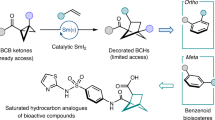
Abstract
Aromatic ring isosteres and rigidified saturated hydrocarbons are important motifs to enable drug discovery. Herein we disclose [2]-ladderanes as a class of meta-substituted aromatic ring isosteres and rigidified cyclohexanes. A straightforward synthesis of the building blocks is presented along with representative derivatization. Preliminary studies reveal that the [2]-ladderanes offer similar metabolic and physicochemical properties thus establishing this class of molecules as interesting motifs.
Similar content being viewed by others
Introduction
The introduction of building blocks is an important direction in modern medicinal chemistry. One key focus is the development of isosteres to manipulate the physicochemical properties of molecules1,2. Classical examples include replacement of C–H bonds with C–F or C–D bonds, typically to prolong in vivo half-life1. More recently, non-classical isosteres have emerged, such as exchange of carbonyls for oxetane rings3,4,5. Along these lines, a recent surge in interest has been directed toward identifying isosteres for substituted aromatic rings6,7. Several approaches have been developed to identify probable isosteres including, but not limited to, the use of exit vector analysis to allow for comparison of chemical space covered by disubstituted scaffolds (Fig. 1A)8. In particular, cubane and [1.1.1]-bicyclopentanes have shown promise as replacements for para-substituted aromatic rings due to the similar positioning of substituents as shown in Fig. 1B9,10,11,12,13,14,15,16. This was exemplified in a 2012 report in which the [1.1.1]-bicyclopentane showed improved properties compared to the parent structure (Fig. 1B)11. As a result, significant effort has been directed toward the synthesis and derivatization of cubane and [1.1.1]-bicyclopentanes17,18,19,20.
A Exit vector analysis to establish the 3D positions of substituents relative to each other. B Known aromatic ring isosteres for para-substituted aromatic rings with a representative example. C Known aromatic ring isosteres for meta-substituted aromatic rings. D [2]-Ladderane as isosteres for aromatic rings E [2]-Ladderane as rigidified cyclohexanes.
Isostere replacement for meta-substituted aromatic rings has primarily seen development along the lines of the 1,4-disubstituted-[2.1.1]-bicyclohexane (Fig. 1C)17. Despite successful exemplification of this isostere, the angle between substituents (ϕ), and hence exit vectors, are deviated from the parent compound. It should be noted that recent efforts have shown that oxa-21 and aza-variants22 are useful in increasing water solubility of this scaffold. In addition, a recent study described the introduction of 1,2-disubstituted-[1.1.1]-bicyclopentanes to serve as meta- and ortho-substituted aromatic ring replacements (Fig. 1C)23. While this study is a notable advance, the positioning of substituents more closely aligns with an ortho-substituted aromatic ring. Other molecules have been proposed to be meta-substituted aromatic ring isosteres, such as 1,3-substituted cubanes, however, synthetic routes have not been established17,24.
Our lab25,26,27 and others28,29,30 have been interested in the chemistry of strained ring systems, particularly cyclobutanes. Within this program of research, we developed routes toward the synthesis of the highly unusual ladderane lipids25,26,27. In the course of these studies, we became interested in the use of [2.2.0]-bicyclohexanes ([2]-ladderanes) as building blocks for discovery chemistry. Here, we report a straightforward synthesis of a cis- and trans-2,6-disubstituted [2]-ladderane as replacements for meta-substituted aromatic rings and cyclohexanes, representative derivatizations, and preliminary ADME comparisons.
Results and discussion
During our investigation into the ladderanes, we made the observation that the positioning of a cis-2,6-disubstituted [2]-ladderane has a similar arrangement of the substituents relative to a meta-substituted aromatic ring (Fig. 1D). This scaffold not only constitutes a hitherto unknown isostere, but also ventures into realms of chemical space that have remained unexplored, to our knowledge, in existing patents. In addition, a 2,6-disubstituted [2]-ladderane can also act as a rigidified 1,3-disubstituted cyclohexane (Fig. 1E). Rigidification of lead compounds is an established strategy in medicinal chemistry31. Anti-1,3-disubstituted cyclohexane are conformationally flexible, however, the analogous [2]-ladderane structure is rigid and is thus of additional value.
After exploration of several strategies, a robust and scalable route to the 2,6-disubstituted [2]-ladderane was developed (Fig. 2A). The general route begins with addition of homoallyl Grignard to either cinnamaldehyde and its derivatives or (E,E)-2,4-decadienal (all common and commercially available reagents). Photosensitized [2 + 2] cycloaddition provided access to cyclobutane 332,33. While this reaction could be conducted in batch (see SI, General Procedure C for batch set-up), scale up of the process was more easily performed in a flow photochemical reactor. Oxidation of the secondary alcohol with DMP followed by diazoketone synthesis provided access to 4. This is the first chromatography performed in the sequence. Irradiation of the diazoketone with 365 nm LEDs in the presence of MeOH provided access to ester 5 with the endo-diastereomer predominating. At this stage, two final elaborations to useful building blocks were established. Epimerization of 5 to the exo-diastereomer when R = Ph followed by esterification and exhaustive oxidation led to synthesis of ester/acid 634. When R = heptene, oxidative cleavage followed by silyl protection and epimerization allowed for the synthesis of acid/silyl ether building block 7. Both routes could easily be conducted to provide hundreds of milligrams of 6 and 7. At this stage of development, the current bottleneck is the conversion of 2 to 3 with our current commercial photochemical flow setup due to challenges with lamp cooling over extended reaction times. Finally, various aryl groups were tolerated and allowed for synthesis of 8–13 (Fig. 2B).
With these building blocks in hand, a variety of derivatizations were carried out to demonstrate synthetic utility (Fig. 3A). It is important to note that the yields shown are reported for the diastereomeric mixture that was isolated. Curtius rearrangement of carboxylic acid 14 induced by DPPA allowed for synthesis of urea 15. In addition, LiAlH4 reduction of 14 and carbamate formation led to 17. Carboxylic acid 7 could also undergo Curtius rearrangement to generate 18. Further elaboration by silyl deprotection and oxidation allowed for the synthesis of 19. In addition, LiAlH4 reduction of 7 led to generation of a mono-protected diol 20.
A Transformation of the ladderane building block to other structures of relevance in medicinal chemistry. B Ni-catalyzed cross-coupling of redox-active esters. C Synthesis of ladder-mazapertine. DPPA diphenylphosphoryl azide, CDI carbonyl diimidazole, TBAF tetrabutylammonium fluoride, DMP Dess Martin periodinane, EDC 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, DMAP 4-dimethylaminopyridine.
Due to the presence of a carboxylic acid moiety, decarboxylative cross-coupling reactions were explored35,36. In the case of redox-active esters derived from [2]-ladderane building blocks that incorporate ester (derived from 6) or Ph substituents (derived from 14), ring opening was observed in addition to desired product. However, in the case of the redox-active ester 21 derived from acid/silyl ether building block 7, cross-coupling was effective (Fig. 3B). In addition, due to the rigid bicyclic nature of the [2]-ladderane, the cross-coupling proceeded with good diastereoselectivity for incorporation of the substituent on the exo-face. The incorporation of alkyl (22)37, aryl (23)15 and alkynyl (24)38 units could all be achieved. Finally, as illustrated by the reactions shown in Fig. 3, as well as additional control studies, the [2]-ladderanes are generally stable to acidic (see the SI stability experiments section), basic (see the SI stability experiments section), reducing (see 7–20, Fig. 3A), and oxidizing conditions (see 5–6 and 7, Fig. 2A).
To demonstrate how these building blocks could be incorporated into discovery chemistry programs, we targeted the [2]-ladderane matched pair of mazapertine (26) (Fig. 3C)39. Simple amide bond formation, deprotection, and substitution with a piperazine allowed for synthesis of ladderane-mazapertine (25) in 39% yield over 4 steps from 7. It is important to note that the present study does not aim to specifically test a biological hypothesis, but rather demonstrate that a [2]-ladderane can serve as an alternative building block to an aromatic ring that can be incorporated to many ongoing medicinal chemistry programs around the world.
Finally, as noted in the synthesis of [2]-ladderane building blocks, the Wolff rearrangement gives rise to the anti-isomer as the major product. When comparing the exit vectors of this structure, it displays similarity to that of an anti-1,3-substituted cyclohexane (Fig. 2E). Due the flexibility of the anti-2,6-substituted cyclohexane, it was envisioned that the anti-[2]-ladderane isomer could act as a rigidified variant, as this is often favorable in medicinal chemistry31. In light of this, we carried out several representative derivatizations to demonstrate the utility of this scaffold (Fig. 4). For example, the [2]-ladderane 5 was easily oxidized to generate building block 27. From this intermediate, amide bond formation, Curtius rearrangement, and chemoselective reduction all proceeded smoothly to generate 28, 29, and 30, respectively. From intermediate 28 (isolated as a single observable diastereomer), depending on reduction conditions employed, 31 and 32 could be prepared. Finally, deprotonation of 5a with LDA and trapping of the enolate with allylbromide (via 34) resulted in the formation of 33 as a single observable isomer.
The metabolic and physicochemical properties of the [2]-ladderanes compared to aryl/cyclohexyl matched pairs were also studied (compare 35 with 36/37 and 38 with 39/40) (Fig. 5). These structures were chosen because they bear functional groups that are common in drug discovery, yet unlikely to pose any inherent risks. In addition, solubility, permeability, rat liver microsomal intrinsic clearance, and LogP were selected to be evaluated as these are common parameters modulated in drug discovery programs to improve the pharmacokinetic/pharmacodynamic and safety profiles of potential drug candidates. Though this is a small data set, the results of these studies demonstrate that [2]-ladderanes behave similarly to that of the aryl/cyclohexyl matched pairs. In fact, there is no appreciable difference in the parameters evaluated, suggesting that [2]-ladderanes should not negatively affect the metabolic or physicochemical properties of a compound when used as an isosteric replacement for a meta-substituted aromatic or cyclohexane ring.
In summary, [2]-ladderanes, has been introduced as a class of building blocks. These can be utilized as isosteres for meta-substituted aromatic rings or rigidified variants of anti-1,3-substituted cyclohexanes. Representative functionalizations provide access to an array of molecular diversity. These studies now establish these structures within the repertoire of building blocks to enable medicinal chemistry.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Experimental procedures, analytical data for all new compounds can be found in the Supplementary Information. The material is available free of charge.
References
Patani, G. A. & LaVoie, E. J. Bioisosterism: a rational approach in drug design. Chem. Rev. 96, 3147–3176 (1996).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Wuitschik, G. et al. Oxetanes as promising modules in drug discovery. Angew. Chem. Int. Ed. 45, 7736–7739 (2006).
Burkhard, J. A., Wuitschik, G., Plancher, J.-M., Rogers-Evans, M. & Carreira, E. M. Synthesis and stability of oxetane analogs of thalidomide and lenalidomide. Org. Lett. 15, 4312–4315 (2013).
Carreira, E. M. & Fessard, T. C. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Rev. 114, 8257–8322 (2014).
Tse, E. G. et al. Nonclassical phenyl bioisosteres as effective replacements in a series of novel open-source antimalarials. J. Med. Chem. 63, 11585–11601 (2020).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
For a study regarding exit vector analysis, see:, Grygorenko, O. O., Demenko, D., Volochnyukac, D. M. & Komarov, I. V. Following Ramachandran 2: exit vector plot (EVP) analysis of disubstituted saturated rings. N. J. Chem. 42, 8355–8365 (2018).
Wei, W., Cherukupalli, S., Jing, L., Liu, X. & Zhan, P. Fsp3: a new parameter for drug-likeness. Drug Discov. Today 25, 1839–1845 (2020).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Lovering, F. Escape from flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Hiesinger, K., Dar’in, D., Proschak, E. & Krasavin, M. Spirocyclic scaffolds in medicinal chemistry. J. Med. Chem. 64, 150–183 (2021).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Zheng, Y.-J. & Tice, C. M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 11, 831–834 (2016).
Zheng, Y., Tice, C. M. & Singh, S. B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 24, 3673–3682 (2014).
Auberson, Y. P. et al. Improving nonspecific binding and solubility: bicycloalkyl groups and cubanes as para‐phenyl bioisosteres. ChemMedChem 12, 590–598 (2017).
For a review, see:, Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
For a review, see:, Reekie, T. A., Williams, C. M., Rendine, L. M. & Kassiou, M. Cubanes in medicinal chemistry. J. Med. Chem. 62, 1078–1095 (2019).
For a review, see:, Ma, X. & Pham, L. N. Selected topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 9, 8–22 (2020).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Ed. 59, 7161–7167 (2020).
Homon, A. A. et al. 4-(di-/trifluoromethyl)-2-heterabicyclo[2.1.1]hexanes: advanced fluorinated phenyl isosteres and proline analogues. Eur. J. Org. Chem. 2021, 6580–6590 (2021).
Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Nat. Acad. Sci. USA 118, e2108881118 (2021).
After acceptance of this manuscript, a related isostere was reported. Frank, N. et. al. Synthesis of meta-Substituted Arene Bioisosteres from [3.1.1]propellane. Nature https://doi.org/10.1038/s41586-022-05290-z (2022).
Hancock, E. N., Kuker, E. L., Tantillo, D. J. & Brown, M. K. Lessons in strain and stability: enantioselective synthesis of (+)-[5]-ladderanoic acid. Angew. Chem. Int. Ed. 59, 436–441 (2020).
Line, N. J., Witherspoon, B. P., Hancock, E. N. & Brown, M. K. Synthesis of ent-[3]-ladderanol: development and application of intramolecular chirality transfer [2+2] cycloadditions of allenic ketones and alkenes. J. Am. Chem. Soc. 139, 14392–14395 (2017).
Hancock, E. N. & Brown, M. K. Ladderane natural products: from the ground up. Chem. Eur. J. 27, 565–576 (2021).
Mascitti, V. & Corey, E. J. Enantioselective synthesis of pentacycloanammoxic acid. J. Am. Chem. Soc. 128, 3118–3119 (2006).
Mascitti, V. & Corey, E. J. Total synthesis of (±)-pentacycloanammoxic acid. J. Am. Chem. Soc. 126, 15664–15665 (2004).
Mercer, J. A. M. et al. Chemical synthesis and self-assembly of a ladderane phospholipid. J. Am. Chem. Soc. 138, 15845–15848 (2016).
Fang, Z., Song, Y., Zhan, P., Zhang, Q. & Liu, X. Conformational restriction: an effective tactic in ‘follow-on’-based drug discovery. Future Med. Chem. 6, 885–901 (2014).
Lu, Z. & Yoon, T. P. Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 51, 10329–10332 (2012).
Elliott, L. D., Kayal, S., George, M. W. & Booker-Milburn, K. Rational design of triplet sensitizers for the transfer of excited state photochemistry from UV to visible. J. Am. Chem. Soc. 142, 14947–14956 (2020).
Carlsen, P. H. J., Katsuki, T., Martin, V. S. & Sharpless, K. B. A greatly improved procedure for ruthenium tetroxide catalyzed oxidations of organic compounds. J. Org. Chem. 46, 3936–3938 (1981).
Sandfort, F., O’Neill, M. J., Cornella, J., Wimmer, L. & Baran, P. S. Alkyl−(hetero)aryl bond formation via decarboxylative cross-coupling: a systematic analysis. Angew. Chem. Int. Ed. 56, 3319–3323 (2017).
Cornella, J. et al. Practical Ni-catalyzed aryl–alkyl cross-coupling of secondary redox-active esters. J. Am. Chem. Soc. 138, 2174–2177 (2016).
Qin, T. et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016).
Smith, J. M. et al. Decarboxylative alkynylation. Angew. Chem. Int. Ed. 56, 11906–11910 (2017).
Scott, M. K. et al. Piperazinylalkyl heterocycles as potential antipsychotic agents. J. Med. Chem. 38, 4198–4210 (1995).
Acknowledgements
We thank Indiana University and the NIH (R35GM131755) for financial support. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF MRI program, CHE-1726633 and CHE-1920026. Equipment Fund from the President of Indiana University and the Office of the Vice President for Research is gratefully acknowledged. This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme Marie Sklodowska Curie Action ITN under Grant Agreement No. 859458.
Author information
Authors and Affiliations
Contributions
R.C.E., E.N.H., and M.K.B designed the study. R.C.E., E.N.H., S.P., and L.H. performed the experiments with oversight from C.S., T.C.F., E.W.B., J.F.L., and M.K.B. The data described in Fig. 5 was collected by D.R.D. and C.G.-M. The manuscript was written by R.C.E., S.P., and M.K.B. with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Antonia Stepan, and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Epplin, R.C., Paul, S., Herter, L. et al. [2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes. Nat Commun 13, 6056 (2022). https://doi.org/10.1038/s41467-022-33827-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33827-3
This article is cited by
-
Analogies between photochemical reactions and ground-state post-transition-state bifurcations shed light on dynamical origins of selectivity
Nature Chemistry (2024)
-
General access to cubanes as benzene bioisosteres
Nature (2023)
-
2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.