Abstract
Human thymic epithelial tumors (TET) are common malignancies in the anterior mediastinum with limited biological understanding. Here we show, by single cell analysis of the immune landscape, that the developmental pattern of intra-tumoral T-cells identify three types within TETs. We characterize the developmental alterations and TCR repertoires of tumor-infiltrating T cells in the context of the distinguishing epithelial tumor cell types. We demonstrate that a subset of tumor cells, featuring medullary thymic epithelial cell (TEC) phenotype and marked by KRT14/GNB3 expression, accumulate in type 1 TETs, while T-cell positive selection is inhibited. Type 2 TETs are dominated by CCL25+ cortical TEC-like cells that appear to promote T-cell positive selection. Interestingly, the CHI3L1+ medullary TEC-like cells that are the characteristic feature of type 3 TETs don’t seem to support T-cell development, however, they may induce a tissue-resident CD8+ T cell response. In summary, our work suggests that the molecular subtype of epithelial tumour cells in TETs determine their tumour immune microenvironment, thus GNB3 and CHI3L1 might predict the immunological behavior and hence prognosis of these tumours.
Similar content being viewed by others
Introduction
Thymic epithelial tumors (TET), including thymomas and thymic carcinomas, are common primary tumors in the human anterior mediastinum1,2. Owing to the lack of relevant cell lines and animal models3,4,5,6, the understanding of the etiology and biology of TETs is far from adequate. All types of TETs are considered potentially malignant in the latest clinical guidelines7 because previously defined benign tumors also exhibit aggressive malignant behavior clinically8. This suggests that further understand the biological characteristics of these heterogeneous malignancies is needed.
Generally, TETs are considered to be transformed from thymic epithelial cells (TEC), which play a crucial role in T-cell development in mammals9. There are two major subtypes of TECs that may be distinguished according to the epithelial origins of human TETs, cortical TECs (cTEC) and medullary TECs (mTEC), which are mainly located in the cortex and medulla structures of the thymus, respectively10. It is well demonstrated that cTECs are essential for the positive selection of T cells during T-cell development in the thymus, while mTECs are mainly involved in negative selection to establish central self-tolerance11,12. Clinical studies have shown that patients with type B thymoma, which may be derived from mTECs, have a higher incidence of autoimmune diseases than patients with type A and type C thymoma13,14. This evidence suggests that the impact of tumor cells on T-cell development in human TETs may be different. Recently, emerging molecular classifications have provided insight into the stratification of prognosis and treatment for patients with TETs at the molecular level15,16.
Here we conduct a comprehensive study using mass cytometry, single-cell sequencing, TCR repertoires, histological analysis, FCM detection and immunofluorescence testing to decode the epithelial origins of human TETs and their impact on immune cell composition. Based on the phenotype of tumor cells, the immune landscape of tumors, intra-tumoral T-cell development pattern and TCR repertoire, we identify three main types of human TETs and uncover the potential mechanisms that lead to the divergent T-cell development in each tumor type.
Results
The immune landscape of human TETs is heterogeneous
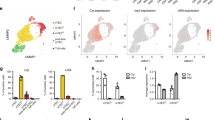
Using multiple approaches, we performed a comprehensive study of the immune landscape of human TETs (Fig. 1a). First, we established a panel of more than 40 markers to detect the cell composition of the human thymus and TETs by CyTOF and found a remarkable difference between the tumor and thymus (Supplementary Fig. 1a, Supplementary Table 1 and Table 2). An apparent discrepancy in the immune landscape also existed among samples of human TETs (Supplementary Fig. 1a), which canonical classifications were not fully aligned (Fig. 1b and Supplementary Fig. 1a). According to the similarity of their cellular composition, we grouped the samples of human TETs into three types (type 1, 2 and 3) (Fig. 1b and Supplementary Fig. 1a). Interestingly, we found that the variance in the immune landscape among the types according to our alternative classification was not fully aligned with the WHO classification and Masaoka stage (Fig. 1b and Supplementary Fig. 1a). The reliability of our alternative classification was further validated by multiple clustering schemes and histological analysis (Supplementary Fig. 1b, c and d). Statistical analysis showed that the quantity of macrocluster cells was significantly different between groups according to our alternative classification (Fig. 1c, d and Supplementary Fig. 1a, e, f). In addition, each type of tumor in our potential alternative classification contained heterogeneous samples according to the WHO subtype and Masaoka stage (Fig. 1e, f).
(a) Schematic diagram of the overall study design and workflow. (b) t-SNE plots of total cells from human TETs (n = 22) and the normal thymus (n = 3) by CyTOF using a Phenograph clustering scheme. Samples were grouped according to WHO histological subtype, Masaoka stage or our alternative classification, and each color represents an independent group or stage (N, normal human thymus; T1, type 1; T2, type 2; T3, type 3; the abbreviations below are consistent with these definitions). (c) t-SNE plot of total cells from all samples (n = 25) by CyTOF using a Phenograph clustering scheme, colored by cell cluster. (d) Bar plots showing the frequencies of cluster 1 and cluster 2 subsets among the four groups of samples (n = 3, 9, 7 and 6 for N, T1, T2 and T3, respectively. Data are presented as the mean ± s.e.m. P values in the figure were determined by an unpaired two-tailed Student’s t test). (e-f) Sheet and pie charts summarizing samples in each alternative classification by WHO histological subtype (e) or Masaoka stage (f). Each color represents a WHO histological subtype or Masaoka stage. (g) Same t-SNE plot as (c), colored by cell type. (h) t-SNE plot of immune cells (EPCAM-CD45 + ) from all samples obtained by CyTOF using a Phenograph clustering scheme, colored by cell type. (i) t-SNE analysis of immune cells from all samples colored by the relative expression of CyTOF markers (CD45, CD3, CD4, CD8a, CD20, CD14, CD141, CD56, CD11c, CD66b, HLA-DR and CD39). (j) Same t-SNE plots of immune cells as (h) from samples of each group (N, T1, T2, T3), colored by cell type. (k) Bar plots showing the frequencies of the main immune cell subsets among the four groups of samples (n = 3, 9, 7 and 6 for N, T1, T2 and T3, respectively. Data are presented as the mean ± s.e.m. P values in the figure were determined by an unpaired two-tailed Student’s t test). (l) Representative flow cytometric plot of CD3+ T cells and CD3-CD4+CD8+ lymphocytes from TETs of each type. Source data are provided as a Source Data file.
Through cell annotation, we found that CD45+ immune cells, but not EPCAM+ tumor cells, constituted the dominant cell subset in TET tumors (Fig. 1g and Supplementary Fig. 1g). In addition, CD45+ immune cells, but not epithelial cells, constituted the major quantitative variate among samples grouped by our alternative classification, WHO classification and Masaoka stage (Fig. 1b, g and Supplementary Fig. 1h). Further cell annotation showed that both TETs and the normal thymus comprised major immune lineages (Fig. 1h, i and Supplementary Fig. 2a), with abundant CD3-CD4+CD8+ and CD3+ T cells (Supplementary Fig. 2b). Intergroup analysis showed obvious differences in the immune cell composition of tumors, especially CD3-CD4+CD8+ and CD3+ T lymphocytes, which are involved in intrathymic T-cell development (Fig. 1j, k). In particular, the proportion of CD3-CD4+CD8+ lymphocytes was the highest in type 1 and lowest in type 3 TETs (Fig. 1k). Moreover, from type 1 to type 3 TETs, there was an obvious tendency toward a decreased abundance of CD3- CD4+ CD8+ lymphocytes in tumors (Fig. 1k). However, the proportions of CD3+ T cells in tissues of both type 2 and type 3 TETs were higher than those in type 1 TETs (Supplementary Fig. 2c). In contrast, the proportions of B cells, dendritic cells (DC), granulocytes and natural killer cells (NK) in tumors was lowest in type 1 and highest in type 3 TETs, with a tendency toward a gradual increase in abundance from type 1 to type 3 TETs (Fig. 1k and Supplementary Fig. 2c). Monocytes were abundant in tumors of type 3 (Supplementary Fig. 2c). It is interesting that except for CD3-CD4+CD8+ lymphocytes, other immune cells related to the peripheral immune response in tumors were more abundant in type 3 TETs (Fig. 1k and Supplementary Fig. 2c). The discrepant abundance of CD3-CD4+CD8+ lymphocytes and CD3+ T-cell tumor types was further validated by flow cytometry (Fig. 1l). Importantly, compared with the WHO classification and Masaoka staging system, our system is focusing on the differences in immune cell subsets in tumors in a comprehensive manner (Supplementary Fig. 3).
Intratumoral T-cell development pattern of human TETs
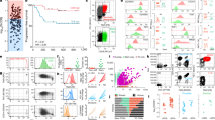
The varying proportions of CD3-CD4+CD8+ and CD3+ T lymphocytes among tumors suggested that there was a different T-cell development pattern in each TET type. To further investigate the change in T-cell development in TETs, we used well-accepted markers, which are highly correlated with T-cell development in the thymus17, to annotate the subsets of CD3+ T cells in the tumor and normal thymus (Fig. 2a and Supplementary Figs. 4a, b). Major subsets of T cells involved in thymic T-cell development were represented12, including double-negative (DN, CD4-CD8-), double-positive (DP, CD4+CD8+), CD4+ single-positive (CD4+ SP), CD8+ single-positive (CD8+ SP), and FOXP3+ regulatory T (Treg) cells (Fig. 2a). Phenotypic analysis showed that the T-cell subsets in thymus tissues and tumors that we defined were representative (Fig. 2b). It is important that the differences in T-cell composition among tumors of different subtypes were well defined by our immuno-based alternative classification, while the WHO classification or Masaoka staging system was more focused on the histopathology and morphology of tumor (Fig. 2c–f). In our alternative classification, immature DP cells dominated in type 1 TETs, while mature SP cells dominated in tumors of type 2 and type 3 (Fig. 2f, g). The proportion of DN and DP cells was the highest in type 1 tumors and lowest in type 3 tumors (Fig. 2g and Supplementary Fig. 4c). Interestingly, the proportion of DN and DP cells in TETs, especially DP cells, showed a tendency to decrease from type 1 to type 3 TETs (Fig. 2g and Supplementary Fig. 4c). In contrast, the proportion of CD4+ and CD8+ T cells was lowest in tumors of type 1 and highest in tumors of type 3 (Fig. 2g). In addition, the proportions of CD4+ and CD8+ T cells in tumors showed an increasing trend from type 1 to type 3 TETs, which was contrary to the pattern observed for DN and DP cells (Fig. 2g). Flow cytometry detection further confirmed the different proportions of DN, DP and SP cells in each TET type (Fig. 2h). Furthermore, immunofluorescence staining showed that DP and SP cells were located in the cortex- and medulla-like structure in type 2 TETs, respectively; however, the typical cortex- and medulla-like structures were not observed in type 1 and type 3 TETs (Fig. 2i).
(a) t-SNE plot of CD45+CD3+ T cells from all samples (n = 25), colored by cell subset. (DN, CD3+CD4-CD8-; DP, CD3+CD4+CD8+). (b) Heatmap showing relative normalized protein expression for the T-cell subsets from CyTOF, normalized total matrix. (c) t-SNE plots of CD45+CD3+ T cells from human TETs (n = 22) and the normal thymus (n = 3) by CyTOF using the Phenograph clustering scheme. Samples were grouped according to WHO classification, Masaoka stage or our alternative classification, and each color represents a T-cell subset. (d–f) Composition of the CD45+CD3+ T-cell compartment showing the average frequencies of major T-cell subsets for each WHO histological subtype (d) (n = 3, 3, 7, 6 and 5 for N, A, AB, B and TC, respectively), Masaoka stage (e) (n = 3, 11, 4, 5 and 2 for N, I, II, III and IV, respectively) or our alternative classification (f) (n = 3, 9, 7 and 6 for N, T1, T2 and T3, respectively). Each color represents a T-cell subset, same as in (c). (g) Bar plots showing the frequencies of the main T-cell subsets among the four groups of samples (n = 3, 9, 7 and 6 for N, T1, T2 and T3, respectively. Data are presented as the mean ± s.e.m. P values in the figure were determined by an unpaired two-tailed Student’s t test). (DP, CD3+CD4+CD8+). (h) Representative flow cytometric plot of CD3+CD4-CD8- T cells, CD3+CD4+ T cells, CD3+CD8+ T cells and CD3+CD4+CD8+ T cells from TETs of three types. (i) Representative immunofluorescence (IF) staining images showing CD3 (pale blue), CD8 (green), CD4 (red) and DAPI (nuclei, blue) in the normal human thymus and TET samples (scale bar: 100 μm). (M: Medulla; C: Cortex; M-like: Medulla-like structure; C-like: Cortex-like structure). Experiment was performed in three independent samples for each group with similar results. Source data are provided as a Source Data file.
Regarding mature T cells, the proportions of naïve SP cells and memory SP cells were lowest in type 1 TETs and highest in type 2 TETs (Fig. 2g and Supplementary Fig. 4c). Interestingly, tissue-resident CD4+ T (CD4+ TRM) cells, tissue-resident CD8+ T (CD8+ TRM) cells and regulatory T cells (Treg), which are typically present in peripheral tissues18, showed an increasing tendency in type 3 TETs (Supplementary Fig. 4c). In addition, we found that the ratio of CD4+ to CD8+ T cells was lowest in tumors of type 1 (Supplementary Fig. 4c). Meanwhile, the differences in mature T-cell composition among WHO classification and Masaoka staging system based on the histopathology and morphology were similar to that in our alternative classification (Supplementary Fig. 5). Taken together, these findings uncovered a unique pattern of T-cell development in each type of tumor according to our alternative classification.
Dysfunction of T-cell development in human TETs
To validate the differences in immune cell composition among tumor types, we performed scRNA-seq and TCRαβ profiling on six paired tumor samples, which validated the immunosubtypes from our alternative classification (2 samples each type) (Fig. 1a). Data from a normal thymus sample published by Jong-Eun Park et al. were also reanalyzed as a normal control17. A total of 52,788 cells from tumors and 2,845 cells from the normal thymus were included (Supplementary Table 3). Twenty-six cell clusters were identified (Supplementary Fig. 6a). Using well-known lineage markers, we further annotated the cell clusters into nine major cell types (Supplementary Fig. 6b–e and Supplementary Data 1). Consistent with the results of CyTOF, the cellular atlas showed that T cells accounted for the major differences in cell types between tumor and normal tissue as well as among types of TETs (Supplementary Fig. 6f and g). In addition, the proportions of B cells and monocytes showed a similar tendency to vary among tumor types as the observed in the CyTOF results (Supplementary Fig. 6h).
To further investigate the differences in T-cell development patterns among tumor types, T cells were reclustered into 18 cell clusters (Supplementary Fig. 7a). Then, the cell clusters were further annotated (Fig. 3a), according to the markers related to T-cell development (Fig. 3b, Supplementary Fig. 7b and Supplementary Data 2). The characteristic molecules of tumor-infiltrating T cells detected by CyTOF were validated by scRNA-seq (Fig. 2b and Supplementary Fig. 7c). In addition, two immature T-cell subsets in tumors (proliferating (P) DP cells and quiescent (Q) DP cells) were identified by using MKI67 and CDK1 according to a previous study17 (Supplementary Fig. 7d–f); the result was partly consistent with the findings obtained via CyTOF (Supplementary Fig. 7g). Interestingly, the expression levels of CD3 genes were not distinct among CD3+ DP cells and CD3- DP cells (Supplementary Fig. 7h), which suggested that these immature DP (P) cells corresponded with the CD3- DP cells in CyTOF analysis.
(a) Uniform manifold approximation and projection (UMAP) visualization of total T cells from the normal human thymus (n = 1) and TET samples (n = 6), colored by the identified cell subpopulation. (DN, double-negative T cells; DP, double-positive T cells; the abbreviations used below are consistent with these definitions). (b) Dot plot of marker gene expression in T-cell types. Here and in later figures, the color represents the maximum normalized mean expression of marker genes in each cell subgroup, and the size indicates the proportion of cells expressing marker genes. (c) Same UMAP plot as (a), colored by groups. (d) Same UMAP plots of total T cells from samples of each group, colored by the identified cell subpopulation as in (a). The immature T cells are indicated by yellow dotted circles, and mature T cells are indicated by blue dotted circles. (e) Composition of the T-cell compartment showing average frequencies of major T-cell subpopulations for each group (n = 1, 2, 2 and 2 for N, T1, T2 and T3, respectively). (f) Pseudotime trajectory for T cells from all samples in a two-dimensional state-space defined by Monocle2, colored by identified T-cell subpopulation. (g) The same pseudotime plots for T cells from samples of each group, colored by group. (h-j) Dot plots depicting the relative expression levels of selected NOTCH (h), WNT (i), or IL-7 (j) signaling pathway genes in total T cells from each group. Source data are provided as a Source Data file.
Compared with the normal thymus, we found that early DP cells increased in type 1 TETs but decreased in type 2 TETs and were almost absent in type 3 TETs (Fig. 3c–e and Supplementary Fig. 7i). In contrast, the abundance of mature SP cells was significantly increased in type 2 and type 3 TETs (Fig. 3c–e and Supplementary Fig. 7i). Moreover, the composition of T-cell subsets involved in T-cell development was significantly different among different types of TETs (Fig. 3c–e). Specifically, early DP cells constituted the largest T-cell subset in type 1 TETs but the smallest subset in type 3 TETs (Fig. 3d, e and Supplementary Fig. 7i). In contrast, mature SP cells constituted the largest T-cell subset in type 3 TETs but the smallest T-cell subset in type 1 TETs (Fig. 3d, e and Supplementary Fig. 7i). Interestingly, naïve T cells were more abundant in type 2 TETs than in type 3 TETs, whereas memory T cells were more abundant in type 3 TETs than in type 2 TETs (Fig. 3d, e and Supplementary Fig. 7i).
To further validate the developmental differences among T cells in tumors, we performed trajectory analysis of the previously defined T-cell subpopulations (Fig. 3f). Consistent with previous findings, trajectory analysis showed an obvious difference in T-cell development in each type of TET compared with the normal thymus (Fig. 3g). Through intergroup analysis, we found that T cells in type 1 TETs were enriched in the early stage of development, while T cells in type 2 TETs were enriched in both the early stage and late stages of development (Fig. 3g). Interestingly, almost all T cells were concentrated in the late stage of development in type 3 TETs (Fig. 3g). The gene expression levels of Notch and Wnt signaling components, which regulate the early stages of T-cell development19,20, were higher in the T cells isolated from type 1 TETs than in those isolated from type 2 and type 3 TETs (Fig. 3h and i). In contrast, IL-7 signaling-related molecules, which could drive intrathymic expansion of positively selected thymocytes prior to their export to the peripheral T-cell pool21, were expressed at higher levels in T cells isolated from type 2 TETs than in those isolated from the normal thymus and other tumor types (Fig. 3j). These results suggested that the course of T-cell development was restrained in the early stage in type 1, promoted in type 2 and almost abolished in type 3 human TETs.
Bias of the TCR repertoire and TCR diversity in human TETs
The TCR repertoire and TCR diversity are closely related to intrathymic T-cell development17. For TCRβ, we found an obvious bias in VJ gene usage among tumor types of TETs (Fig. 4a), which resembled the VJ gene usage pattern of DP and SP cells (Supplementary Fig. 8a). Consistently, the VJ pairs of T cells were also significantly different among different types of TETs (Fig. 4b) and were consistent with DP and SP cells (Supplementary Fig. 8b). For the TCRα locus, we found a clear association between developmental timing and V-J pairing (Supplementary Fig. 8b), as previously described17,22. During the T-cell developmental stage, recombination of proximal pairs at the TCRα locus was mainly observed in the DP stage, whereas recombination of distal pairs was mainly observed in the SP stage (Supplementary Fig. 8c). This bias in recombination in turn restricted the V-J pairing of TCRα in DP and SP cells (Supplementary Fig. 8d). We also observed that the VJ gene usage and pairing of TCRα in each tumor type were in a similar pattern as DP and SP cells respectively. (Fig. 4c, d). Furthermore, we found that the clonotypes of tumor-infiltrating T cells were strongly associated with the T-cell developmental states defined by scRNA-Seq (Fig. 4e). It is worth noting that clonotypic amplification was mainly observed in mature SP cells but was less frequent in immature DN and DP cells (Fig. 4e). This also indicated that immature T cells (DN and DP cells) had higher clonal diversity than mature T cells (SP cells), which was consistent with the finding of low clonal diversity and high numbers of SP cells in type 3 TETs (Fig. 4f). Together, these findings uncovered the potential effects of the T development differences among human TET types on the TCR repertoire and clonal amplification.
(a) Heatmap showing the proportion of each TCRβ V and J gene segment present in total T cells from each group, normalized per column. Gene segments positioned according to genomic location. (b) Frequency of V-J gene pairs at the TCRβ locus of each group. (c) Same scheme as in (a) applied to TCRα V and J gene segments, normalized per column. (d) Same scheme as in (b) applied to TCRα V-J gene pairs. (e) UMAP plot of all T cells from TET samples (n = 6), colored by the number of TCR clonotypes detected. (f) Bar plot showing clonotypic diversity among the three groups of TET samples (n = 2, 2 and 2 for T1, T2 and T3, respectively). Source data are provided as a Source Data file.
Epithelial origin decodes T-cell developmental dysfunction in human TETs
Intrathymic T-cell development is tightly orchestrated by heterogeneous TECs10. To further investigate the epithelial origin of each type of TET and its potential impact on T-cell development, the subsets of epithelial cells (tumor cells) in the thymus and tumors were further analyzed (Fig. 5a and Supplementary Fig. 9a). A varying epithelial cell composition among human TETs was observed (Fig. 5a). Epithelial cells were most abundant in type 3 TETs, while they were much less abundant in type 1 and type 2 TETs (Fig. 5a, b). Gene expression analysis showed that epithelial cells in type 3 TETs expressed high levels of cancer stem cell (CSC)-related markers (NOTCH2, CD24, ALDH1B1 and PROM1) (Fig. 5c). At the protein level, the epithelial cells in type 3 TETs expressed higher levels of Ki67, further suggesting malignancy (Supplementary Fig. 9b). In addition, trajectory analysis showed that the epithelial cells in type 1 and type 2 TETs were at discrete developmental stages compared with those in type 3 TETs (Fig. 5d).
(a) UMAP visualization of total cells from samples in each group, colored by the cell types of epithelial cells and other cells (n = 2, 2 and 2 for T1, T2 and T3, respectively). (b) Bar plot showing the frequencies of epithelial cells among the TET groups (n = 2, 2 and 2 for T1, T2 and T3, respectively). (c) Violin plots showing the expression of the marker genes for CSCs on epithelial cells among different types of TETs. (d) Pseudotime trajectory for epithelial cells from all samples in a two-dimensional state-space defined by Monocle2, colored by group. (e) UMAP plot of all epithelial cells from the normal human thymus (n = 1) and TET samples (n = 6), colored by the identified cell subpopulation (left). Same UMAP plot, colored by group (right). (f) Dot plot of the expression of marker genes in epithelial cell types. (g) UMAP visualization of the expression of marker genes used for epithelial cell cluster identification. The color scale represents normalized expression. Gray to brown: low to high expression. (h) Composition of the epithelial cell compartment showing average frequencies of major epithelial cell subpopulations for each group of TETs; each color represents an epithelial cell subset (n = 2, 2 and 2 for T1, T2 and T3, respectively). (i) Representative IF staining images showing CCL25 (pink), CHI3L1 (red), KRT14 (green) and DAPI (nuclei, blue) in TET samples (scale bar: 100 μm). Experiment was performed in three independent samples for each group with similar results. Source data are provided as a Source Data file.
To further uncover the heterogeneity of tumor cells among TET subtypes, the epithelial cells in TETs were reclustered into 17 cell clusters (Supplementary Fig. 9c). The cell clusters were annotated into seven subsets based on a combination of known TEC markers (Fig. 5e–g and Supplementary Fig. 9d and Supplementary Data 3). Through gene expression analysis, we found that GNB3+ mTEC-like cells expressed high levels of OVOL3, PLCB2, and TRPM5 but low levels of PLCG2, CHAT, and DCLK1, all of which are marker genes of tuft-like mTEC cells in the normal thymus17,23,24 (Supplementary Fig. 9e). Similarly, KRT14+ mTEC-like cells expressed high levels of CLU, FN1, and IGFBP5 but not ITGA6, KRT17, or SOX417,24 (Supplementary Fig. 9f). Furthermore, we found that CTSL and PRSS16 were mainly expressed in cTEC-like cells and mcTEC-like cells in TETs (Supplementary Fig. 9g). FEZF2 and AIRE, which are responsible for thymic T-cell negative selection, were mainly expressed in CHI3L1+ mTEC-like cells, MYOG+ TEC-like cells, GNB3+ mTEC-like cells and CHGA+ TEC-like cells (Supplementary Fig. 9g). Intergroup analysis showed that the composition of tumor cell subsets and TEC marker gene expression in different TET subtypes was significantly different (Fig. 5e, h and Supplementary Fig. 10). In addition, our results identified the dominant tumor cell subsets in each type of TET (Fig. 5e and h). The main tumor cell subsets in each TET type were further confirmed by immunofluorescence (Fig. 5i). Interestingly, mcTEC-like cells present in both type 1 and type 2 tumors exhibited more molecular characteristics of thymic epithelial progenitor cells (TEPCs) than KRT14+ mTEC-like and CCL25+ cTEC-like cells25 (Supplementary Fig. 11a). RNA velocity indicated that KRT14+ and GNB3 + mTEC-like cells might be differentiated from mcTEC-like cells (Supplementary Fig. 11b). Through pathway analysis, we found that NF-κB signaling in mcTEC-like cells of type 1 TETs was obviously activated, while it was inhibited in type 2 TETs (Supplementary Fig. 11c), which implied that the differentiation of mcTEC-like cells into KRT14 + and GNB3 + mTEC-like cells in type 1 TETs was promoted, while it was suppressed in type 2 TETs25. Moreover, AIRE expression in type 2 TETs was significantly lower than that in the other two types (Supplementary Fig. 12a and b), which was correlated with the incidence of autoimmune disease13. Consistently, we also observed a higher proportion of type 2 TET patients presenting with myasthenia gravis (MG) (Supplementary Fig. 12c). It is interesting that expression of CHRNA1 was only detected in MYOG + TEC-like cells of type 3 TETs, which indicated that the loss of CHRNA1 + MYOG + TEC-like cells may involve in the MG development in type 1 and type 2 TETs26,27 (Supplementary Fig. 12d, e).
Interestingly, CXCL12 and CCL25, the key chemokines recruiting blood-borne lymphoid progenitor cells into the thymus25, were almost not expressed in CHI3L1+ mTEC-like cells (Supplementary Fig. 13a). Then, we focused on the tumor cell and lymphocyte interactions mediated by chemokines and demonstrated that mTEC-like and mcTEC-like tumor cells in TETs could induce DP cell migration for positive selection through CCL25:CCR9 and CXCL12:CXCR3 interactions (Supplementary Fig. 13b and Supplementary Data 4). Interestingly, cTEC-like tumor cells also played a role in inducing SP cell migration through the CCL19:CCR7 interaction (Supplementary Fig. 13c), which differed from cTECs in the normal thymus28.
Through Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, we found that the genes highly expressed in DP cells of type 2 TETs were significantly enriched in signaling pathways related to T-cell expansion and positive selection, including oxidative phosphorylation, the NF-κB signaling pathway, Th17-cell differentiation, Th1 and Th2 cell differentiation and the TNF signaling pathway25,29 (Supplementary Fig. 13d). High expression of genes in apoptosis-related pathways also indicated that more DP cells in type 2 TETs were undergoing positive selection (Supplementary Fig. 13d). Compared with normal thymus tissue, DP cells of type 2 TETs showed high expression of genes enriched in signaling pathways relevant for positive selection (Supplementary Fig. 13e). Moreover, we analyzed the expression of marker genes that indicated the pre- or ongoing state for positive selection of DP cells29 and found that DP cells in type 1 TETs mainly expressed preselection-related genes, while DP cells in type 2 TETs highly expressed genes related to the ongoing state of positive selection (Supplementary Fig. 13 f). These results indicated that positive selection during T-cell development was promoted in type 2 TETs but blocked in type 1 TETs, which was further supported by the observation that the expression level of HLA-DR in tumor cells in type 1 TETs was lower than that in other TET types (Supplementary Fig. 13 g). Furthermore, we performed a coculture experiment using sorted tumor epithelial cells and DP cells of type 1 and type 2 TETs (Supplementary Fig. 13h-j) and showed that tumor cells could directly promote T-cell development in vitro (Supplementary Fig. 13k–m). Taken together, these findings suggested that the specific epithelial origin of each type of TET may lead to a distinctive dysfunction of epithelial-cell-T-cell interactions and consequently to T-cell developmental dysfunction.
Tumor cells induce a CD8+ TRM cell-mediated immune response in type 3 TETs
Among the three types of TETs, it was interesting that precursor T cells were almost absent in type 3 TETs, whereas mature T cells were still abundant (Fig. 2c, f, g). A possible reason for this phenomenon was the immune recruitment induced by malignant cells, which is commonly observed30. We confirmed that CD8+ TRM cells, which play a central role in the immune surveillance network of peripheral tissues31, were significantly enriched in type 3 TETs (Fig. 6a, b). The unique abundance of CD8+ TRM cells in type 3 TETs was further confirmed by flow cytometry analysis (Supplementary Fig. 14a). Compared with other epithelial subpopulations, CHI3L1+ mTEC-like cells, which were most highly enriched in type 3 TETs, were predicted to have the largest number of interactions with CD8+ TRM cells (Fig. 6c and Supplementary Data 4). Specific ligand–receptor interactions between CHI3L1+ mTEC-like tumor cells and CD8+ TRM cells were identified (Fig. 6d and Supplementary Data 4). The higher expression level of CXCR3 on CD8+ TRM cells was confirmed by flow cytometry (Fig. 6e, f). CD8+ TRM cells in type 3 TETs also expressed high levels of activation- and proliferation-related molecules (Fig. 6g and Supplementary Fig. 14b). Moreover, genes related to activation were also significantly upregulated in CD8+ TRM cells compared with other types of T cells in the TETs (Fig. 6h). At the protein level, CD8+ TRM cells expressed higher levels of activation-related molecules than other T-cell subsets (Fig. 6i, j and Supplementary Fig. 14c). Pathway analyses revealed that the upregulated genes of CD8+ TRM cells were significantly enriched in the TCR signaling pathway, antigen processing and presentation, cytokine–cytokine receptor interaction and the chemokine signaling pathway (Fig. 6k). Further analysis demonstrated that CD8+ TRM in type 3 TETs could potentially recruit T cells, B cells, DCs and macrophages via multiple chemokines (Fig. 6l and Supplementary Data 4). Through flow cytometry, we confirmed that CD8+ TRM cells could secrete more of the chemokine CXCL13 than other T-cell subsets (Fig. 6m, n). Immunofluorescence staining further confirmed the colocalization of epithelial cells and CD8+ TRM cells, as well as CD8+ TRM cells and DCs and B cells, in type 3 TETs (Fig. 6o, p and Supplementary Fig. 14d). These results suggested that CHI3L1+ mTEC-like tumor cell-induced CD8+ TRM cell activation plays a central role in the immune response and the formation of the immune landscape in type 3 TETs.
(a) Same t-SNE plots as (Fig. 2c), colored by CD8+ TRM cells and other cells. (b) Bar plot showing the frequencies of CD8+ TRM cells among the three groups (n = 3, 16 and 6 for N, T1 + T2 and T3, respectively. Data are presented as the mean ± s.e.m. P values in the figure were determined by an unpaired two-tailed Student’s t test). (c) Heatmap showing the number of interactions between CD8+ TRM cells and epithelial cell subpopulations. (d) Heatmap of chemokine interactions among T cells and epithelial cells, normalized per column. (e–f) Flow cytometric analysis of CXCR3 expression among CD4+ T cells, CD8+ non-TRM cells and CD8+TRM cells in TETs. (g) Heatmap showing the expression of activation-associated proteins in CD8+ TRM cells in each group by CyTOF and normalized total matrix. (h) Dot plot of the expression of cell activation- and TCR activation-associated genes within CD8+ TRM cells and other T-cell subsets. (i-j) Flow cytometric analysis of IFN-γ and CD39 expression among CD4+ T cells, CD8+ non-TRM cells and CD8+TRM cells in TETs. (k) KEGG pathway analysis of genes that were significantly (P < 0.01) upregulated in CD8+ TRM cells compared to other T cells. (l) Heatmap of chemokine interactions among CD8+ TRM cells and other cell types, normalized per column. (m–n) Flow cytometric analysis of CXCL13 expression among CD4+ T cells, CD8+ non-TRM cells and CD8+ TRM cells in TETs. (o–p) Representative IF staining images showing CD103 (red), EPCAM (green), CD11c (green), CD20 (pale blue) and DAPI (nuclei, blue) in type 3 TET samples. The interactions between epithelial cells and TRM cells are indicated by white arrows (o). The interactions between TRM cells and DCs are indicated by yellow arrows, and those between TRM cells and B cells are indicated by white arrows (p). Scale bar: 20 μm. Experiment was performed in three independent samples with similar results. (f, j, n) n = 8(f), 8 (j-left), 6 (j-right),6(n), respectively. Data are presented as the mean ± s.e.m. P values in the figure were determined by a two-tailed paired Student’s t test. Source data are provided as a Source Data file.
The molecular markers of tumor cell correlate with survival of human TETs
Finally, we tried to establish a molecular classification system for human TETs based on the epithelial origin of tumor cells. First, we selected representative genes that represented the unique epithelial cell type in each TET type based on scRNA-seq results (Fig. 5f) and found that GNB3 and CHI3L1 were highly expressed in tumor cells of type 1 and type 3 TETs, respectively (Fig. 7a, b). Consistently, the expression of GNB3 and CHI3L1 in tumors was further validated by IF staining (Fig. 7c). Therefore, we reclassified 119 TET patients from the TCGA cohort into three groups according to the expression levels of GNB3 and CHI3L1 (Supplementary Fig. 15a). Kaplan–Meier survival analysis demonstrated that the molecular classification we established was closely correlated with the survival of TET patients (P = 0.01409) (Fig. 7d). However, the survival prediction effects of Masaoka staging system and WHO classification were not significant (P = 0.1698, P = 0.2912) (Supplementary Fig. 15b, c). Collectively, based on our findings, we proposed a tripartite framework to explain the origin of tumor cells and their impact on T-cell development in the tumor microenvironment of human TETs (Fig. 7e).
(a-b) Dot plots depicting the relative levels of the GNB3 (a) and CHI3L1 (b) genes in total cells from each group. (c) Representative IF staining images showing GNB3 (green), CHI3L1 (red), and DAPI (nuclei, blue) in TET samples. Scale bar: 50 μm. Experiment was performed in three independent samples for each group with similar results. (d) Overall survival (OS) examined in each of the three types of TETs in the TCGA cohort. The P value was calculated using the log-rank test. (e) Schematic model summarizing the developmental patterns of three types of TETs, including interactions between different types of cells. The ligand is secreted by the cell at the beginning of an arrow, and the receptor is expressed by the cell at the end of that arrow. Source data are provided as a Source Data file.
Discussion
TETs are heterogeneous tumors considered to originate from TECs32. However, the exact cellular origin of TETs is largely unknown9. Growing evidence has shown that TECs in the human thymus are extraordinarily heterogeneous25. Therefore, it is reasonable to speculate that the epithelial origin of human TETs may also be heterogeneous. The findings of discrepant tumor cell compositions among human TET types in our study supported this hypothesis. In addition, we identified the exact tumor cell subset of each human TET type that represented its epithelial origin. These findings suggested that there was a remarkable heterogeneity in the epithelial origin of human TETs, which may determine the biological nature and prognosis of human TETs.
Genetic aberrations were reported in human TETs a decade ago33. Subsequently, a specific missense mutation in GTF2I was demonstrated to occur at high frequency in type A and type AB thymomas but rarely in the aggressive subtypes34. In contrast, thymic carcinomas carry a higher number of recurrent mutations of known cancer genes, such as TP53, CYLD, CDKN2A, BAP1 and PBRM1, than thymomas34. Consistently, another study identified recurrent somatic mutations in TET2, CYLD, SETD2, TP53, FBXW7, HRAS and RB1 in thymic carcinoma and no mutations in GTF2I35. However, the details of how these driver oncogenes promote tumor development and the key driver genes in each type of TET are still unknown. Recently, GTF2I mutation was reported to induce cell transformation and metabolic alterations in TECs, which provided direct evidence of the role of GTF2I mutation in promoting tumorigenesis of TETs36. By decoding the epithelial origin of each human TET type, we identified GNB3 and CHI3L1, which were specifically expressed in type 1 and type 3 tumors, respectively. This evidence suggests that GNB3 and CHI3L1 may be candidate driver oncogenes in the original TECs of type 1 and type 3 human TETs, respectively.
There are various histological classifications and clinical staging systems for human TETs, including the WHO classification and Masaoka staging system9. Despite improvements in these classification systems, some longstanding controversies remain unresolved37,38,39. Recently, genomic analysis of tumors showed that according to mutations in GTF2I, T-cell signaling mRNA signatures and somatic copy number alteration (SCNA) levels, human TETs could be classified into four types associated with disease-free and overall survival of patients15. A pioneer study uncovered the integrated genomic landscape of TETs and established new molecular classifications based on the gene mutation rates and expression levels in the tumor mass, which could predict outcomes and the incidence of autoimmune diseases in TET patients16. Here, we proposed an alternative molecular classification for human TETs according to the molecules specifically expressed in the unique tumor cell subset that reflected the epithelial origin of each tumor type. Although our proposed molecular classification showed promise in prognostic prediction in a cohort of 119 TETs patients, further clinical validation was needed.
A high incidence rate of autoimmune disease is one of the most remarkable clinical features of human TETs10. However, the cellular and molecular mechanisms of immune disorders in TETs are still largely unclear. We hypothesized that the epithelial origin of human TETs, which plays a critical role in thymic T-cell development and central self-tolerance13, accounted for the generation of immune disorders in patients. As expected, we found that the abundance of cTEC-like cells increased in type 2 TETs; in contrast, that of mTEC-like cells decreased. Consistently, AIRE, the key molecule for the negative selection of T cells, was not expressed in the major subsets of tumor cells in type 2 TETs. This evidence suggested that the increased abundance of cTEC-like cells, decreased abundance of mTEC-like cells and decreased AIRE expression in type 2 TETs led to the dysfunction of central self-tolerance and the generation of autoimmune disease in patients. In addition, the finding of a relative lack of mature T cells in tumors suggested that defects in T-cell maturity in tumors may account for the generation of acquired T-cell deficiency in type 1 TETs. The findings obtained in our study provide a new clue for understanding the generation of immune disorders in TET patients.
Taken together, we report a comprehensive study that is the first to use multiple omics approaches to decode the biological characteristics of human TETs, from the immune phenotype to the underlying mechanism determined by the epithelial origin of tumors. We uncovered an epithelium-T-cell loop that reflected the core differences in biological characteristics among tumor types. Finally, according to the immune landscape of tumors and molecular markers of tumor cell, we proposed a potential alternative classification of human TETs. Although the sample size of our study is substantially smaller than the canonical classifications rely on, we believe that our findings provide insights into the biology of human TETs and will support translational research on human TETs.
Methods
Patients and specimens
Tumor tissues (T, homogeneous cellularity, without necrotic foci) were obtained from patients with TETs who underwent surgical resection at the Department of Thoracic Surgery, Second Affiliated Hospital, Zhejiang University School of Medicine. None of the patients had received radiotherapy or chemotherapy before surgery. The pathological results of all patients indicated TETs. Normal human thymus samples were obtained from the Department of Cardiac Surgery, Second Affiliated Hospital, Zhejiang University following cardiothoracic surgery on adults with heart disease, as thymic tissue is routinely removed and discarded to achieve adequate exposure of the retrosternal operative field. All samples were anonymously coded in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki), written informed consent was obtained, and the protocol was approved by the Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine.
Preparation of cell suspensions
Freshly excised tissues were stored in sterile RPMI (Corning) supplemented with 10% FBS (Life Technologies) and 1% streptomycin and penicillin (Life Technologies) and processed within 2 hours. The tissues were cut into small pieces and then digested in RPMI containing 10% FBS, type I collagenase (1 mg/ml), and type IV collagenase (1 mg/ml) for 1 hour at 37 °C using a gentleMACSTM Dissociator (Miltenyi Biotech) according to the manufacturer’s instructions. The resulting single-cell suspension was filtered sequentially through sterile 70 μm cell strainers. Then, the cell suspensions were stored in complete medium at 4 °C for subsequent experiments.
HE staining and image acquisition
For HE analyses, TETs and normal thymus tissues were fixed in 10% formalin, embedded in paraffin and sectioned transversely. The 5 μm-thick formalin-fixed paraffin-embedded (FFPE) sections on glass slides were incubated at 37 °C overnight, dewaxed in xylene, rehydrated through decreasing concentrations of ethanol, and washed in PBS. Then, the sections were stained with hematoxylin and eosin. After staining, the sections were dehydrated through increasing concentrations of ethanol and xylene. The slides were scanned with a KF-PRO-120 scanner (KFBIO).
Immunofluorescence staining and image acquisition
The 5 μm-thick FFPE sections were also incubated at 37 °C overnight, dewaxed in xylene, rehydrated through decreasing concentrations of ethanol, and washed in PBS. Antigen retrieval was performed in Target Retrieval Solution (TRS) citrate buffer (pH 6.0) using a pressure cooker. Then, the sections were blocked with a blocking buffer solution (5% FBS, 1% BSA and 0.2% Triton) for 2 h at room temperature and incubated with the primary antibody in blocking buffer (BioLegend) at 4 °C overnight. After washing with PBS (pH 7.4), polymer horseradish peroxidase (HRP) conjugated secondary antibody staining was performed at room temperature for 1 h. Tyramide signal amplification (TSA)-based visualization was performed with Opal fluorophores. Next, the sections were washed three times with PBST, and DAPI reagent was added for 10 min to detect cell nuclei. Antibodies against CD3 (ab16669, diluted 1:100), CD8a (ab17147, diluted 1:100), CD4 (ab133616, diluted 1:500), CD103 (ab129202, diluted 1:800), CD11c (ab52632, diluted 1:500), CD20 (ab78237, diluted 1:2000) and EPCAM (ab223582, diluted 1:500) were procured from Abcam. Antibodies against CD45 (60287-1-IG, diluted 1:500), CCL25 (25285-1-AP, diluted 1:500), CHI3L1 (12036-1-AP, diluted 1:400), AIRE (22517-1-AP, diluted 1:500), KRT14 (60320-1-IG, diluted 1:800) and GNB3 (12036-1-AP, diluted 1:400) were procured from Proteintech Group. HRP conjugated Goat Anti-Mouse/Anti-Rabbit secondary antibodies (ab2891, 1:200) were procured from Abcam. Epifluorescence multispectral whole-slide images of all sections were acquired through the NIKON ECLIPSE C1 system (Nikon Corporation) and scanned at high resolution on a Pannoramic SCAN II system (3DHISTECH Ltd.).
Flow cytometry
The antibodies CD45-BV510 (2D1, diluted 1:100), CD45-APCCY7 (HI30, diluted 1:100), CD3-BV421 (UCHT1, diluted 1:100), CD3-BV605 (UCHT1, diluted 1:100), CD3-APCCY7 (UCHT1, diluted 1:100), CD3-PECY7 (UCHT1, diluted 1:100), CD4-APC (RPA-T4, diluted 1:100), CD4-PECY7 (RPA-T4, diluted 1:100), CD8a-APCCY7 (RPA-T8, diluted 1:100), CD8a-AF700 (RPA-T8, diluted 1:100), CD8a-FITC (RPA-T8, diluted 1:100), CD103-BV605 (Ber-ACT8, diluted 1:100), CD103-FITC (Ber-ACT8, diluted 1:100), CD69-PECY7 (FN50, diluted 1:100), CD69-PerCP-Cy5.5 (FN50, diluted 1:100), CD39-PE (A1, diluted 1:100), IFN-γ-PE (B27, diluted 1:50), CXCR3-BV421 (G025H7, diluted 1:100), EPCAM-PE (9C4, diluted 1:100), EPCAM-APC (CO17-1A, diluted 1:100), and 7-AAD (Cat#:420404, diluted 1:200) were procured from BioLegend. CXCL13-APC (Cat#: IC801A, diluted 1:20) was procured from R&D Systems. We preincubated fresh tissue cells (1×106/ml) in a mixture of PBS, 2% fetal calf serum, and 0.1% (w/v) sodium azide with FcgIII/IIR-specific antibody to block nonspecific binding and stained them with different combinations of fluorochrome-coupled antibodies for 15 minutes at room temperature. IFN-γ and CXCL13 were stained for 30 minutes at room temperature after fixation and permeabilization. Cells were washed with PBS and passed through a 70-μm filter, and data were collected on a FACSCanto II system and FACSFortessa system (BD Biosciences) and analyzed using FlowJo software (version 10.0.7). Gating strategies used for the flow cytometry were presented in supplementary Fig. 16.
Mass cytometry (CyTOF) sample preparation
The 42 metal-conjugated antibodies used in this study are shown in Supplementary Table 1. Briefly, the cells derived from the TET samples were stained with 5 µM cisplatin (Fluidigm) in PBS without BSA for viability staining. Then, the samples were washed in PBS containing 2.5% BSA and blocked for 30 minutes at 4 °C. After that, they were stained with cell-surface antibodies in PBS containing 5% goat serum and 30% bovine serum albumin (BSA) for 30 min at 4 °C. Next, the samples were washed, fixed and permeabilized using the Foxp3 fix and permeabilization kit (eBioscience) as well as 100 nM Iridium nucleic acid intercalator (Fluidigm) according to the manufacturer’s instructions at 4 °C overnight. The cells were then washed twice with Foxp3 permeabilization buffer and incubated with intracellular antibodies in permeabilization buffer for 30 min at 4 °C. Finally, the cells were washed twice with ddH2O to prepare them for analysis.
CyTOF data acquisition and analysis
Immediately before the acquisition, samples were washed and resuspended at a concentration of 1 million cells/ml in water containing EQ Four Element Calibration Beads (Fluidigm). Samples were acquired on a Helios CyTOF System (Fluidigm) at an event rate of <500 events/second. EQ beads (Fluidigm) were used as a loading control. All data were produced on a Helio3 CyTOF Mass Cytometer (Fluidigm). Mass cytometry data files were normalized using bead-based normalization software, which uses the intensity values of a sliding window of bead standards to correct for instrument fluctuations over time and between samples. CyTOF analyses were performed by PLTTech Inc. (Hangzhou, China) according to a previously described protocol40. The data were gated to exclude residual normalization beads, debris, dead cells and doublets for subsequent clustering and high dimensional analyses. Forty-two immune cell markers were all applied for clustering and visualization. The Phenograph41, PARC and Xshif algorithms were used to cluster the cells. A total of 50,000 cells were selected randomly for visualization by the t-distributed stochastic neighbor embedding (t-SNE) dimension reduction algorithm42 using the R package cytofkit (version 0.13). Immune subset cells were defined by the median values of specific expression markers in hierarchical clustering. Heatmaps of normalized marker expression, relative marker expression, and relative differences in population frequency were generated using the pHeatmap R package and Python (https://www.python.org/). Comparisons between two groups were performed by unpaired Student’s t tests using GraphPad Prism (v8). The use of these tests was justified based on an assessment of the normality and variance of the distribution of the data.
Single-cell RNA sequencing
Trypan blue was used for quality evaluations of single-cell suspensions prepared as outlined earlier, and the cell survival rate was generally above 80%. The cells that passed the test were washed and resuspended to prepare a suitable cell concentration of 1000 cells/µl for 10x Genomics ChromiumTM. Approximately 10,000 cells were loaded onto the 10X Chromium Single Cell Platform (Single Cell 5′ library and Gel Bead Kit v.3) as described in the manufacturer’s protocol. The generation of gel beads in emulsion (GEMs), barcoding, GEM-RT clean-up, complementary DNA amplification and library construction were all performed according to the manufacturer’s protocol. Qubit (Agilent 2100) was used for library quantification before pooling. The final library pool was sequenced on an Illumina NovaSeq 6000 instrument using 150-base-pair paired-end reads.
Single-cell gene expression analysis
Raw gene expression matrices were generated using CellRanger (10x Genomics) and analyzed using the Seurat v3 R package43,44. All cells expressing <500 genes were removed, as well as cells that contained <500 unique molecular identifiers (UMIs) and >25% mitochondrial counts. Samples were merged and normalized. Because every cell has a unique barcode, the scRNA-seq data could be linked with the scTCR-seq data.
scRNA-seq clustering analysis
The LogNormalize method of the “Normalization” function of Seurat software was used to calculate the expression values of genes. Principal component analysis (PCA) was performed using the normalized expression value. Among all the PCs, the top 10 PCs were used to perform clustering and t-SNE analysis. We used the shared nearest neighbor (SNN) graph-based clustering algorithm from the Seurat package (FindClusters function with default parameters) to identify clusters of cells. Among them, 18 clusters demonstrated high expression of genes specific to epithelial and T cells and were thus selected for the final reclustering analysis. These clusters were then identified as distinct cell subtypes based on highly expressed genes from a literature review.
Differential expression and pathway analysis
DEGs were identified using the bimod test with the FindMarkers and FindAllMarkers functions in Seurat with a threshold of log2 (fold change) (log2FC) ≥ 0.26 and expression in more than 10% of cells. In addition, we selected 0.01 as the cutoff of the p value. KEGG45 analyses were performed using the OmicStudio tools (https://www.omicstudio.cn/tool) to identify gene set enrichment with a hypergeometric test.
TCR analysis
Full-length TCR V(D)J segments were enriched from amplified cDNA via PCR amplification using a Chromium Single-Cell V(D)J Enrichment kit according to the manufacturer’s protocol (10X Genomics). 10X TCR-enriched libraries were mapped with the Cell Ranger Single-Cell Software Suite (version 4.0.0, 10x Genomics) to the custom reference provided by the manufacturer (version 2.0.0 GRCh38 VDJ reference). All assembled contigs were filtered to retain only those that were assigned a raw clonotype ID and categorized as being both full-length and productive. Each clonotype was assigned a unique identifier, consisting of the predicted amino acid sequences of the CDR3 regions of these two chains, which was used to match clonotypes across samples. Clonality, which reflects the dominance of particular clones across the TCR repertoire, was calculated for each sample. To visualize the proportion of TCR clonotypes shared between T-cell phenotypes, we used barcode information to project T cells with prevalent TCR clonotypes on UMAP plots.
Pseudotime reconstruction and trajectory inference
The R package Monocle (version 2) algorithm was used to reconstruct pseudotime trajectories to determine the potential lineage development among diverse T-cell subsets and epithelial cell subsets46. For each analysis, PCA-based dimension reduction was performed on DEGs of each phenotype, followed by two-dimensional visualization on UMAP. Graph-based clustering (Louvain) sorted the T cells into twelve subsets. The cell differentiation trajectory was then captured using the orderCells function. As described previously for differential gene expression analyses, the R package MAST was used to detect genes significantly covarying with pseudotime based on a log-likelihood ratio test between the model formula, including cell pseudotime, and a reduced model formula. Additional model covariates were included in the residual model formula. Benjamini–Hochberg multiple testing correction was used to calculate the FDR, and genes with a FDR < 5% were considered to vary significantly with pseudotime. For T cells from different groups, the same process with the same signature genes and Monocle parameters was used to construct the clone-based trajectories.
RNA velocity analysis
The spliced and unspliced reads were counted from aligned bam files generated by CellRanger and fed into the velocyto.R (version 0.6) to calculate RNA velocity values for each gene in each cell. The resulting RNA velocity vector was then embedded into the UMAP space.
Cell-to-cell communication analysis of scRNA-seq data
CellPhoneDB (www.CellPhoneDB.org) was used to assess putative receptor–ligand interactions between epithelial cells and T-cell subsets, CD8+ TRM cells and other cell subsets (epithelial cells, B cells, DCs, fibroblast cells, monocytes, macrophages, and VSMCs). Briefly, the algorithm allows the detection of ligand–receptor interactions between cell types in scRNA-seq data using the statistical framework described in previous studies47,48. The tool was run for 1000 iterations. The normalized interaction score was calculated by multiplying the average expression level of ligands and receptors for all cell pairs and maximum normalizing these values. The total number of pairwise paracrine interactions between CD8+ TRM cell and epithelial cell subsets obtained using the CellphoneDB scoring method were visualized as heatmaps in the R package pheatmap.
Cell isolation and in vitro coculture
After antibody staining, CD45-EPCAM+ epithelial cells and CD45+CD3+CD4+CD8+ T cells were sorted by an Aria II cell sorter (BD Biosciences), and 7-AAD was used to eliminate dead cells. The purity of all sorted cells was greater than 90%. CD45-EPCAM+ epithelial cells and CD45+CD3+CD4+CD8+ T cells were mixed in 96-well plates for coculture in the presence of rhIL-7 (10 ng/ml) (Cat#: GMP200-07). The sorted cells were cocultured at a ratio of epithelial:CD4+CD8+ T cells of 1:2 (25000:50000). Another group containing CD4+CD8+ T cells was prepared as a negative control by adding rhIL-7 alone without epithelial cells. After coculture in vitro for 8 days, the proportions of DP (CD3+CD4+CD8+ T) cells and SP (CD3+CD4+ T and CD3+ CD8+ T) cells among CD3+ T cells were analyzed by flow cytometry.
RNA isolation and reverse-transcription quantitative PCR
Total RNA was extracted from CD3-CD4+CD8+ and CD3+CD4+CD8+ cells sorted by an Aria II cell sorter (BD Biosciences) using TRIzol reagent (Thermo Fisher Scientific K.K.). The cDNA was reverse-transcribed from 500 ng of total RNA using PrimeScriptTM RT reagent Kit (Takara) according to the manufacturer’s instructions. Quantitative PCR was performed on a Bio–Rad MyiQ Real-Time PCR Detection System using TB Green Premix Ex TaqTM II(Takara). The relative mRNA expression of CD3E and CD3G was normalized to the mRNA levels of the housekeeping gene GAPDH. The real-time PCR primers were synthesized by Integrated DNA Technologies. Primer sequences can be found in Supplemental Table 8.
Survival analysis
TCGA TET data were used to evaluate the prognostic effect of individual genes or gene sets derived from specific cell clusters. The gene expression and survival data were downloaded from UCSC Xena (http://xena.ucsc.edu/). A total of 119 patients with records containing GNB3 and CHI3L1 gene expression information were included in the survival analysis (of these, 117 had a WHO classification and Mosaok stage). The expression profile was normalized by log2 (normalized_count+1) to exclude potential bias. For each gene, patient cohorts were grouped into high- and low-expression groups by the top 20% of the normalized average expression. Kaplan–Meier survival curves were plotted to show differences in survival time, and P values were reported from the log-rank test using GraphPad Prism (v8).
Statistical analysis
Statistical analysis was performed using Prism version 8.3 (GraphPad Software). Two-sided Student’s t tests were used to identify differences between two groups. All results are presented as the mean ± SEM, and P values of <0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The raw scRNA-seq data reported in this paper has been deposited in the Genome Sequence Archive in National Genomics Data Center under the accession number HRA002334. For analysis of the normal thymus, Jong-Eun Park et al.’s dataset17 were downloaded from ArrayExpress (accession number E-MTAB-8581). The remaining data were available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
Code availability
Custom codes were not created for data analyses in this study. Analysis of CyTOF followed publicly available instructions from PhenoGraph (https://github.com/jacoblevine/PhenoGraph), Xshift (https://github.com/ginberg/xshift_operator) and PARC (https://github.com/ShobiStassen/PARC). Analysis of scRNA-seq followed publicly available instructions from Cellranger (https://github.com/10XGenomics/cellranger), Seurat (http://satijalab.org/seurat/), Monocle (http://coletrapnell-lab.github.io/monocle-release/docs/), Velocyto (http://velocyto.org) and CellPhoneDB (https://www.cellphonedb.org). Any additional information required for the analysis of data in this manuscript is available from the authors upon request.
References
Engels, E. A. Epidemiology of thymoma and associated malignancies. J. Thorac. Oncol. 5, S260–S265 (2010).
de Jong, W. K. et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur. J. Cancer 44, 123–130 (2008).
Marx, A. et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp. Neurol. 270, 55–65 (2015).
Huang, B. et al. Anti-Apoptotic Signature in Thymic Squamous Cell Carcinomas - Functional Relevance of Anti-Apoptotic BIRC3 Expression in the Thymic Carcinoma Cell Line 1889c. Front Oncol. 3, 316 (2013).
Ehemann, V. et al. Establishment, characterization and drug sensitivity testing in primary cultures of human thymoma and thymic carcinoma. Int J. Cancer 122, 2719–2725 (2008).
Gokmen-Polar, Y. et al. Establishment and characterization of a novel cell line derived from human thymoma AB tumor. Lab Invest 92, 1564–1573 (2012).
Marx, A. et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J. Thorac. Oncol. 10, 1383–1395 (2015).
Strobel, P. et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J. Clin. Oncol. 22, 1501–1509 (2004).
Conforti, F., Pala, L., Giaccone, G. & De Pas, T. Thymic epithelial tumors: From biology to treatment. Cancer Treat. Rev. 86, 102014 (2020).
Abramson, J. & Anderson, G. Thymic Epithelial Cells. Annu Rev. Immunol. 35, 85–118 (2017).
Cumano, A. et al. New Molecular Insights into Immune Cell Development. Annu Rev. Immunol. 37, 497–519 (2019).
Starr, T. K., Jameson, S. C. & Hogquist, K. A. Positive and negative selection of T cells. Annu Rev. Immunol. 21, 139–176 (2003).
Shelly, S., Agmon-Levin, N., Altman, A. & Shoenfeld, Y. Thymoma and autoimmunity. Cell Mol. Immunol. 8, 199–202 (2011).
Weksler, B. & Lu, B. Alterations of the immune system in thymic malignancies. J. Thorac. Oncol. 9, S137–S142 (2014).
Lee, H. S. et al. Genomic Analysis of Thymic Epithelial Tumors Identifies Novel Subtypes Associated with Distinct Clinical Features. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 23, 4855–4864 (2017).
Radovich, M. et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 33, 244–258.e210 (2018).
Park, J. E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
Masopust D. & Soerens A. G. Tissue-Resident T Cells and Other Resident Leukocytes. Ann. Rev. Immunol. 37, 521–546 (2019).
Taghon, T., Waegemans, E. & Van de Walle, I. Notch signaling during human T cell development. Curr. Top. Microbiol Immunol. 360, 75–97 (2012).
Weerkamp, F., van Dongen, J. J. & Staal, F. J. Notch and Wnt signaling in T-lymphocyte development and acute lymphoblastic leukemia. Leukemia 20, 1197–1205 (2006).
Aspinall, R. T cell development, ageing and Interleukin-7. Mech. Ageing Dev. 127, 572–578 (2006).
Carico, Z. M., Roy Choudhury, K., Zhang, B., Zhuang, Y. & Krangel, M. S. Tcrd Rearrangement Redirects a Processive Tcra Recombination Program to Expand the Tcra Repertoire. Cell Rep. 19, 2157–2173 (2017).
Miller, C. N. et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559, 627–631 (2018).
Bornstein, C. et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559, 622–626 (2018).
Kadouri, N., Nevo, S., Goldfarb, Y. & Abramson, J. Thymic epithelial cell heterogeneity: TEC by TEC. Nat. Rev. Immunol. 20, 239–253 (2020).
Marx, A. et al. Thymus and autoimmunity. Semin. Immunopathol. 43, 45–64 (2021).
Chia R. et al. Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: a genome-wide association study. Proc Natl Acad Sci USA 119, e2108672119 (2022).
Bunting, M. D., Comerford, I. & McColl, S. R. Finding their niche: chemokines directing cell migration in the thymus. Immunol. Cell Biol. 89, 185–196 (2011).
Chopp, L. B. et al. An Integrated Epigenomic and Transcriptomic Map of Mouse and Human alphabeta T Cell Development. Immunity 53, 1182–1201.e1188 (2020).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Masopust, D. & Soerens, A. G. Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev. Immunol. 37, 521–546 (2019).
Oramas D. M.,& Moran C. A. Thymoma: Histologically a heterogenous group of tumors. Semin Diagn Pathol 39, 99–104 (2021).
Lee, G. Y. et al. Genome-wide genetic aberrations of thymoma using cDNA microarray based comparative genomic hybridization. BMC Genomics 8, 305 (2007).
Petrini, I. et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat. Genet. 46, 844–849 (2014).
Saito, M. et al. The genomic and epigenomic landscape in thymic carcinoma. Carcinogenesis 38, 1084–1091 (2017).
Kim, I. K. et al. Mutant GTF2I induces cell transformation and metabolic alterations in thymic epithelial cells. Cell death Differ. 27, 2263–2279 (2020).
Weis, C. A. et al. The impact of thymoma histotype on prognosis in a worldwide database. J. Thorac. Oncol. 10, 367–372 (2015).
Roden, A. C. et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J. Thorac. Oncol. 10, 691–700 (2015).
Ahmad, U. The eighth edition TNM stage classification for thymic tumors: What do I need to know? J. Thorac. Cardiovasc Surg. 161, 1524–1529 (2021).
Han, G., Spitzer, M. H., Bendall, S. C., Fantl, W. J. & Nolan, G. P. Metal-isotope-tagged monoclonal antibodies for high-dimensional mass cytometry. Nat. Protoc. 13, 2121–2148 (2018).
Levine, J. H. et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell 162, 184–197 (2015).
Amir, E.-aD. et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 31, 545–552 (2013).
Stuart, T. et al. Comprehensive Integration of Single-. Cell Data. Cell 177, 1888–1902.e1821 (2019).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 45, D353–D361 (2017).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 (2018).
Acknowledgements
We are grateful to the tissue donors and the clinical staff at The Second Affiliated Hospital of Zhejiang University School of Medicine who made tissue collection possible. We thank the Jian Huang lab for technical assistance and helpful support. We thank all the members of the Pin Wu lab for discussions and comments on the manuscript. This work was supported by funding from the National Natural Science Foundation of China (81572800 and 82073141 to PW, 82073142 to DW), the Fundamental Research Funds for the Central Universities (2019QNA7025 to PW) and the Natural Science Foundation of Zhejiang Province (LY15H160041 and LR22H160006 to PW, LY19H160050 to DW).
Author information
Authors and Affiliations
Contributions
P.W. designed the study. Z.W.X., M.J.L., Z.X.H., D.C., Y.Y.C. and performed the experiments. Z.W.X., M.J.L. and Z.X.H. collected the samples and clinical data. X.X. and J.F.L. reviewed the histological types of the samples. Z.W.X., D.W. and P.W. analyzed the data, interpreted the data, and wrote the manuscript. Y.C. and P.W. supervised the project, and all authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xianwen Ren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xin, Z., Lin, M., Hao, Z. et al. The immune landscape of human thymic epithelial tumors. Nat Commun 13, 5463 (2022). https://doi.org/10.1038/s41467-022-33170-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33170-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.