Abstract
Atmospheric ozone has long been a threat to human health, however, rational design of high-performance O3-decomposition catalysts remains challenging. Herein, we demonstrate the great potential of a series of isomorphous bimetallic MOFs denoted as PCN-250(Fe2M) (M = Co2+, Ni2+, Mn2+) in catalytic O3 decomposition. Particularly, PCN-250(Fe2Co) showed 100% O3 removal efficiency for a continuous air flow containing 1 ppm O3 over a wide humidity range (0 ‒ 80% RH) at room temperature. Mechanism studies suggested that the high catalytic performance originated from the introduction of open Co(II) sites as well as its porous structure. Additionally, at low pressures around 10 Pa, PCN-250(Fe2Co) exhibited high adsorption capacities (89 ‒ 241 mg g−1) for most VOCs, which are not only a class of hazardous air pollutants but also the precursor of O3. This work opens up a new avenue to develop advanced air purification materials for O3 and VOCs removal in one.
Similar content being viewed by others
Introduction
Ground-level ozone (O3) has long been a threat to human health, especially in urban area1,2,3. Outdoor O3 is mostly generated from the photochemical reactions between nitrogen dioxides and volatile organic compounds (VOCs) in the air under sunlight4,5. Indoor O3 is from indoor-outdoor air exchange as well as some emission sources include photocopiers, laser printers, ultraviolet lamps, and O3 disinfectors6,7,8. Although being highly reactive as a very strong oxidizing agent, O3 itself is relatively stable and nearly does not spontaneously decompose to oxygen (O2) at the concentrations (ppb level) that are typically encountered in ambient air6. Plenty of studies have demonstrated the potential risk of respiratory and cardiovascular mortality for the human body after long-term exposure to the air with a trace of O39,10. The World Health Organization has recommended that the 8-h daily maximum O3 concentration in ambient air exceeding 100 μg m−3 (∼51 ppb) poses health risks11. However, the occurrence of severe warm-season O3 pollution has become more frequent in the past few years for both developing and developed countries7,12. For example, field observations and satellite retrievals reveal that the observed hourly maximum O3 concentrations in China frequently exceeded 150 ppb (severe O3 pollution)13,14. The study to reduce O3 pollution is thus in high demand for the protection of the environment and human health.
The existing approaches to remove O3 from ambient air are typically based on activated carbon adsorption, chemical absorption, and catalytic decomposition methods15,16,17,18. Among them, catalytic decomposition of O3 into O2 at low temperatures is promising because of its ease in operation, high efficiency, good persistence, and environmental friendliness19,20. Over the past decades, significant advances have been made in catalytic O3 degradation using transition metal (Mn21, Fe22, Co23, Ni24, and Cu25) oxides mostly. As a class of newly emerged materials, metal-organic frameworks (MOFs) have attracted great interest in various catalytic reactions because of their tunable structure and diverse functionality, including O3 catalytic decomposition26,27,28,29,30,31. In addition, compared with transition metal oxides, MOFs can have highly porous structures, thus may offer extra catalytically active sites on interior pore surface, and even serve as advanced multifunctional materials to decompose O3 and remove other airborne hazardous molecules by adsorption simultaneously32,33,34,35,36. Indeed, there exist kinds of air pollutants in some real application scenarios. For example, non-thermal plasma (NTP) is a highly efficient technology for VOCs removal and has been widely used for the past two decades, but the exhausted gases of NTP reactors usually contain the by-product O3 as well as some incompletely decomposed VOCs37. It is thus highly desirable but still challenging to develop highly efficient, porous, and stable MOFs as such multifunctional air filtering materials.
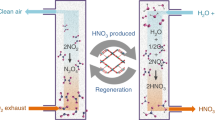
The catalytically active sites in MOFs are commonly metal centers, and most reported MOFs are monometallic. Generating multimetallic building units in MOFs has been recently proposed as an effective strategy to regulate their catalytic properties38,39. Exploring multimetallic MOFs for catalytic O3 decomposition would be intriguing. The MOF PCN-250(Fe3)40 (also known as MIL-127(Fe)41) constructed from Fe3-µ3-oxo clusters and ABTC4− ligands (H4ABTC = 3,3′,5,5′-azobenzenetetracarboxylic acid) is a preferred platform for such a study because one Fe(III) ion of the Fe3-µ3-oxo cluster can be readily substituted by a common divalent transition metal ion, e.g., Co(II), Ni(II), and Mn(II), and this MOF can be facilely prepared with high stability and high porosity. Herein, we demonstrate the great potential of these bimetallic MOFs PCN-250(Fe2M) (M = Co2+, Ni2+, Mn2+) for catalytic O3 removal from ambient air, especially PCN-250(Fe2Co). It is also found that PCN-250(Fe2Co) shows a high capacity in the adsorptive removal of volatile organic compounds (VOCs) (Fig. 1), which are not only a class of hazardous air pollutants but also the precursor of O3. It is an attribution not available for most of the other O3-decomposition catalysts. This study suggests the great potential of MOF-based materials for O3 pollution control and also offers insights into the design of new air purification materials.
Results
Synthesis and characterization
Three bimetallic MOFs PCN-250(Fe2M) (M = Co2+, Ni2+, Mn2+), as well as their monometallic counterpart PCN-250(Fe3), were prepared by the reported method with some modifications to evaluate their performances on catalytic O3 decomposition. PXRD patterns of the four samples all showed strong peaks and well matched the simulated PXRD pattern of PCN-250(Fe3) (Supplementary Fig. 2), confirming that their structures are isomorphous and the samples are in high crystallinity and purity. N2 sorption isotherms of the four MOFs recorded at 77 K were all classic type-I isotherms for microporous materials (Supplementary Figs. 3–6). The pore volumes and BET surface areas of PCN-250(Fe3), PCN-250(Fe2Co), PCN-250(Fe2Ni), and PCN-250(Fe2Mn) were estimated to be 0.52, 0.59, 0.58, and 0.57 cm3 g−1, and 1408, 1478, 1465, and 1443 m2 g−1 with a pore size distribution of 7.0–11.5 Å, respectively. Thermal gravimetric analysis (TGA) curves suggested that the four MOFs thermally decomposed at about 400 °C (Supplementary Fig. 7). X-ray photoelectron spectra (XPS) analyses confirmed the presence of only Fe(III) ions in PCN-250(Fe3) and the presence of both divalent metal ions (Co2+, Ni2+, or Mn2+) and trivalent Fe3+ ions in the three bimetallic MOFs (Supplementary Figs. 8–11), which was further confirmed by the results of inductively coupled plasma-atomic emission spectroscopy (ICP-AES) measurements for digested samples of the MOFs (Supplementary Table 7) and EDS mapping images for the PCN-250 crystals (Supplementary Fig. 12).
Catalytic activity for O3 decomposition
Catalytic O3 decomposition experiments were conducted with continuous-flow fixed-bed reactors of the tested materials (30 mg) at ambient temperature and pressure, and the inlet gas flow rate was 1 L min−1 (see supplementary information for details). Considering the concentration of O3 in ambient air is normally in the range of tens to hundreds of ppb, the O3 concentration of inlet gas was set to be 1 ppm (balance air). For dry inlet gas (zero air), the three bimetallic MOFs PCN-250(Fe2Co), PCN-250(Fe2Mn), and PCN-250(Fe2Ni) completely removed the O3 of inlet gas during 12 h of continuous testing, while the O3 removal efficiency of PCN-250(Fe3) was around 85% (Fig. 2a). When the humidity of the inlet gas increased to RH = 40% (relative humidity at room temperature), the performances of the four MOFs became more different (Fig. 2b). For PCN-250(Fe2Mn), PCN-250(Fe2Ni), and PCN-250(Fe3), the initial O3 removal efficiencies were 97%, 89%, and 78%, which gradually decreased in the first 2 h and stabilized at 92%, 85%, and 75% in the following 10 h, respectively. In contrast, PCN-250(Fe2Co) still retained 100% O3 removal efficiency throughout the test. These results revealed that the three bimetallic MOFs were more catalytically active than PCN-250(Fe3) for the decomposition reaction of O3, and the O3 removal performances of PCN-250(Fe3), PCN-250(Fe2Ni), and PCN-250(Fe2Mn) could be affected by the humidity of inlet gas. It is common that the O3 decomposition performances of catalysts reduce under more humid conditions21,22. Notably, PCN-250(Fe2Co) showed 100% O3 removal efficiency under both dry and humid conditions, suggesting that it has the highest catalytic activity among the four MOFs.
a RH < 1% (zero air) and b RH = 40% for the PCN-250 samples. c RH < 1% (zero air) and d RH = 40% for PCN-250(Fe2Co), ZZU-281, MIL-100(Fe), α-Fe2O3, Co3O4, CoFe2O4, and activated charcoal. e Varied RHs for PCN-250(Fe2Co). Other conditions for all the above tests: 0.03 g catalyst diluted with 0.2 g quartz sand, concentration of O3 = 1 ppm, flow rate = 1 L min−1, room temperature. f O3 test strips used in the tests under humid condition (RH = 40%) and the color scale.
For comparison, the O3 removal performances of some representative materials were also evaluated under the same experimental conditions, including two MOFs previously reported to show high O3 degradation activities, ZZU-28131 and MIL-100(Fe)30, three metal oxides, α-Fe2O315, Co3O422, and CoFe2O442, and a common adsorbent widely used in air purification, activated charcoal43. The structure, purity, and/or porosity of these reference materials were confirmed (Supplementary Figs. 16–22). As shown in Fig. 2c, d, the O3 removal efficiency of Co3O4 was around 45% under both dry and humid conditions. The O3 removal efficiencies of MIL-100(Fe) and α-Fe2O3 were close after 12 h continuous testing, being ∼75% and 65% under dry and humid conditions, respectively. The O3 removal efficiencies of activated charcoal and CoFe2O4 decreased from 91 to 70% and 100 to 40% within 12 h under humid conditions, respectively, although their O3 removal efficiencies under dry conditions were >99% throughout the test. The continuous decline in O3 removal performance of activated charcoal under both dry and humid conditions may be attributed to the gradual consumption of O3 reactive sites (e.g., hydroxyl groups) on the sample surface44. In addition, it is noteworthy that the O3 removal performance of the bimetallic oxide CoFe2O4 is quite different from that of the physical mixture of Co3O4 and α-Fe2O3 (Supplementary Fig. 23). Among these reference materials, only ZZU-281 completely removed O3 in inlet gases under both dry and humid conditions as PCN-250(Fe2Co) did. To further validate the experimental results, commercial O3 test strips were used to detect the O3 concentration of outlet gases that respectively passed through the materials under humid conditions (RH = 40%). As the O3 concentration of test gas increases, the strips change from white (ozone-free) to light-yellow (O3 concentrations around 90 µg m−3) and then to brown (O3 concentrations higher than 210 µg m−3) (Fig. 2f, right). The observed colors of the strips applied to the materials confirmed their different O3 removal efficiencies (Fig. 2f, left).
The O3 removal performances of PCN-250(Fe2Co) under various humidity and longer testing duration were also investigated. As shown in Fig. 2e, PCN-250(Fe2Co) kept 100% O3 removal efficiency within 100 h of continuous testing when the RH of inlet gas varied from <1% (dry zero air) to 80%. We further explored the effects of the weight amount of PCN-250(Fe2Co) and the O3 concentration of inlet gas on the O3 removal efficiency of PCN-250(Fe2Co). Control experiments were carried out after the weight amount of PCN-250(Fe2Co) was reduced from 30 mg to 15 mg or the O3 concentration of inlet gas was increased from 1 ppm to 50 ppm. For dry inlet gas, the O3 removal efficiency of PCN-250(Fe2Co) maintained 100% in both cases; for humid inlet gas (RH = 40% at room temperature), the O3 removal efficiency of PCN-250(Fe2Co) reduced to around 65% and 35%, respectively (Supplementary Fig. 24). These results revealed that the catalytic O3 degradation capacity of PCN-250(Fe2Co) under dry condition was higher than that under humid condition, as also observed for the other three PCN-250 MOFs. The different O3 removal efficiencies of PCN-250(Fe2Co) for dry and humid gases were also confirmed by cycling tests where the humidity of the gas flow was alternately changed (Supplementary Fig. 25). Under the dry condition, the O3 removal efficiency of PCN-250(Fe2Co) was 100% even if the O3 concentration of inlet gas was further increased to 200 ppm, even much higher than that of ZZU-281 under the same condition (Supplementary Fig. 26). The different O3 degradation performance of PCN-250(Fe2Co) under dry and humid conditions may be originated from two aspects. On the one hand, PCN-250(Fe2Co) is moderately hydrophilic as indicated by its water vapor adsorption isotherm at 298 K, where the water uptake gradually increases to 27 mmol g−1 at 0.6 P/P0 and keeps nearly unchanged at higher pressures (Supplementary Fig. 27). The water contact angle of the powder sample of PCN-250(Fe2Co) is about 11° (Supplementary Fig. 28), indicating that the crystal surface is also moderately hydrophilic. Under humid conditions, adsorbed water molecules would partially or even fully block the pores of PCN-250(Fe2Co), reducing the amount of accessible catalytically active sites on the interior pore surface to contact with O3 molecules. On the other hand, water molecules may participate in the catalytic O3 decomposition reaction, and the resulting new reaction pathway requires higher activation energy. Indeed, it was found that the O3 removal efficiency of PCN-250(Fe2Co) under humid conditions could be largely improved by generally heating the reactor to 45 °C (Supplementary Fig. 29).
For the practical application of O3 removal, the long-term stability of catalysts under real working conditions is highly important45. The stability issue is especially outstanding for MOF-type catalysts since a large number of MOFs have been proven unstable even in ambient air46. In the last few years, a few MOFs showing high stability in water, even under harsh acidic or basic conditions, have been reported, whereas there are very little knowledge about the stability of MOFs when exposed to O3-containing air47,48,49,50,51,52. The high hydrolytic stability of PCN-250 MOFs has been well documented in the literature53. The long-term hydrolytic stability of PCN-250(Fe2Co) was further tested by soaking it in water at room temperature for 30 days and by exposing it to ambient air for 4 months. PXRD and N2 sorption measurement results revealed that the PCN-250(Fe2Co) samples well maintained their high crystallinity and porosity after these treatments (Fig. 3a, b). No obvious change was observed by SEM for the crystal morphology and surface of PCN-250(Fe2Co) (Fig. 3c).
a PXRD patterns, b N2 sorption isotherms, and c SEM images of the pristine and stability-tested PCN-250(Fe2Co) samples. d O3 removal efficiencies for PCN-250(Fe2Co) after 50 ppm O3 treatment. Test conditions: 0.03 g catalyst diluted with 0.2 g quartz sand, concentration of O3 = 1 ppm, flow rate = 1 L min−1, room temperature.
As a strong oxidizing agent, O3 is highly reactive to many substances, e.g., destroying organic compounds containing carbon-carbon double bonds, carbon-carbon triple bonds, and porphyrin rings54,55,56. Therefore, besides hydrolytic stability, the materials used for O3 removal should also be able to resist long-term O3 attack. The tolerance of PCN-250(Fe2Co) to airborne O3 (1 ppm) was indicated by its stable O3 removal efficiency in the 100 h tests discussed above (Fig. 2e). Its tolerance was further evaluated by exposing it to a continuous flow (flow rate: 0.5 L min−1) of dry or humid air (RH = 40% at room temperature) containing 50 ppm O3 for 100 h (50 h for wet gas, and 50 h for dry gas) at room temperature. The comparison of PXRD patterns, N2 adsorption isotherms, and SEM images for the O3 treated and pristine PCN-250(Fe2Co) samples suggested a high tolerance of PCN-250(Fe2Co) to O3 containing ambient air (Fig. 3a–c). Further experiments indicated that the four PCN-250 samples all are highly tolerant of O3 (Supplementary Figs. 2–11). Moreover, the 50 ppm O3 treated PCN-250(Fe2Co) still showed 100% O3 removal efficiency to dry or humid air (RH = 40% at room temperature) containing 1 ppm O3 as the pristine sample did (Fig. 3d). Unexpectedly, it was found that some MOFs commonly regarded to be highly stable easily degraded after exposure to O3 containing ambient air, e.g., ZIF-8, ZIF-L, and MIL-101(Cr). After being exposed to the flow of humid air containing 1 ppm O3 for 24 h, the sample of ZIF-8 changed from white powder to light-yellow viscous liquid and completely lost its initial crystalline structure (Supplementary Fig. 30). The change was faster (in 4 h) when the O3 concentration was increased to 50 ppm. Similar results were also observed for ZIF-L, which is built from the same metal ions and organic ligands as ZIF-8 (Supplementary Fig. 31). Although being more tolerant of O3 in humid air than the two ZIFs, MIL-101(Cr) also lost crystallinity and changed color after exposing to the flow of O3 containing humid air for 24 h (Supplementary Fig. 32). Therefore, PCN-250(Fe2Co) represents a rare and excellent catalyst for O3 decomposition due to its high stability and activity.
O3 decomposition mechanism
To explore the source of the high O3 decomposition activity of PCN-250(Fe2Co), O3 removal tests were also conducted for its starting materials, namely, the H4ABTC ligand and the [Fe2Co(μ3-O)(CH3COO)6] complex. As shown in Supplementary Fig. 33, the O3 removal efficiency of H4ABTC was nearly negligible, but the [Fe2Co(μ3-O)(CH3COO)6] complex could remove 60–90% O3 in inlet gas under dry and humid conditions. These results indicated that the catalytic active sites of PCN-250(Fe2Co) for O3 decomposition were the metal centers of Fe2Co-µ3-oxo clusters which are bonding with labile water molecules. It is also revealed that assembling the starting materials into the highly open MOF structure was beneficial to achieve a higher O3 removal efficiency due to the increase of accessible catalytic active sites exposed on the pore surface. Since the four PCN-250 MOFs are isomorphous and PCN-250(Fe2Co) showed the highest O3 removal efficiency, Co(II) center should be the most active catalytic site for O3 decomposition among the four types of metal centers (Fe3+, Co2+, Ni2+, and Mn2+). In addition, the catalytic reaction mechanisms under humid and dry conditions should be different according to the experimental results. Density functional theory (DFT) calculations were carried out with a [Fe2Co(μ3-O)(PhCOO)6] cluster model to reveal the O3 decomposition mechanism at the Co(II) site of PCN-250(Fe2Co) and the following reaction pathways were proposed (see supplementary information for details). Under the humid condition, as shown in Fig. 4a, c, the H atom of H2O* adsorbed on the Co(II) atom first attacks O3 along with the formation of •OOOH and *•OH radicals (* indicates the atom bonded to the Co atom). Subsequently, the remaining H atom of *•OH is transferred to •OOOH generating O2, H2O, and •O* (State 3 in Fig. 4a and INT1-2, S = 16 in Fig. 4c). Then, another O3 molecule attacks •O* releasing two O2 molecules. Afterward, the Co(II) atom becomes coordinatively unsaturated and prefers to coordinate with H2O over O3 under the humid condition due to the favorable adsorption energy of the former (22.4 vs. 9.0 kcal mol−1). As a result, the initial state is regenerated, and the catalysis repeats. Throughout the entire catalytic process, the first H transfer is a rate-determining step with a barrier of 15.9 kcal mol−1 (TS1-1, S = 14 in Fig. 4c, and Supplementary Table 2).
Proposed reaction pathways, energy profiles, and stationary-point structures along the calculated reaction pathways for the O3 decomposition catalyzed at the Co(II) site of PCN-250(Fe2Co) under both a, c humid and b, d dry conditions. Energy profiles and stationary-point structures along the calculated reaction pathways at the Fe(III) site of the PCN-250(Fe2Co)-catalyzed O3 decomposition under e humid and f dry conditions. Energy levels of the total spin state of S = 14 and S = 16 are shown in red and light blue, respectively. Color code: Co, blue; Fe, turquoise; C, gray; O, red; and H, pink.
Differently, under dry conditions, the reaction pathway essentially starts from the coordinatively unsaturated Co(II) center, which is generated by the reaction pathway under humid conditions (from State 1 to State 5 in Fig. 4a). O3 first binds to the exposed Co(II) center and then the *O3 splits into O2 and •O* with a barrier of 9.4 kcal mol−1 (State 3 in Fig. 4b, TS2-1, S = 14 in Fig. 4d, and Supplementary Table 3). Afterward, another O3 molecule attacks •O*, resulting in two O2 molecules and a coordinatively unsaturated Co(II) center (State 1 in Fig. 4b, and PROD2, S = 14 in Fig. 4d). The barrier of the rate-determining step in the dry condition is estimated to be 9.4 kcal mol−1. It is clear from the above computational results that the rate-determining step in both humid and dry conditions is related to their first elementary reaction, namely the H transfer in the former and the O3 decomposition in the latter. Importantly, the barrier of the rate-determining step is smaller in the dry condition than that in the humid condition (9.4 and 15.9 kcal mol−1). In addition, it is an endergonic reaction step in the humid condition (∆G = 11.8 kcal mol−1), while it becomes exergonic in the dry condition (∆G = − 5.4 kcal mol−1). Taken together, the dry condition is more favorable for the O3 removal, which is consistent with the experimental results.
Moreover, we also calculated the rate-determining barriers of the O3 decomposition at the Fe(III) site of [Fe2Co(μ3-O)(PhCOO)6] and [Fe3(μ3-O)(PhCOO)6]. In brief, both the Co(II) and Fe(III) sites share a similar catalytic mechanism. However, the Fe(III) site has larger barriers under either humid or dry conditions compared with the Co(II) site. Under humid and dry conditions, the barriers were calculated to be 21.5 and 14.8 kcal mol−1 for [Fe2Co(μ3-O)(PhCOO)6] (Fig. 4e, f and Supplementary Table 5); while they were estimated to be 24.7 and 11.7 kcal mol−1 for [Fe3(μ3-O)(PhCOO)6] (Supplementary Fig. 34 and Supplementary Table 6). The calculations clearly showed that the enhanced O3 decomposition performance of PCN-250(Fe2Co) originated from the introduction of open Co(II) sites.
VOCs adsorption of PCN-250(Fe2Co)
Due to its high porosity and moderate pore size, PCN-250(Fe2Co) is potentially capable of removing other pollutants in the air by adsorption besides O3. VOCs are not only a class of hazardous air pollutants but also the precursor of O3, the removal of VOCs from air is thus of high importance35. To evaluate its performance on VOCs removal, adsorption isotherms of PCN-250(Fe2Co) were recorded at 25 °C for ten common VOCs, including methanol, acetone, two aliphatic hydrocarbons (n-hexane and cyclohexane) and six aromatic hydrocarbons (benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene). Type-I adsorption isotherms were obtained for all the tested VOCs (Fig. 5a, b), showing an abrupt increase of uptakes at low vapor pressures below P/P0 = 0.002 (Supplementary Fig. 35). At 0.01 kPa, the uptakes of methanol, acetone, n-hexane, cyclohexane, benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene were 89, 177, 216, 123, 175, 241, 179, 114, 130, and 164 mg g−1 (Fig. 5c), respectively, corresponding to 18–75% of the maximum uptakes at 0.90‒0.99 P/P0 (174‒652 mg g−1). The high VOCs adsorption capacities of PCN-250(Fe2Co) suggest that the MOF may serve as a general adsorbent to remove various VOCs even if the concentrations of VOCs are low. In practical application, the humidity of air can significantly affect the performance of adsorbents for VOCs removal. To check the effect of humidity on PCN-250(Fe2Co), breakthrough experiments of dry and humid air flows containing 3000 ppm acetone vapor passing through a column of the MOF adsorbent (20 mg) were carried out at room temperature (see supplementary information for details). As shown in Fig. 5d, for dry gas, acetone started to penetrate the MOF column after about 190 s (160 min g−1), corresponding to an acetone capture capacity of ca. 355 mg g−1 in the dynamic adsorption process, which is close to the theoretical uptake at 0.3 kPa (400 mg g−1) from the static adsorption isotherm measurement. When the humidity of the gas mixture increased to RH = 40%, about 93% of the acetone capture capacity of PCN-250(Fe2Co) retained, suggesting a good tolerance to the humidity of the MOF adsorbent for acetone vapor capture. Moreover, PXRD measurements revealed that PCN-250(Fe2Co) also showed excellent stability toward the VOCs (Supplementary Fig. 36).
a for relatively low boiling point VOCs (methanol, acetone, benzene, n-hexane, and cyclohexane) and b for relatively high boiling point VOCs (toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene) recorded at 25 °C. c Zoom-in view for adsorption data at the low-pressure range (below 0.4 kPa). d Breakthrough curves for dry and humid air (RH = 40% at room temperature) containing acetone vapor (C0 = 3000 ppm) flowed through a column packed with PCN-250(Fe2Co). Test conditions: 0.02 g PCN-250(Fe2Co) diluted with 0.4 g quartz sand, concentration of acetone = 3000 ppm, flow rate = 0.2 L min−1, room temperature.
It is noteworthy that previously reported materials showing high performance on catalytic O3 removal, such as the above-mentioned ZZU-281 and some advanced transition metal oxides19,22,24, are of low porosity or nonporous and thus have limited capacity to adsorptively remove non-O3 air pollutants like VOCs. In addition, compared with the most common and prevalent adsorbent for VOCs removal, activated charcoal, PCN-250(Fe2Co), shows more stable performance and higher activity on catalytic O3 removal. The high O3 removal performance, high porosity, high stability, and facile preparation make PCN-250(Fe2Co) a unique and promising multifunctional material for air purification.
Discussion
The great potential of isomorphous bimetallic MOFs PCN-250(Fe2M) as high-performance porous catalysts for both O3 degradation and VOCs removal is demonstrated. Particularly, PCN-250(Fe2Co) showed 100% O3 removal efficiency for a continuous airflow containing 1 ppm O3 over a wide humidity range (0‒80% RH) at room temperature. Under the same conditions, the O3 removal efficiency of PCN-250(Fe2Co) was higher than those of many representative materials, including α-Fe2O3, Co3O4, CoFe2O4, activated charcoal, and MIL-100(Fe). PCN-250(Fe2Co) also showed higher catalytic activity for O3 decomposition than ZZU-281 under dry conditions. The highly porous and crystalline structure of PCN-250(Fe2Co) remained intact after it was treated by soaking in water for 30 days, or exposing to ambient air for 4 months at room temperature, and its O3 decomposition capacity retained after exposure of the MOF to relatively high concentration O3 in dry or humid air (50 ppm). The O3 decomposition reaction mechanisms catalyzed by PCN-250(Fe2Co) under dry and wet conditions were proposed based on DFT calculations. It was suggested that the high catalytic performance of PCN-250(Fe2Co) originated from the introduction of open Co(II) sites as well as its porous structure. Meanwhile, PCN-250(Fe2Co) is highly potential in the adsorptive removal of VOCs from the air, which is not only a class of hazardous air pollutants but also the precursor of O3. It is an attribution not available for the other O3-decomposition catalysts. At a low vapor pressure round 10 Pa and room temperature, PCN-250(Fe2Co) showed high adsorption capacities (89‒241 mg g−1) for ten common VOCs, namely methanol, acetone, n-hexane, cyclohexane, benzene, toluene, ethylbenzene, o-xylene, m-xylene, and p-xylene. The VOC capture capacity of PCN-250(Fe2Co) from both dry and humid air was confirmed by breakthrough experiments. PCN-250(Fe2Co) may serve as a unique and promising multifunctional material for air purification. This work opens up a new avenue to develop new materials for air pollution control, and the introduction of high porosity to O3-decomposition catalysts may endow the O3-decomposition catalysts with multifunctionality, like adsorption, separation, and sensing, which could be beneficial in practical air purification applications.
Methods
Synthesis of PCN-250(Fe2M) (M = Fe, Co, Ni, Mn)
PCN-250(Fe2M) was synthesized following the literature with some modifications40. Trinuclear complexes [Fe2M(μ3-O)(CH3COO)6] (M = Fe, Co, Ni, Mn) (45 mg), H4ABTC (30 mg), and acetic acid (2.0 mL) were ultrasonically dissolved in DMF (6 mL) in a 15 mL high-pressure vessel. The vessel was then tightly sealed, stirred, and heated in an oil bath of 140 °C for 12 h. After cooling down to room temperature, the as-synthesized solid was washed successively with DMF (3 × 40 mL) at 80 °C for 48 h and methanol (3 × 40 mL) at 60 °C for 48 h, and then dried under vacuum at 80 °C for 8 h.
O3 decomposition measurement
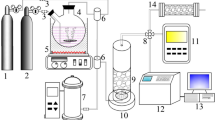
Catalytic O3 decomposition experiments were conducted with continuous-flow fixed-bed reactors (quartz tube, inner diameter: 4 mm; length: 15 cm) of the tested materials at room temperature (unless otherwise specified) and the inlet gas flow rate of 1 L min−1. Before the test, all tested samples were dried in a vacuum at 80 °C for 12 h. Then a mixture of 30 mg tested materials and 200 mg quartz sand (40–60 mesh) were loaded into the fixed-bed reactors and immobilized by quartz wool. A humidity generator was used to control the humidity of inlet gas. The temperature of the reactor was maintained by a thermostatic water bath. O3 in the inlet gas was generated by passing zero air through an ultraviolet lamp, and its concentration was controlled by the flow rate of zero air. The O3 concentration in inlet and outlet gas was detected by an O3 monitor with a detection limit of 2 ppb. O3 removal efficiency was calculated as 100% × (Cinlet – Coutlet)/Cinlet. A schematic representation of the O3 decomposition measurement setup is shown in Supplementary Fig. 14.
Breakthrough experiments
The breakthrough experiments were carried out with zero air containing ca. 3000 ppm acetone vapor by a setup shown in Supplementary Fig. 15. Quartz tubes (4 mm inner diameter and 15 cm length) were packed with a mixture of 20 mg PCN-250(Fe2Co) and 400 mg quartz sand (40–60 mesh). A humidity generator was used to control the humidity of inlet gas (flow rate = 198 mL min−1). The flow rate of air containing saturated acetone vapor at room temperature was controlled by a mass flow controller (Alicat, flow rate = 2 mL min−1). The concentration of acetone in the gases passing through the MOF column was monitored with a Hiden HPR20 mass spectrometer gas analysis system.
Theoretical calculation
A PCN-250(Fe2Co) cluster model is constructed by cutting from its periodic structure as done in recent works30 (Supplementary Fig. 2a), and it includes the Fe2Co core, six phenyl carboxylic groups coordinated with one Co atom and two Fe atoms, and two water molecules occupying two exposed Fe sites. All calculations presented in this work are performed using the unrestricted density functional theory (DFT) with the hybrid functional PBE0 as implemented in the Gaussian09 package57,58,59. Geometry optimizations are carried out with the LANL2DZ basis sets for the Fe and Co atoms and the 6-31 G* basis sets for the other atoms60,61,62. Dispersion correction is included in geometry optimizations using the empirical formula by Grimme et al.63. Frequency calculations are performed at the same level of theory to confirm the nature of stationary points and to obtain zero-point energies (ZPE) and entropy effects. The reported free energies are corrected for dispersion, ZPE, and entropy effects.
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Dickerson, R. R. et al. The impact of aerosols on solar ultraviolet radiation and photochemical smog. Science 278, 827–830 (1997).
Paoletti, E., De Marco, A., Beddows, D. C., Harrison, R. M. & Manning, W. J. Ozone levels in European and USA cities are increasing more than at rural sites, while peak values are decreasing. Environ. Pollut. 192, 295–299 (2014).
Li, P. et al. Nationwide ground-level ozone measurements in China suggest serious risks to forests. Environ. Pollut. 237, 803–813 (2018).
Cooper, O. R., Langford, A. O., Parrish, D. D. & Fahey, D. W. Challenges of a lowered U.S. ozone standard. Science 348, 1096–1097 (2015).
Lu, X. et al. Severe surface ozone pollution in China: a global perspective. Environ. Sci. Technol. Lett. 5, 487–494 (2018).
Weschler, C. J. Ozone in indoor environments: concentration and chemistry. Indoor Air 10, 269–288 (2000).
Chang, L.-T. et al. Indoor ozone levels, houseplants and peak expiratory flow rates among healthy adults in Taipei, Taiwan. Environ. Int. 122, 231–236 (2019).
Guo, C., Gao, Z. & Shen, J. Emission rates of indoor ozone emission devices: a literature review. Build. Environ. 158, 302–318 (2019).
Hubbell, B. J., Hallberg, A., McCubbin, D. R. & Post, E. Health-related benefits of attaining the 8-hr ozone standard. Environ. Health Perspect. 113, 73–82 (2005).
Turner, M. C. et al. Long-term ozone exposure and mortality in a large prospective study. Am. J. Respir. Crit. Care Med. 193, 1134–1142 (2016).
WHO. Air quality guidelines: global update 2005. (World Health Organization, 2006).
Chen, Z. et al. Understanding the causal influence of major meteorological factors on ground ozone concentrations across China. J. Clean. Prod. 242, 118498 (2020).
Li, G. et al. Widespread and persistent ozone pollution in eastern China during the non-winter season of 2015: observations and source attributions. Atmos. Chem. Phys. 17, 2759–2774 (2017).
Wang, T. et al. Ozone pollution in China: a review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 575, 1582–1596 (2017).
Mehandjiev, D. & Naidenov, A. Ozone decomposition on α-Fe2O3 catalyst. Ozone. Sci. Eng. 14, 277–282 (1992).
lvarez, P. M. A., Masa, F. J., Jaramillo, J., Beltra´n, F. J. & Go´mez-Serrano, V. Kinetics of ozone decomposition by granular activated carbon. Ind. Eng. Chem. Res. 47, 2545–2553 (2008).
Yang, S., Nie, J., Wei, F. & Yang, X. Removal of ozone by carbon nanotubes/quartz fiber film. Environ. Sci. Technol. 50, 9592–95928 (2016).
Ma, J., Wang, C. & He, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B 201, 503–510 (2017).
Mathew, T. et al. Mesoporous ferrihydrite-based iron oxide nanoparticles as highly promising materials for ozone removal. Angew. Chem. Int. Ed. 123, 7519–7522 (2011).
Zhu, G. et al. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B 209, 729–737 (2017).
Cao, R., Li, L. & Zhang, P. Macroporous MnO2-based aerogel crosslinked with cellulose nanofibers for efficient ozone removal under humid condition. J. Hazard. Mater. 407, 124793 (2021).
Gong, S. et al. Highly active and humidity resistive perovskite LaFeO3 based catalysts for efficient ozone decomposition. Appl. Catal. B 241, 578–587 (2019).
Tang, W.-X., Liu, H.-D., Wu, X.-F. & Chen, Y.-F. Higher oxidation state responsible for ozone decomposition at room temperature over manganese and cobalt oxides: effect of calcination temperature. Ozone. Sci. Eng. 36, 502–512 (2014).
Ma, J., Chen, Y., He, G. & He, H. A robust H-transfer redox mechanism determines the high-efficiency catalytic performance of layered double hydroxides. Appl. Catal. B 285, 119806 (2021).
Gong, S. et al. Gram-scale synthesis of ultra-fine Cu2O for highly efficient ozone decomposition. RSC Adv. 10, 5212–5219 (2020).
Zhou, H.-C., Long, J. R. & Yaghi, O. M. Introduction to metal-organic frameworks. Chem. Rev. 112, 673–674 (2012).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Lv, X.-L. et al. A base-resistant metalloporphyrin metal-organic framework for C-H bond halogenation. J. Am. Chem. Soc. 139, 211–217 (2017).
Al-Attas, T. A. et al. Ligand-engineered metal–organic frameworks for electrochemical reduction of carbon dioxide to carbon monoxide. ACS Catal. 11, 7350–7357 (2021).
Wang, H. et al. An iron-containing metal-organic framework as a highly efficient catalyst for ozone decomposition. Angew. Chem. Int. Ed. 57, 16416–16420 (2018).
Sun, Z.-B., Si, Y.-N., Zhao, S.-N., Wang, Q.-Y. & Zang, S.-Q. Ozone decomposition by a manganese-organic framework over the entire humidity range. J. Am. Chem. Soc. 143, 5150–5157 (2021).
Zhou, D.-D. et al. Intermediate-sized molecular sieving of styrene from larger and smaller analogues. Nat. Mater. 18, 994–998 (2019).
Cao, J. W. et al. One-step ethylene production from a four-component gas mixture by a single physisorbent. Nat. Commun. 12, 6507 (2021).
Pei, J. et al. Dense packing of acetylene in a stable and low-cost metal–organic framework for efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 60, 25068–25074 (2021).
Xie, L.-H., Liu, X.-M., He, T. & Li, J.-R. Metal-organic frameworks for the capture of trace aromatic volatile organic compounds. Chem. 4, 1911–1927 (2018).
Lai, C. et al. Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev. 427, 213565 (2021).
Liu, B. et al. Catalytic ozonation of VOCs at low temperature: a comprehensive review. J. Hazard. Mater. 422, 126847 (2022).
Abednatanzi, S. et al. Mixed-metal metal-organic frameworks. Chem. Soc. Rev. 48, 2535–2565 (2019).
Chen, L., Wang, H.-F., Li, C. & Xu, Q. Bimetallic metal-organic frameworks and their derivatives. Chem. Sci. 11, 5369–5403 (2020).
Feng, D. et al. Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal-organic frameworks. Nat. Commun. 5, 5723 (2014).
Dhakshinamoorthy, A. et al. Iron(iii) metal–organic frameworks as solid Lewis acids for the isomerization of α-pinene oxide. Catal. Sci. Technol. 2, 324–330 (2012).
Cai, C. et al. Efficient degradation of clofibric acid by heterogeneous catalytic ozonation using CoFe2O4 catalyst in water. J. Hazard. Mater. 410, 124604 (2021).
Subrahmanyam, C., Bulushev, D. A. & Kiwi-Minsker, L. Dynamic behaviour of activated carbon catalysts during ozone decomposition at room temperature. Appl. Catal. B 61, 98–106 (2005).
Ondarts, M., Outin, J., Reinert, L., Gonze, E. & Duclaux, L. Removal of ozone by activated carbons modified by oxidation treatments. Eur. Phys. J. Spec. Top. 224, 1995–1999 (2015).
He, T., Kong, X.-J. & Li, J.-R. Chemically stable metal-organic frameworks: rational construction and application expansion. Acc. Chem. Res. 54, 3083–3094 (2021).
Burtch, N. C., Jasuja, H. & Walton, K. S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 114, 10575–10612 (2014).
Lv, X.-L. et al. Ligand rigidification for enhancing the stability of metal-organic frameworks. J. Am. Chem. Soc. 141, 10283–10293 (2019).
He, T. et al. Kinetically controlled reticular assembly of a chemically stable mesoporous Ni(II)-pyrazolate metal-organic framework. J. Am. Chem. Soc. 142, 13491–13499 (2020).
Yang, H. et al. Ultrastable high-connected chromium metal-organic frameworks. J. Am. Chem. Soc. 143, 14470–14474 (2021).
Guillerm, V., Xu, H., Albalad, J., Imaz, I. & Maspoch, D. Postsynthetic selective ligand cleavage by solid-gas phase ozonolysis fuses micropores into mesopores in metal-organic frameworks. J. Am. Chem. Soc. 140, 15022–15030 (2018).
Yue, C. et al. Study on the stability, evolution of physicochemical properties, and postsynthesis of metal-organic frameworks in bubbled aqueous ozone solution. ACS Appl. Mater. Interfaces 13, 26264–26277 (2021).
Cozzolino, A. F. et al. Ligand redox non-innocence in the stoichiometric oxidation of Mn2(2,5-dioxidoterephthalate) (Mn-MOF-74). J. Am. Chem. Soc. 136, 3334–3337 (2014).
Chen, Y. et al. Unusual moisture-enhanced CO2 capture within microporous PCN-250 frameworks. ACS Appl. Mater. Interfaces 10, 38638–38647 (2018).
Oyama, S. T. Chemical and catalytic properties of ozone. Catal. Rev. 42, 279–322 (2000).
Campestrini, S., Robert, A. & Meunier, B. Ozone epoxidation of olefins catalyzed by highly robust manganese and iron porphyrin complexes. J. Org. Chem. 56, 3725–3727 (1991).
Sharma, M., Meehan, E., Mercado, B. Q. & Bruckner, C. β-Alkyloxazolochlorins: revisiting the ozonation of octaalkylporphyrins, and beyond. Chem. Eur. J. 22, 11706–11718 (2016).
Parr, R. G. & Yang, W. Density-Functional Theory of Atoms and Molecules. (Oxford University Press, New York, 1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Frisch, M. J. et al. Gaussian 09, Revision A.02. (Gaussian, Inc., 2009).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 82, 299–310 (1985).
Ditchfield, R., Hehre, W. J. & Pople, J. A. Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 54, 724–728 (1971).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chem. Acc. 28, 213–222 (1973).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22038001, receipted by J.R.L., 51621003, receipted by J.R.L., and 21688102, receipted by W.H.F.).
Author information
Authors and Affiliations
Contributions
J.R.L., L.H.X., and C.D. conceived the project. C.D. and L.H.X. designed the experiments. C.D. prepared and characterized the samples. C.D., L.H.X., and J.R.L. analyzed the experimental data and discussed the results. J.J.Y., W.H.F., and G.C. carried out DFT calculations. C.D., J.J.Y., and L.H.X. wrote the manuscript. W.H.F., G.C., and J.R.L. guided and supervised the project. All authors reviewed and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, C., Yang, JJ., Xie, LH. et al. Catalytic ozone decomposition and adsorptive VOCs removal in bimetallic metal-organic frameworks. Nat Commun 13, 4991 (2022). https://doi.org/10.1038/s41467-022-32678-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32678-2
This article is cited by
-
Performance of an Aliovalent-Doping MnCeOx/Cordierite Monolithic Catalyst Derived from MOFs for o-Xylene Oxidation
Catalysis Letters (2024)
-
Catalytic ozonation mechanism over M1-N3C1 active sites
Nature Communications (2023)
-
Interface engineering of ZnSnO3-based heterojunctions for room-temperature methanol monitoring
Rare Metals (2023)
-
Removal of Toluene by Non-thermal Plasma Combined with CoxNiy-MOF-74 Catalyst
Catalysis Letters (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.