Abstract
The determination of durability and vaccine-associated protection is essential for booster doses strategies, however data on the stability of SARS-CoV-2 immunity are scarce. Here we assess anti-SARS-CoV-2 immunogenicity decay and incident cases six months after the 2nd dose of Sinovac-CoronaVac inactivated vaccine (D210) in 828 autoimmune rheumatic diseases patients compared with 207 age/sex-balanced control individuals. The primary outcome is the presence of anti-S1/S2 SARS-CoV-2 IgG at 6 months compared to 6 weeks after 2nd vaccine dose for decay evaluation. Secondary outcomes are presence of neutralizing antibodies, percent inhibition by neutralizing, geometric mean titers and cumulative incident cases at 6 months after 2nd dose. Anti-S1/S2 IgG positivity and titers reduce to 23.8% and 38% in patients (p < 0.001) during the six-month follow up and 20% and 51% in controls (p < 0.001), respectively. Neutralizing antibodies positivity and percent inhibition declines 41% and 54% in patients (p < 0.001) and 39.7% and 47% in controls (p < 0.001). Multivariate logistic regression analysis show males (OR = 0.56;95% CI0.40-0.79), prednisone (OR = 0.56; 95% CI0.41-0.76), anti-TNF (OR = 0.66;95% CI0.45-0.96), abatacept (OR = 0.29; 95% CI0.15-0.56) and rituximab (OR = 0.32;95% CI0.11-0.90) associate with a substantial reduction in IgG response at day 210 in patients. Although cellular immunity was not assessed, a decrease of COVID-19 cases (from 27.5 to 8.1/100 person-years; p < 0.001) is observed despite the concomitant emergence and spread of the Delta variant. Altogether we show a reduction in immunity 6-months of Sinovac-CoronaVac 2nd dose, particularly in males and those under immunosuppressives therapies, without a concomitant rise in COVID-19 cases. (CoronavRheum clinicaltrials.gov:NCT04754698).
Similar content being viewed by others
Introduction
Mass vaccination is the main measure to control the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread and the emergence of new viral variants of concern1. While the pandemic drags on, the determination of immunogenicity durability is an essential step to establish booster dose strategies.
Data on the medium- and long-term persistence of immunity after vaccination against SARS-CoV-2 are scarce. Only a few cases series are reported with messenger RNA (mRNA) and viral vector vaccines, and the data have demonstrated a variable decline of antibody levels 2–6 months after two doses of SARS-CoV-2 vaccination in the general population2,3,4,5,6,7,8. In addition, a large prospective study in healthcare workers reported a substantial decrease of mRNA vaccine-induced antibodies by 6 months8. Some of these studies identified age and sex as associated with reduced durability of vaccine humoral immune response3,8.
The inactivated Sinovac-CoronaVac vaccine is currently used in the most populated countries of the world, and its protective effect against hospitalization and death related to coronavirus infectious disease 2019 (COVID-19) was demonstrated in more than 10 million subjects9. The short-term waning of antibody response to this vaccine was evaluated in 159 healthcare workers with persistent seropositivity up to 98 days after vaccination, although with a significant reduction in antibody titers after 42 days10.
The durability of vaccine immunity was also evaluated in a population of immunocompromised individuals composed of a few cancer patients under active therapy. The follow-up after full vaccination lasted solely 3–4 months. The study reported decay of IgG titers or inability to sustain IgG levels above the threshold11,12. With regard to autoimmune diseases, one study assessed 242 patients with a wide range of different conditions using a general computer-based questionnaire. They identified that participants with immunosuppression had a 65% reduction in IgG levels and 70% in neutralizing antibody (NAb) concentrations compared to those without these therapies, up to 6 months after vaccination with mRNA vaccine8.
The deleterious impact of immunosuppressive therapy in a large autoimmune rheumatic diseases (ARD) population was reported for primary Sinovac-CoronaVac vaccination in a prospective study13,14. However, there is no report evaluating the long-term durability of anti-SARS-CoV-2 immunity in COVID-19 vaccinated ARD patients.
Here, we described the analysis of a large ARD population, that was conducted to assess prospectively the 6-month durability of SARS‐CoV‐2 immunity in fully vaccinated adults with Sinovac-CoronaVac compared with age- and sex-balanced control individuals without the rheumatic disease. We further evaluated incident symptomatic COVID-19 cases confirmed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR). We also assessed risk factors for reduced 6-month durability of anti-SARS-CoV-2 immunity.
Results
Study design and participants
Participants originated from a large single center (Sao Paulo, Brazil) phase 4 controlled prospective study (no. NCT04754698, CoronavRheum) of immunogenicity and safety of two doses of Sinovac-CoronaVac vaccine in ARD patients and control group (CG)13. After applying the exclusion criteria, the final study groups consisted of 828 ARD patients and 207 healthy controls vaccinated with two doses (Fig. 1). ARD group included patients with: 27.5% (n = 228) rheumatoid arthritis (RA), 24.9% (n = 206) systemic lupus erythematosus (SLE), 23.7% (n = 196) axial spondyloarthritis, 6.2% (n = 51) primary vasculitis, 4.8% (n = 40) idiopathic inflammatory myopathies, 4.3% (n = 36) systemic sclerosis (SSc), and 4.3% (n = 36) primary Sjögren’s syndrome. Regarding ARD current therapy at 6 weeks after the second dose (D69) the most frequently used were: 62.0% (n = 513) immunosuppressive drugs [209 (25.2%) methotrexate (MTX), 115 (13.9%) leflunomide (LEF), 109 (13.2%) mycophenolate mofetil (MFF), 90 (10.9%) azathioprine (AZA), 20 (2.4%) tofacitinib, 10 (1.2%) tacrolimus, 9 (1.1%) cyclosporine and 6 (0.7%) cyclophosphamide (CYC)], 36.5% (n = 302) prednisone (PRED) [median dose 5 (IQR 0.5–10) mg/day] and 35.7% (n = 296) biologic therapy [126 (15.2%) TNF inhibitor (35/126 combined to MTX), 46 (5.6%) tocilizumab (TCZ), 43 (5.2%) abatacept (ABA), 34 (4.1%) secukinumab, 26 (3.1%) belimumab, 17 (2.1%) rituximab (RTX), and 4 (0.5%) ustekinumab]. ARD and CG groups were comparable regarding median current age (p = 0.898), female sex (p > 0.999), and Caucasian ethnicity (p = 0.163) (Table 1).
The figure depicts enrollment, exclusions, and analysis of Anti-SARS-CoV-2 antibodies decay and COVID-19 incidence in patients with autoimmune rheumatic disease (ARD) and controls (CG). The participants allocated to vaccination were followed from the day of inclusion, which is the day of first dose vaccination with CoronaVac vaccine (DO) up to 10 days after the second dose vaccination (T1). The second period of follow-up (T2) ranged from T1 to 6 months after the second dose. PLWHA people living with HIV/AIDS, RT-PCR real-time polymerase chain reaction, SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2, D0 inclusion of participants and vaccine first dose, D40 10 days after the vaccine second dose, D69 6 weeks after the vaccine second dose, D210 6 months after the vaccine second dose.
Anti-SARS-CoV-2 S1/S2 IgG responses in ARD patients and CG
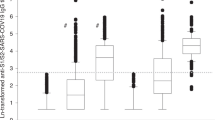
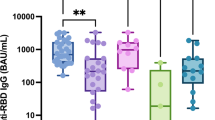
Anti-SARS-CoV-2 S1/S2 IgG assessed at D0, D69 (6 weeks after vaccine second dose), and D210 (6 months after the 2nd dose) are presented in Table 2. At D0, ARD patients had significantly lower anti-S1/S2 IgG seropositivity compared to CG [142 (17.1%) vs. 68 (32.9%), p < 0.001]. From D69 to D210 (6 months after second dose), anti-S1/S2 IgG seropositivity rates reduced by 23.8% in ARD [650 (78.5%) vs. 495 (59.8%), p < 0.001] and 20% in CG [202 (97.6%) vs. 161 (77.8%), p < 0.001], with moderate but lower IgG persistence in ARD compared to CG at D210 (p < 0.001). IgG GMT from D69 to D210 declined significantly after the second dose in ARD [41.8 (38.0–46.0) vs. 26.1 (23.2–29.4) AU/mL, p < 0.001] and in CG [99.6 (88.2–112.6) vs. 48.8 (40.3–59.0) AU/mL, p < 0.001], with lower IgG levels in the former group at D210 (p < 0.001) (Table 2). The decrease in IgG titer, calculated as 1–Ln(IgG210/IgGD69), was significantly lower in ARD compared to CG [38% (95% CI 32–43%) vs. 51% (95% CI 43–58%), p = 0.004].
A subanalysis of ARD patients with positive anti-SARS-CoV-2 serology before vaccination demonstrated a significant increase in anti-S1/S2 GMT from D0 to D69 (p < 0.001) with a subsequent decrease from D69 to D210 (p < 0.001) [74.7 (95% CI 64.4–86.6) vs. 165.7 (95% CI 143.2–191.7) vs. 104.8 (95% CI 88.5–124.2) AU/mL, p < 0.001].
Neutralizing antibodies responses in ARD patients and CG
From D69 to D210, NAb positivity declined by 41% in ARD [539 (65.1%) vs. 318 (38.4%), p < 0.001] and 39.7% in CG [181 (87.4%) vs. 109 (52.7%), p < 0.001]. There was a significantly greater reduction in NAb percent inhibition in those with positive NAb titers at D69 in the ARD vs. the CG [54% (95% CI 51–57%) vs. 47% (95% CI 41–52%), p = 0.024].
Factors associated with negative IgG 6 months after second dose
At D210, negative anti-S1/S2 IgG in ARD group was associated with older age (p = 0.001), lower frequencies of SLE (p = 0.005) and SSc (p = 0.024) and higher frequency of male sex (p = 0.007) and RA (p < 0.001). Regarding the influence of current therapy, patients seronegative for IgG at D210 were more often under PRED (43.7% vs. 31.1%, p < 0.001), at higher median dose [7.5 (5–10) vs. 5 (5–10) mg/day, p = 0.043], anti-TNF (19.2% vs. 13.2%, p = 0.012), ABA (9.8% vs. 2.7%, p < 0.001), and RTX (3% vs. 1.1%, p = 0.028), while hydroxychloroquine (HCQ) use was more frequent in ARD with positive anti-S1/S2 IgG (Table 3). Multivariate logistic regression analysis using IgG positivity at D210 as the dependent variable and as independent variables those with p < 0.2 in univariate analysis (current age, male sex, RA, SLE, SSc, HCQ, sulfasalazine, PRED, LFN, anti-TNF, ABA, and RTX use), revealed that male sex (OR = 0.56, 95% CI 0.40–0.79, p < 0.001), PRED use (OR = 0.56; 95% CI 0.41–0.76, p < 0.001), anti-TNF use (OR = 0.66; 95% CI 0.45–0.96, p = 0.031), ABA use (OR = 0.29; 95% CI 0.15–0.56, p < 0.001), and RTX use (OR = 0.32; 95% CI 0.11–0.90, p = 0.031) were significantly associated with absence of anti-S1/S2 IgG 6 months after vaccination in ARD patients (Table 4).
Factors associated with negative NAb 6 months after 2nd dose
For NAb analysis at D210, SLE diagnosis was less frequent in seronegative ARD patients (p = 0.019) whereas biologic therapy (p = 0.031), particularly ABA use (p = 0.018) was higher in patients without NAb (Table 3). After multivariate logistic regression analysis using NAb positivity as the dependent variable and as independent variables, those with p < 0.2 in univariate analysis (RA, SLE, sulfasalazine, anti-TNF, ABA, TCZ, and RTX use), only ABA use (OR = 0.48; 95% CI 0.24–0.97, p = 0.041) remained significantly associated with the absence of NAb at D210 in ARD patients (Table 4).
Kaplan–Meier curve of COVID-19 cases and hospitalizations
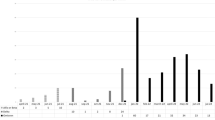
Analysis of incident cases of RT-PCR confirmed COVID-19 was performed from the 1st dose to 10 days after the second dose (T1) and thereafter to 180 days after the second dose (T2). Evaluation of incident cases among ARD patients (n = 1193) revealed n = 79 cases (n = 33 in T1 and n = 46 in T2) of COVID-19 with n = 14 hospitalizations (n = 7 in T1 and n = 7 in T2) and n = 4 deaths (n = 1 in T1 and n = 3 in T2). The incident symptomatic COVID-19 cases reduced from 27.5 (95% CI 18.9–38.6)/100 person-years in T1 to 8.1 (95% CI 6.0–10.9)/100 person-years in T2, with an incidence rate decline of 19.4 (95% CI 12.7–26.1) cases/100 person-years (p < 0.001). Likewise, in the same time periods, hospitalizations due to COVID-19 decreased from 5.8 (95% CI 2.3–12.0)/100 person-years to 1.2 (95% CI 0.5–2.6)/100 person-years, with an incidence rate decline of 4.6 (95% CI 1.8–7.4) hospitalizations/100 person-years (p = 0.001).
Deaths due to COVID-19 occurred in n = 4 ARD patients: n = 1 in T1 (before the second vaccine dose) and n = 3 in T2 (p = 0.694), after the second dose of Sinovac-CoronaVac. Among these three later patients, two were negative for anti-S1/S2 IgG and NAb at D69, and only one was positive for both at moderate levels.
Further comparative survival analysis of ARD and CG (p = 0.152), with a random selection of age and sex comparable subjects, pointed that n = 33 (ARD n = 31 and CG n = 2) RT-PCR confirmed COVID-19 incident symptomatic cases were reported during T1 (40 days) and n = 52 cases (ARD n = 44 in CG n = 8) during T2 (180 days) evenly distributed along this period in ARD patients (Fig. 2).
The figure depicts real-time polymerase chain reaction (RT-PCR) confirmed COVID-19 cases in patients with autoimmune rheumatic disease (ARD) and controls (CG). All participants were followed from the day of inclusion, which is the day of first dose vaccination with CoronaVac vaccine (DO) up to 10 days after the second dose vaccination (T1). The second period of follow-up (T2) ranged from T1 to 6 months after the second dose. Kaplan–Meier curves were compared with long-rank test and if p-value was <0.05 then the difference was considered significant.
Discussion
The present study demonstrated a substantial decline of anti-SARS-CoV-2 immunogenicity in ARD patients 6 months after the full inactivated vaccine schedule without a simultaneous increase in breakthrough cases. We further identified male sex and immunosuppression as deleterious for long-term antibody persistence in these patients.
A large number of ARD patients with a balanced age and sex CG included in this study was the main strength since it provided a unique opportunity to define more accurately risk factors for vaccine-induced anti-SARS-CoV-2 durability. In fact, age and sex were identified previously as determinants of the inability to sustain SARS-CoV-2 antibody levels in health population3. The use of internationally well-established disease classification criteria for the diagnosis of each disease in patients included in the study allowed a more accurate definition of the impact of different conditions and their therapy. The inclusion of a broad spectrum of non-rheumatologic immunosuppressed participants and the use of a generic computer-based questionnaire about existing conditions and treatment precluded a definitive conclusion about the subgroup of ARD and their treatment in a previous study8.
The period of 6 months with a parallel observation of incident cases is endorsed by the report of breakthrough infection 4-month post-BTN162b2 vaccination associated with reduced levels of antibodies peri-infection and a significantly diminished humoral response in 6 months1,8. The uniform post-vaccination follow-up was an essential parameter for a more accurate definition of vaccine-induced antibody persistence at 6 months. In fact, previous studies have demonstrated that vaccine humoral response waning dynamics vary over time and are also distinct for IgG and NAb level8. An important limitation of the present study is the non-assessment of cellular immunity that also contributes to vaccine efficacy in this population. Even though NAb evaluated herein were reported to be associated with protective immunity, they do not surpass the T-cell evaluation15.
We provide data of a reduction of anti-SARS-CoV-2 antibodies positivity over 6 months post second dose of Sinovac-CoronaVac in ARD patients with a magnitude of ∼20% for patients and controls. We also confirmed herein with CoronaVac vaccination the previously reported observation of a robust (≥4 times) decrease in SARS-CoV-2 mRNA vaccine IgG levels after 6 months in healthcare workers8. We further demonstrated that the same phenomena, although less intense, occurred for the Sinovac-CoronoVac with a more expressive decrease in controls (51%) than in ARD patients (38%) at 6 months post second dose. In fact, a substantial decrease in anti-SARS-CoV-2 IgG titers has been previously reported in the healthy population4,8. In spite of that, the decrease in the IgG seropositivity rates was similar in both groups (24% in ARD and 20% in the control group) herein.
The analysis of NAb positivity, reported being a strong correlate of protection15, revealed ~40% reduction for ARD patients and controls with a parallel waning of 54% in percent inhibition by NAb, after 6 months of Sinovac-CoronaVac vaccination. A more substantial decrease of 70% in NAb titer was reported 6-month post-mRNA vaccination for participants with immunosuppression8. This finding may be explained by the distinct immunocompromised populations analyzed in both studies. Regarding deleterious factors for vaccine-induced immunogenicity durability, we have identified in the multivariate logistic regression analysis that male sex, PRED and anti-TNF had a moderate impact on immunogenicity persistence, whereas a major harmful effect was evidenced for ABA and RTX after 6 months. These results are in line with previous evidence of our group demonstrating that PRED, MTX, anti-TNF, ABA, and RTX had the greatest negative impact on vaccine-induced anti-S1/S2 IgG response3. Additionally, ABA and RTX were also reported as harmful to other vaccines' immunogenicity16. With regard to anti-TNF, one-third of patients were in combination with MTX, and this latter drug may partially account for this finding since it was reported to induce a reduced antibody response3. For NAb positivity, only ABA was associated with the absence of these antibodies at D210 in ARD patients. Likewise, a significantly lower NAb activity level was reported in men and immunosuppressed health worker participants 6 months after receipt of the second dose8.
The predominance of incident cases in the 38 days after the vaccine's first shot (including 10 days after the second dose) contrasts with the significant drop in infection and hospitalization in the study participants in the subsequent 40 days. This last phase coincided with the second peak of COVID-19 cases in São Paulo city (45% increase in the same time period)17. Interestingly, the emergence of Delta (B.1.617.2) variant in Sao Paulo in July18 with a rapid spread in the following months did not lead to a parallel upsurge of COVID-19 breakthrough cases in our cohort which remained with a homogeneous distribution of cases throughout the study period, supporting the notion that Sinovac-CoronaVac may maintain its effectiveness during 6 months.
In conclusion, we provide data on the long-term Sinovac-CoronaVac immunogenicity in ARD patients, demonstrating a significant decrease in IgG and NAb levels 6 months after the 2nd dose without a corresponding rise in symptomatic COVID-19 cases in the same period. Male, PRED, and biological therapy were identified as the main contributing factors to the reduced durability of the vaccine-induced humoral response.
Methods
Study design and population
This work is within a phase 4 prospective longitudinal study (CoronavRheum clinicaltrials.gov #NCT04754698) conducted at a large tertiary hospital in Sao Paulo, Brazil. The protocol complies with all relevant ethical regulations, and it was approved by the National and Institutional Ethical Committee of Hospital das Clinicas HCFMUSP, Faculdade de Medicina, University of Sao Paulo, Sao Paulo, Brazil (CAAE: 42566621.0.0000.0068). For this study, only ARD patients followed at our Outpatient Rheumatology Clinics were included, irrespective of their SARS-CoV-2 serologic status before vaccination. The patients were diagnosed according to international guidelines and disease classification criteria, and the population included were rheumatoid arthritis19, systemic erythematosus lupus20, axial ankylosing spondylitis21, psoriatic arthritis22, primary vasculitis23,24, primary Sjogren’s syndrome25, systemic sclerosis26, idiopathic Inflammatory myopathies27, and primary antiphospholipid syndrome28. After the selection of the ARD group, hospital services workers, health professionals, and hospital administrative service employees or their relatives, without ARD or immunosuppressive therapy, were selected as a healthy control group (CG). All participants were ≥18 years old, and the controls were balanced with patients by sex and age (up to 5 years differences) at the entry (1 control: 4 patients) using an in-house program run on Excel (Microsoft 2018) for random selection of individuals in each group. All ARD patients and CG received the inactivated Sinovac-CoronaVac vaccine (Sinovac Life Sciences, Beijing, China, batch #20200412) first dose between February 9 and 17, 2021, and the second dose between March 9 and 17, 2021 (28 days apart). No strategy for immunogenicity improvement was applied. The exclusion criteria at vaccination included previous vaccination with any SARS-CoV-2 vaccine, previous history of anaphylactic events after vaccine administration, history of previous vaccination with live virus up to 4 weeks, or with inactivated virus vaccine up to 2 weeks, COVID-19-related symptoms up to 4 weeks before entry, acute febrile illness, Guillain-Barré syndrome, decompensated heart failure, demyelinating disease, people living with HIV/AIDS (PLWHA), history of blood products administration up to 6 months before the study start. Hospitalized patients or controls were also excluded. The full flow diagram with all exclusions is presented in Fig. 1. The final study groups for immunogenicity analyses consisted of 828 ARD patients (mean age 49.8 ± 12.3 years, 75.5% female sex) and 207 healthy controls (mean age 49.7 ± 12.3 years, 75.4% female sex). Blood collections for this study were performed 6 weeks after the second dose (April 19, 2021, D69) and 6 months after the second dose (September 18, 2021, D210) for ARD and CG. All participants signed the written informed consent prior to the vaccination and blood collection. Compensation for participation was not provided. Study protocol with data collection and outcomes information is available in the Supplementary Information.
A rigorous follow-up of incident cases was performed for all participants (1115 ARD patients, mean age 49.7 ± 12.7 years, 76.7% female sex and 223 CG, mean age 49.6 ± 12.5 years, 76.7% female sex) with COVID-19 symptoms from vaccine first dose to 6 months after the second dose (D210). Four in-person visits (vaccine first dose, vaccine second dose, D69, and D210) were performed, with careful checking of a standardized diary regarding COVID-19 history at each visit. All ARD patients and controls were instructed to communicate any manifestation associated with COVID-19 through telephone, smartphone instant messaging, or email. A medical team was available for 24 h to provide a proper follow-up. Suspicious cases of COVID-19, even if mild, were instructed to seek medical care near the residence and to come to our tertiary hospital to undergo a RT-PCR test for SARS-Cov-2. Participants who were unable to come to our center were instructed to go to an independent laboratory near their homes. COVID-19 incident cases were followed from the first vaccine dose to 10 days after the second dose (T1) and thereafter, T2, for the following 170 days (from D40 to D210).
These participants were tested using a real-time reverse transcriptase-polymerase chain reaction (RT-PCR) test for SARS-CoV-2 by naso- and oropharyngeal swabs.
Primary and secondary outcomes
The primary outcome was humoral immunogenicity assessed by the presence of anti-S1/S2 SARS-CoV-2 IgG 6 months after the second vaccine dose (D210) compared to the same parameter at D69 (6 weeks after second vaccine dose) for decay evaluation. Secondary outcomes were: the presence of neutralizing antibodies (NAb), percent inhibition by NAb, and anti-S1/S2 SARS-CoV-2 IgG GMT. Incident cases confirmed by RT-PCR for SARS-CoV-2 infection were also a secondary outcome.
Serologic assays
Serology was assessed for all participants prior to the first vaccine dose, at D69 (interval from second dose to serum collection = 6 weeks) and 6 months after the second dose (D210). The blood samples were collected, centrifuged, and stored at −80 °C until analysis. The serologic assay consisted of the measurement of the total IgG antibodies against the SARS-CoV-2 S1 and S2 proteins performed by chemiluminescent immunoassay on the ETI-MAX-3000 equipment (DiaSorin, Italy) using the Indirect ELISA, LIAISON® SARS-CoV-2 S1/S2 IgG kit Cat# 311450 and Cat# 311451 (DiaSorin, Italy), and the measurement of the circulating NAb against SARS-CoV-2 using the SARS-CoV-2 sVNT Kit, Cat# L00847-A (GenScript, Piscataway, NJ, USA). The two assays were performed following the manufacturer’s instructions. Samples with 15.0 UA/mL or more for total anti-S1/S2 IgG and with 30% or more inhibition for neutralizing assay, were considered seropositive according to the manufacturer’s guide29,30. Furthermore, quantitative results were reported, attributing the value of 1.9 UA/mL (half of the lower limit of quantification 3.8 UA/mL) to undetectable levels (<3.8 UA/mL) of total IgG and 15% (half of the lower limit of quantification 30%) to undetectable levels (<30%) of neutralizing antibodies.
RT-PCR for SARS-CoV-2 analysis
Naso- and oropharyngeal swabs were obtained whenever the participants reported COVID-19-related symptoms, and SARS-CoV-2 RT-PCR was performed using the SuperScriptTM III Platinum® One-Step Quantitative RT-PCR System Cat#11732-088 (Invitrogen, Carlsbad, CA, USA). A m2000sp equipment (Abbott, Des Plaines, IL, USA) was for RNA extraction and a m2000rt (Abbott, Des Plaines, IL, USA) was for RNA amplification and detection. The assay was carried out according to the protocol reported by Corman and colleagues as previously reported assay31.
Statistical analysis and reproducibility
Participants originated from a large phase 4 controlled prospective study of immunogenicity and safety of two doses of Sinovac-CoronaVac vaccine, with its proper sample size calculation13. No statistical method was used to predetermine the sample size in the current research. Demographical data were presented as number (percentage) for categorical variables and as mean ± standard deviation, and median (interquartile ranges) for continuous variables. The comparison between ARD and CG was performed by the chi-square or Fisher’s exact tests, as appropriate for categorical variables, and by Mann–Whitney test for continuous variables.
Immunogenicity assessment
ARD patients and the controls were balanced with patients by sex and age (up to 5 years differences) at the entry (1 control: 4 patients) using an in-house program run on Excel (Microsoft 2018) for random selection of individuals in each group. Seropositivity (SP) rates of anti-S1/S2 IgG and NAb were presented as numbers (percentage) of positive samples and were compared between groups (ARD and CG) and between timepoints (D69 vs. D210) using generalized estimating equations (GEE) with binomial distribution and logit link function, assuming autoregressive correlation matrix between moments. IgG antibody titers are expressed as geometric means with 95% confidence interval (95% CI). Total anti-S1/S2 SARS-CoV-2 IgG was expressed as geometric mean titers (GMT) and 95% confidence intervals, and it was compared using Generalized Estimating Equations (GEE) with two factors [2 groups (ARD and CG) at two timepoints (D69 vs. D210)] followed by Bonferroni’s multiple comparisons in neperian logarithm (ln)-transformed data. Percent inhibition by NAb was expressed as medians (interquartile range) of the percentage of inhibition and was compared using Generalized Estimating Equations (GEE) with two factors [2 groups (ARD and CG) at two timepoints (D69 vs. D210)] followed by Bonferroni’s multiple comparisons. Multivariate logistic regression analyses were performed using as dependent variables SC or NAb positivity at D210 and as independent variables those with p < 0.2 in each univariate analysis (for IgG SC: current age, male sex, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, hydroxychloroquine, sulfasalazine, prednisone, leflunomide, anti-TNF, abatacepte, and rituximabe use; for Nab: rheumatoid arthritis, systemic lupus erythematosus, sulfasalazine, anti-TNF, abatacept, tocilizumab, and rituximab use).
COVID-19 incident cases
Confirmed COVID-19 cases and hospitalization incident-density data (along with 95% confidence intervals) were estimated using Poisson distribution and test-based methods and compared on ARD patients at T1 (from the 1st dose to 10 days after the second dose) and T2 (180 days after the second dose), with significance related to incident differences. Survival analysis of ARD and CG subjects free of confirmed COVID-19 cases was performed using Kaplan–Meier curves and compared with the long-rank test. Statistical significance was defined as p < 0.05.
Most statistical analyses were performed using Statistical Package for the Social Sciences, version 20.0 (IBM-SPSS for Windows. 20.0. Chicago, IL, USA). For incident-density data, MedCalc version 20.015 was used.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Raw data for each figure or table have been deposited as a Source Data file. All data used in these analyses can be found at (https://figshare.com/s/b25763a08f5d782f50ce). The raw personal data are protected and are not available due to data privacy laws. Source data are provided with this paper.
References
Bergwerk, M. et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 385, 1474–1484 (2021).
McDade, T. W. et al. Durability of antibody response to vaccination and surrogate neutralization of emerging variants based on SARS-CoV-2 exposure history. Sci. Rep. 11, 17325 (2021).
Widge, A. T. et al. Durability of responses after SARS-CoV-2 mRNA-1273 Vvaccination. N. Engl. J. Med. 384, 80–82 (2021).
Shrotri, M. et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 398, 385–387 (2021).
Barouch, D. H. et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N. Engl. J. Med. 385, 951–953 (2021).
Doria-Rose, N. et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N. Engl. J. Med. 384, 2259–2261 (2021).
Pegu, A. et al. Durability of mRNA-1273-induced antibodies against SARS-CoV-2 variants. bioRxiv [Preprint]. 2021.05.13.444010. https://doi.org/10.1101/2021.05.13.444010 (2021).
Levin, E. G. et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2114583 (2021).
Jara, A. et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 385, 875–884 (2021).
Cucunawangsih, C., Wijaya, R. S., Lugito, N. P. H. & Suriapranata, I. Antibody response to the inactivated SARS-CoV-2 vaccine among healthcare workers, Indonesia. Int. J. Infect. Dis. https://www.sciencedirect.com/science/article/pii/S1201971221007876?via%3Dihub (2021).
Eliakim-Raz, N., Massarweh, A., Stemmer, A. & Stemmer, S. M. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. https://jamanetwork.com/journals/jamaoncology/fullarticle/2783031 (2021).
Tadmor, T. et al. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia 35, 2727–2730 (2021).
Medeiros-Ribeiro, A. C. et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat. Med. 27, 1744–1751 (2021).
Aikawa, N. E. et al. Immunogenicity and safety of two doses of the CoronaVac SARS-CoV-2 vaccine in SARS-CoV-2 seropositive and seronegative patients with autoimmune rheumatic diseases in Brazil: a subgroup analysis of a phase 4 prospective study.Phase 4 prospective analysis of immunogenicity and safety of two doses of Sinovac-CoronaVac COVID-19 vaccine in SARS-CoV-2-exposed and naÏve autoimmune rheumatic disease patients. Lancet Rheumatol. https://doi.org/10.1016/S2665-9913(21)00327-1In (2021).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Ribeiro, A. C. et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res. (Hoboken). 65, 476–480 (2013).
Coronavírus—Boletim Completo. Governo do Estado de São Paulo. https://www.seade.gov.br/coronavirus/# (2020).
Lamarca, A. P. et al. Genomic surveillance tracks the first communitary outbreak of Delta (B.1.617.2) variant in Brazil. https://virological.org/t/genomic-surveillance-tracks-the-first-communitary-outbreak-of-delta-b-1-617-2-variant-in-brazil/733 (2022).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Tillett, W. et al. The ClASsification for Psoriatic ARthritis (CASPAR) Criteria—a retrospective feasibility, sensitivity, and specificity study. J. Rheumatol. 39, 154–156 (2012).
Arend, W. P. et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 33, 1129–1134 (2010).
Leavitt, R. Y. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 33, 1101–1107 (2010).
Vitali, C. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 61, 554–558 (2002).
van den Hoogen, F. et al. Classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 72, 1747–1755 (2013).
Lundberg, I. E. et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 76, 1955–1964 (2017).
Miyakis, S. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 4, 295–306 (2006).
Taylor, S. C. et al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J. Clin. Microbiol. 59, e02438-20 (2021).
Lustig, Y. et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 9, 999–1009 (2021).
Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT–PCR. Eurosurveillance 25, 2000045 (2020).
Acknowledgements
This study was sponsored by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (#2015/03756–4 to C.A.S., S.G.P., N.E.A., and E.B.; #2019/17272-0 to L.V.K.K.; #2018/09937-9 to V.O.A.M., #2020/09367-8 to L.E.B.V.), Conselho Nacional de Desenvolvimento Científico e Tecnológico (#304984/2020-5 to C.A.S.; #305556/2017-7 to R.M.R.P.; #303379/2018-9 and to S.K.S. #305242/2019-9 to E.B.), and B3-Bolsa de Valores do Brasil and Instituto Todos pela Saúde (ITPS 01/2021, C1313 to C.A.S., S.G.P., N.E.A., and E.B.). Instituto Butantan supplied the study product and had no other role in the trial. We thank the volunteers for participating in all in-person visits and for handling the biological material. We also thank the contribution of the Central Laboratory Division, Registry Division, Security Division, IT Division, Superintendence, Pharmacy Division, and Vaccination Center for their technical support.
Author information
Authors and Affiliations
Contributions
C.A.S., A.C.M.R., L.V.K.K., N.E.A., C.G.S., E.F.N.Y., S.G.P., C.G.S., E.G.K., and E.B. conceived and designed the study and participated in data collection and analysis, supervised clinical data management, writing of the manuscript, and revision of the manuscript. S.R.G.F. organized and supervised blood collection and vaccination. A.J.S.D. supervised sera processing, SARS-CoV-2-specific antibody ELISAs/neutralization assays, and SARS-CoV-2 RT-PCR. C.A.S., A.C.M.R., L.V.K.K., N.E.A., C.G.S., E.F.N.Y., S.G.P., C.G.S., E.B., S.R.G.F., R.M.R.P., S.K.S., A.S.R.H., E.F.B., F.H.C.S., L.K.N.G., R.M., K.R.B., D.S.D., A.Y.S., D.C.O.A., L.P.C.S., R.F., P.D.S.B., A.P.L.A., J.C.B.M., C.G.S., H.A.M.G., H.C.S., V.A.O.M., L.E.B.V., R.S.N., L.P.S., C.S.R.A., M.S.R.S., and D.M.N.F. collected epidemiological and clinical data and assisted with the identification of SARS-CoV-2 infection and follow-up of patients. M.H.L. organized and supervised the vaccination protocol. A.J.S.D. supervised and performed the assays for measurement of anti-SARS-CoV-2 antibodies. All authors helped to edit the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Fabrizio Conti, Anna Vilella and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, C.A., Medeiros-Ribeiro, A.C., Kupa, L.V.K. et al. Immunogenicity decay and case incidence six months post Sinovac-CoronaVac vaccine in autoimmune rheumatic diseases patients. Nat Commun 13, 5801 (2022). https://doi.org/10.1038/s41467-022-33042-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33042-0
This article is cited by
-
Biological and glucocorticoids treatment impair the medium-term immunogenicity to SARS-CoV-2 mRNA vaccines in autoimmune inflammatory rheumatic diseases
European Journal of Medical Research (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.