristine BiVO4 slows down the relaxation of OCPlight to OCPdark (Fig. 8c). On the other hand, the doped sample relaxed much faster back to the initial OCPdark (Fig. 8f), indicating a reduced number of deep trap states at the surface. Based on the above discussions, we again suggest that phosphorus-doped BiVO4 samples have unique properties including efficient light absorption, superior conductivity (carrier density and electron mobility), steeper band bending for hole collection, and enlarged photovoltage. These characteristics promote charge separation, transport, and collection and finally enable considerable charge carriers to travel long distances at low-bias voltage.
Band structures and band bending schematics of the BiVO4 and phosphorus-doped BiVO4 samples. a, d Dark equilibrium state, b, e light-irradiation state, and c, f secondary-dark equilibrium state. The relative positions of conduction band minimum (CBM), valence band maximum (VBM), OCP in the dark (OCPdark), OCP under illumination (OCPlight), depletion width (WD), deep defects (solid purple remark beneath CBM) and polaron states (solid blue remark beneath CBM) are constructed from the UV–vis, Mott-Schottky, TRMC, OCP and cNEB simulation data. Adapted and reprinted with permission from Ye, K.-H. et al. Enhancing photoelectrochemical water splitting by combining work function tuning and heterojunction engineering. Nat. Commun. 10, 3687 (2019). Published under (CC-BY) license.
In summary, this work has attempted to fundamentally understand the multiple roles of substitutional phosphorus incorporation in mediating charge transport of the nanoporous BiVO4 samples. The synergistic effects have allowed the phosphorus-doped BiVO4 to exhibit record high photon-to-current conversion efficiencies with APCE of 87% and ηsep of 80% at 0.6 VRHE. NiFeOx water oxidation catalyst was coated to facilitate charge injection at the phosphorus-doped BiVO4 surface, demonstrating a high ABPE of 2.21%. This study extends the understanding of the doping effect of metal oxide photoelectrodes beyond the increased carrier density to now include charge transport dynamics, particularly with mediating trap states and polaron hopping.
Methods
Synthesis of BiVO4 and phosphorus-doped BiVO4 samples
The BiVO4 sample was fabricated following the typical procedure reported in the literature4. In brief, BiOI nanoflakes were firstly electrodeposited on the pre-cleaned FTO substrate from a 0.04 M Bi(NO3)3 solution containing 0.4 M KI and 0.23 M p-benzoquinone for 5 mins. Subsequently, 150 µL of DMSO solution containing 0.2 M vanadyl acetylacetonate was impregnated on the prepared BiOI sample at 60 oC and then calcined in Air at 450 oC for 2 h with a ramping rate of 2 oC min−1. The obtained samples were soaked in 1 M NaOH solution for 30 mins with gentle stirring to remove the impurities. Lastly, the samples were sequentially rinsed with water and ethanol. Phosphorus-doped BiVO4 samples were prepared by a gas phase implementation method. In a typical procedure, 0.5 mg, 1 mg, or 5 mg of NaH2PO2·H2O was placed in the middle of a furnace tube and the fabricated BiVO4 sample was located at the downstream side with a distance of 26 cm. Ar flow was purged in the furnace tube for 30 min before elevating the temperature. Subsequently, the temperature of the furnace tube was elevated with a ramping rate of 2 oC min−1 and was held at 300 oC for 30 mins under Ar atmosphere. Thermal decomposition of NaH2PO2·H2O led to the formation of phosphine gas. The phosphorus-doped BiVO4 samples were carefully taken out from the furnace tube after the temperature naturally cooled down to room temperature. The corresponding treated samples are denoted as P-BiVO4−0.5 mg, P-BiVO4−1 mg, and P-BiVO4−5 mg, respectively.
Depositing of NiFeOx cocatalysts on phosphorus-doped BiVO4 samples
NiFeOx water oxidation cocatalyst was deposited on the photoactive surfaces of BiVO4 samples by a pulsed photoelectrodeposition method reported14. In brief, NiFeOx catalyst was deposited on the prepared BiVO4 samples at 0 VRHE in 0.4 mM Fe(CH3COO)2 and 0.04 mM Ni(CH3COO)2 solution. The backside of the BiVO4 sample was irradiated by AM 1.5 G light source with a chopped on/off cycle of 10 s/20 s for 20 min.
Characterization
Microscopic morphologies were characterized by a scanning electron microscope (FEI Nova Nano SEM 450). The crystal structure of the prepared samples was studied by a PANalytical Empyrean II X-Ray diffractometer (XRD) with a Cu Kα radiation source (λ=1.54060 Å). X-ray photoelectron spectroscopy (XPS) measurements were done using a Thermo Scientific (ESCALAB220i-XL) instrument with a monochromatic Al Kα X-ray source at 1486.68 eV. The presented XPS data and binding energies were calibrated by the carbon 1 s peak at 284.6 eV. The high-resolution transmission electron microscopy (HRTEM) images and corresponding energy-dispersive X-ray spectroscopy (EDX) mapping results were obtained using FEI Talos FE200x G2 instrument. The optical absorption properties of the samples were recorded using a UV–Vis–NIR spectrophotometer equipped with an integrating sphere (UV-3600, Shimadzu).
PEC measurements
PEC measurements were carried out using a CHI 660E potentiostat. A three-electrode cell with a flat quartz window was used, in which a Pt wire and an Ag/AgCl electrode were used as the counter and the reference electrodes, respectively. The electrolyte was 0.1 M KPi buffer solution (pH \(=\)7). For sulfite oxidation studies, 1 M Na2SO3 was added to the electrolyte. The electrolytes were purged with argon gas for 30 min before measurement to remove any dissolved oxygen. While the simulated solar irradiance was acquired by illuminating light from a 300 W Xe arc lamp (Oriel Newport) through a neutral density filter and an AM 1.5 G filter. The intensity of the light source was calibrated to 100 mW cm−2 at the surface of the FTO side by a Newport optical power meter. The output spectral distribution of the Oriel Newport lamp is calibrated with the standard AM 1.5 G spectrum. J–V curves were obtained using linear sweep voltammetry which was performed from 0 to 1.23 VRHE at a scan rate of 10 mV s−1. The potential was converted from Ag/AgCl potentials by Eq. (1)
The conductance-frequency curves were determined from EIS results by applying an alternating potential with 5 mV amplitude in the frequency from 1 to 105 Hz at 0.6 VRHE. Mott–Schottky results were obtained at a frequency of 103 Hz and a scan rate of 10 mV s−1 in the dark. The ND can be determined using Eq. (2)
where q is the electron charge, \(\varepsilon\) is the dielectric constant of BiVO4 semiconductor, \({\varepsilon }_{0}\) is the permittivity of vacuum, c is the capacitance of the depletion region, V is the applied voltage, and \(d(1/{c}^{2})/dV\) is the slope of the Mott–Schottky plot. The WD for materials sharing similar morphology is calculated using Eq. (3)
where EFB is the flat band position. Open circuit potentials were collected in 0.1 M KPi solution with 1 M Na2SO3 after testing 2800 s in the dark and under AM 1.5 G irradiation. Temperature-dependent EIS measurement was performed on CHI 660E potentiostat equipped with an oil bath and hot-plate. Under the dark condition, conductance-frequency data of BiVO4 samples were determined at different temperatures with an applied bias of 0.6 VRHE.
IPCE was examined under monochromatic irradiation from a 300 W Xe lamp (Newport Oriel) equipped with a monochromator (Newport Oriel) at 0.6 VRHE in 0.1 M KPi with 1 M Na2SO3. The intensity of light (P(λ)) was measured by a Newport optical power meter connected with a calibrated silicon photodiode detector. The IPCE values were obtained by Eq. (4)
where λ is the wavelength and I is the photocurrent density. APCE data at each wavelength was calculated according to Eq. (5)
Jabs is the photon absorption rate presented as the photocurrent density of BiVO4 samples, which is obtained according to Eq. (6)
where h is the Plank constant, c is the light speed, \({\eta }_{{{{{{\rm{abs}}}}}}}\) is the light absorbance of the BiVO4 samples. ηsep was calculated by Eq. (7)
where \({J}_{s{{{{{\rm{ulfite}}}}}}}\) is the photocurrent density obtained in sulfite oxidation (assuming the surface charge injection efficiency for sulfite oxidation is nearly 100%). The ABPE values were determined from the J-V curves in a three-electrode system using Eq. (8)
where\(\,{J}_{{{{{{{\rm{H}}}}}}}_{2}{{{{{\rm{O}}}}}}}\) is the photocurrent density measured in water oxidation, P is the light intensity of AM 1.5 G (100 mW cm−2) and Vbias is the applied bias potential versus RHE.
Gas evolution measurements were performed by side illumination on a gas-tight PEC reactor through a quartz window. The reactor was purged with argon for 30 min before the reaction to remove the dissolved oxygen. The reactions were conducted at 0.6 VRHE for 3 h in 0.1 M KPi under simulated solar irradiation (AM 1.5 G, 100 mW cm−2). The accumulated charge (C) was recorded by a potentiostat (CHI 660E). The evolved H2 and O2 gases were monitored by gas chromatography (Shimadzu GC-8A, HayeSep DB column) using a 5 Å molecular sieve column and Ar as the carrier gas. The stability measurement was conducted by chronoamperometry at 0.6 VRHE for 10 h under simulated solar irradiation (AM 1.5 G, 100 mW cm−2).
TRMC measurements
TRMC results were collected using a wavelength-tunable optical parametric oscillator (OPO) coupled to a diode-pumped Q-switched Nd:YAG laser at wavelengths of 410, 450, and 470 nm as the excitation source with a 3 ns pulse width and an X-band (8400–8700 MHz) microwave probe48,49. The samples were mounted in a microwave cavity cell. The signal change (\(\triangle P/P\)) in the microwave cavity cell correlates to the photoinduced variation in the conductance of the BiVO4 sample (\(\triangle G\)) using Eq. (9)
where K is the sensitivity factor derived from the resonance characteristics of the cavity and the dielectric properties of the medium. The product of the charge carrier generation yield (\(\phi\)) and the sum of electron and hole mobilities (∑µ) can be obtained according to Eq. (10)
where I0 is the incident intensity per pulse, q is the elementary charge, \(\beta\) is the ratio between the inner broad and narrow dimensions of the waveguide, and FA is the fraction of incident photons absorbed within the sample. The laser pulse intensities were adjusted using calibrated filters and varied from ~1013 to 4.5 × 1014 photons cm−2 pulse−1. The hole diffusion length (Lh) was calculated using Eq. (11) and Eq. (12)
where \(\mu\), \({\mu }_{{{{{{\rm{h}}}}}}}\), m0, q, T, and k are the carrier mobility, the hole mobility, the effective mass, the electron charge, the temperature, and the Boltzmann constant, respectively48.
Computational details
All density functional theory (DFT) calculations were carried out by Vienna Ab-initio Simulation Package (VASP)50. The Perdew-Burke-Ernzerhof (PBE)51 type of generalized gradient approximation (GGA)52 was applied for exchange-correlation. The electron-core interactions were described by the projector augmented wave (PAW) method53. The van der Waals (vdW) interaction was treated by the Grimme method54. The plane-wave cutoff energy was set to 400 eV, and the first Brillouin zone was sampled by a 2 × 3 × 3 Monkhorst-pack k-points grid55. The effective Hubbard U of 2.7 eV was added to the d orbitals of V atoms according to Dudarev’s method56. The structures were fully optimized until the total energy and residual forces were converged to 10−6 eV and 0.005 eV Å−1, respectively. The polaron hopping was simulated by cNEB method57,58 with 7 images, where the first and last images correspond to the initial and final positions of the polaron hopping, respectively.
The formation energy (\({E}_{{{{{{\rm{f}}}}}}}\)) of phosphorus doping defects including phosphorus interstitial (Pint), phosphorus on V site (PV), phosphorus on Bi site (PBi), and phosphorus on O site (PO) was defined as Eq. (13)
where \({E}_{{tot}}\left[{BVO},\,{pristine}\right]\) is the total energy of a perfect supercell (2×1×2 unit cells with 96 atoms) and \({E}_{{tot}}\left[{BVO},\,{doped}\right]\) is the total energy of a supercell doped with one phosphorus atom in charge state q. \({n}_{i}\) is the number of atoms (in our case is 1) that were removed (\({n}_{i}\) < 0) or added (\({n}_{i}\) > 0) to the supercell.\(\,{E}_{i}\) and \({\mu }_{i}\) are the average energy of the elemental phase and the chemical potential, respectively. \({E}_{V}\) represents the energy at the valence band maximum of the defect free structure. \({E}_{{Fermi}}\) is the Fermi energy relative to the \({E}_{V}\), ranging from 0 to 2.1 eV (band gap of pristine BVO). The final term\(\,{E}_{{corr}}\) represents the correction term for the electrostatic finite-size effect, following the scheme proposed by Freysoldt59. The 0, +1, +2, +3, +4, and +5 charge states were considered for Pint; the 0, +1, and +2 charge states were considered for PBi; the 0 and −1 charge states were considered for PO. Only the neutral state of PV was considered because P possesses the same number of valence electrons as V. To maintain thermal equilibrium, the \({\mu }_{{Bi}}\), \({\mu }_{V}\) and \({\mu }_{O}\) should satisfy the Eq. (14)
where \({H}^{f}\left[{{{{{\rm{BVO}}}}}}\right]\) is the formation enthalpy of BiVO4. The \({\mu }_{i}\) should also be smaller than zero so that the source elements would not aggregate to the elemental phases. To avoid the formation of secondary phases (Bi2O3, VO2, and V2O5), the \({\mu }_{{Bi}}\), \({\mu }_{V}\) and \({\mu }_{O}\) shall meet Eqs. (15–17)
where \({H}^{f}\left[{{{{{{\rm{Bi}}}}}}}_{2}{{{{{{\rm{O}}}}}}}_{3}\right]\), \({H}^{f}\left[{{{{{\rm{V}}}}}}{{{{{{\rm{O}}}}}}}_{2}\right]\), and \({H}^{f}\left[{{{{{{\rm{V}}}}}}}_{2}{{{{{{\rm{O}}}}}}}_{5}\right]\) are the formation enthalpy of Bi2O3, VO2, and V2O5, respectively. Considering all these constraints, the available range of \({\mu }_{{Bi}}\), \({\mu }_{V}\) and \({\mu }_{O}\) is limited to the grey area in Supplementary Fig. 10. In our calculations, two representative conditions shown as A (O rich) and B (O poor) were considered. The chemical potential of phosphorus element is constrained by \({\mu }_{P}\) <0 and \({2\mu }_{P}+5{\mu }_{O} < {H}^{f}\left[{{{{{{\rm{P}}}}}}}_{2}{{{{{{\rm{O}}}}}}}_{5}\right]\).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided as a Source Data file. Source data are provided with this paper.
References
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Kuang, Y. et al. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy 2, 16191 (2017).
Kudo, A., Omori, K. & Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 121, 11459–11467 (1999).
Woo Kim, T. & Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Jian, J. et al. Activating a semiconductor–liquid junction via laser-derived dual interfacial layers for boosted photoelectrochemical water Splitting. Adv. Mater. 34, 2201140 (2022).
Lu, Y. et al. Boosting charge transport in BiVO4 photoanode for solar water oxidation. Adv. Mater. 34, 2108178 (2022).
Park, H. S., Ha, H. W., Ruoff, R. S. & Bard, A. J. On the improvement of photoelectrochemical performance and finite element analysis of reduced graphene oxide-BiVO4 composite electrodes. J. Electroanal. Chem. 716, 8–15 (2014).
Grigioni, I. et al. Photoinduced charge-transfer dynamics in WO3/BiVO4 photoanodes probed through midinfrared transient absorption spectroscopy. J. Am. Chem. Soc. 140, 14042–14045 (2018).
Han, H. S. et al. Boosting the solar water oxidation performance of a BiVO4 photoanode by crystallographic orientation control. Energy Environ. Sci. 11, 1299–1306 (2018).
Meng, A. Q. et al. Efficient BiVO4 photoanodes by postsynthetic treatment: remarkable improvements in photoelectrochemical performance from facile borate modification. Angew. Chem. Int. Ed. 58, 19027–19033 (2019).
Wu, H., Qu, S., Xie, Z. & Ng, Y. H. Surface modulation inducing bismuth-rich surface composition in BiVO4 for efficient photoelectrochemical water splitting. ACS Appl. Energy Mater. 5, 8419–8427 (2022).
Wu, H. et al. Unveiling carrier dynamics in periodic porous BiVO4 photocatalyst for enhanced solar water splitting. ACS Energy Lett. 6, 3400–3407 (2021).
Kim, T. W., Ping, Y., Galli, G. A. & Choi, K. S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 6, 8769 (2015).
Ye, K.-H. et al. Enhancing photoelectrochemical water splitting by combining work function tuning and heterojunction engineering. Nat. Commun. 10, 3687 (2019).
Zhang, K. et al. Black phosphorene as a hole extraction layer boosting solar water splitting of oxygen evolution catalysts. Nat. Commun. 10, 2001 (2019).
Zhou, L. et al. High light absorption and charge separation efficiency at low applied voltage from Sb-doped SnO2/BiVO4 core/shell nanorod-array photoanodes. Nano Lett. 16, 3463–3474 (2016).
Qiu, W. et al. Freeing the polarons to facilitate charge transport in BiVO4 from oxygen vacancies with an oxidative 2D precursor. Angew. Chem. Int. Ed. 8, 19087–19095 (2019).
Selim, S. et al. Impact of oxygen vacancy occupancy on charge carrier dynamics in BiVO4 photoanodes. J. Am. Chem. Soc. 141, 18791–18798 (2019).
Passerone, D. & Erni, R. Direct evidence of surface reduction in monoclinic BiVO4. Chem. Mater. 27, 3593–3600 (2015).
Wiktor, J., Ambrosio, F. & Pasquarello, A. Role of polarons in water splitting: the case of BiVO4. ACS Energy Lett. 3, 1693–1697 (2018).
Rettie, A. J. E. et al. Anisotropic small-polaron hopping in W:BiVO4 single crystals. Appl. Phys. Lett. 106, 022106 (2015).
Rettie, A. J. E., Chemelewski, W. D., Emin, D. & Mullins, C. B. Unravelling small-polaron transport in metal oxide photoelectrodes. J. Phys. Chem. Lett. 7, 471–479 (2016).
Zhang, W. et al. Unconventional relation between charge transport and photocurrent via boosting small polaron hopping for photoelectrochemical water splitting. ACS Energy Lett. 3, 2232–2239 (2018).
Walsh, A. et al. In situ observation of picosecond polaron self-localisation in α-Fe2O3 photoelectrochemical cells. Nat. Commun. 10, 3962 (2019).
Lohaus, C. & Klein, A. Limitation of Fermi level shifts by polaron defect states in hematite photoelectrodes. Nat. Commun. 9, 4309 (2018).
Carneiro, L. M. et al. Excitation-wavelength-dependent small polaron trapping of photoexcited carriers in α-Fe2O3. Nat. Mater. 16, 819–825 (2017).
Hu, Z., Shen, Z. & Yu, J. C. Covalent fixation of surface oxygen atoms on hematite photoanode for enhanced water oxidation. Chem. Mater. 28, 564–572 (2016).
Wang, G. et al. Computational and photoelectrochemical study of hydrogenated bismuth vanadate. J. Phys. Chem. C. 117, 10957–10964 (2013).
Cooper, J. K. et al. Role of hydrogen in defining the n-Type character of BiVO4 photoanodes. Chem. Mater. 28, 5761–5771 (2016).
Kim, J. H. & Lee, J. S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 31, 1806938 (2019).
Wang, S. et al. In situ formation of oxygen vacancies achieving near-complete charge separation in planar BiVO4 photoanodes. Adv. Mater. 32, 2001385 (2020).
Jian, J. et al. Embedding laser generated nanocrystals in BiVO4 photoanode for efficient photoelectrochemical water splitting. Nat. Commun. 10, 2609 (2019).
Zhang, Z., Karimata, I., Nagashima, H., Sugimoto, K. & Tachikawa, T. Interfacial oxygen vacancies yielding long-lived holes in hematite mesocrystal-based photoanodes. Nat. Commun. 10, 4832 (2019).
Zhang, Z., Nagashima, H. & Tachikawa, T. Ultra‐narrow depletion layers in hematite mesocrystal‐based photoanode for boosting multihole water oxidation. Angew. Chem. Int. Ed. 59, 9047–9054 (2020).
Pradhan, D. K. et al. Correlation of dielectric, electrical and magnetic properties near the magnetic phase transition temperature of cobalt zinc ferrite. Phys. Chem. Chem. Phys. 19, 210–218 (2017).
Deshpande, S. K., Shrikhande, V. K., Jugad, M. S., Goyal, P. S. & Kothiyal, G. P. Conductivity studies of lithium zinc silicate glasses with varying lithium contents. Bull. Mater. Sci. 30, 497–502 (2007).
Miyata, K. et al. Large polarons in lead halide perovskites. Sci. Adv. 3, 1701217 (2017).
Zhang, W. et al. Anomalous conductivity tailored by domain-boundary transport in crystalline bismuth vanadate photoanodes. Chem. Mater. 30, 1677–1685 (2018).
Tang, S. et al. Harvesting of infrared part of sunlight to enhance polaron transport and solar water splitting. Adv. Funct. Mater. 32, 2110284 (2022).
Subramanian, V., Wolf, E. E. & Kamat, P. V. Catalysis with TiO2/Gold nanocomposites. effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 126, 4943–4950 (2004).
Melo, M. A. et al. Surface photovoltage measurements on a particle tandem photocatalyst for overall water splitting. Nano Lett. 18, 805–810 (2018).
Zhang, Y. et al. Nonmetal P-doped hematite photoanode with enhanced electron mobility and high water oxidation activity. Energy Environ. Sci. 8, 1231–1236 (2015).
Wu, F. & Ping, Y. Combining Landau-Zener theory and kinetic Monte Carlo sampling for small polaron mobility of doped BiVO4 from first-principles. J. Mater. Chem. A 6, 20025–20036 (2018).
Kokott, S. et al. First-principles supercell calculations of small polarons with proper account for long-range polarization effects. N. J. Phys. 20, 033023 (2018).
Kweon, K. E., Hwang, G. S., Kim, J., Kim, S. & Kim, S. Electron small polarons and their transport in bismuth vanadate: A first principles study. Phys. Chem. Chem. Phys. 17, 256–260 (2015).
Zachäus, C., Abdi, F. F., Peter, L. M. & Van De Krol, R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 8, 3712–3719 (2017).
Zhong, M. et al. Surface modification of CoOx loaded BiVO4 photoanodes with ultrathin p-type NiO layers for improved solar water oxidation. J. Am. Chem. Soc. 137, 5053–5060 (2015).
Jang, J. W. et al. Enhancing charge carrier lifetime in metal oxide photoelectrodes through mild hydrogen treatment. Adv. Energy Mater. 7, 1701536 (2017).
Song, A. et al. Evaluation of copper vanadate (β-Cu2V2O7) as a photoanode material for photoelectrochemical water oOxidation. Chem. Mater. 32, 2408–2419 (2020).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Allouche, A. Software news and updates gabedit -a graphical user interface for computational chemistry softwares. J. Comput. Chem. 32, 174–182 (2012).
Pack, J. D. & Monkhorst, H. J. ‘special points for Brillouin-zone integrations’-a reply. Phys. Rev. B 16, 1748–1749 (1977).
Dudarev, S. & Botton, G. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Freysoldt, C. et al. First-principles calculations for point defects in solids. Rev. Mod. Phys. 86, 253–305 (2014).
Acknowledgements
This project was financially supported by the Hong Kong Research Grant Council (RGC) General Research Fund (GRF) CityU 11305419, CityU 11306920, CityU 11308721 and the General Program of Science and Technology Innovation Committee of Shenzhen Municipality JCYJ20190808181805621.
Author information
Authors and Affiliations
Contributions
H.W. conceived the original concept with discussion with Y.H.N. H.W. and Y.H.N. supervised the project. H.W. carried out the materials synthesis, characterization, and PEC experiments. R.I. carried out the TRMC experiments with the supervision of R.v.d.K. and F.F.A. L.Z. performed the modeling and data analysis with the help of A.D. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiangjiu Guan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, H., Zhang, L., Du, A. et al. Low-bias photoelectrochemical water splitting via mediating trap states and small polaron hopping. Nat Commun 13, 6231 (2022). https://doi.org/10.1038/s41467-022-33905-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33905-6
This article is cited by
-
Induced dipole moments in amorphous ZnCdS catalysts facilitate photocatalytic H2 evolution
Nature Communications (2024)
-
Liquid metal-embraced photoactive films for artificial photosynthesis
Nature Communications (2024)
-
Accelerating Oxygen Electrocatalysis Kinetics on Metal–Organic Frameworks via Bond Length Optimization
Nano-Micro Letters (2024)
-
Novel approaches to the degradation of nitrophenols using TiO2-biopolymer-ligand-metal complex as photocatalysts
Journal of Materials Science: Materials in Electronics (2024)
-
Bias-free driven ion assisted photoelectrochemical system for sustainable wastewater treatment
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.