Abstract
Lakes are a major emitter of the atmospheric greenhouse gas methane (CH4); however, their roles in past climate warming episodes remain poorly understood owing to a scarcity of geological records. Here we report the occurrence of sustained and intensified microbial CH4 cycling in paleo-Lake Junggar in northwestern China, one of the largest known Phanerozoic lakes, during Early Permian climate warming. High-precision U-Pb geochronology refines the age of the upper Lucaogou Formation to the Artinskian, which marks a major glacial-to-postglacial climate transition. The 13C-enriched authigenic dolomites indicate active methanogenesis in the anoxic lake sediments, and 13C-depleted hopanes suggest vigorous methanotrophy in the water column. The intensification of CH4 cycling coincided with increasing global temperature, as evidenced from elevated continental chemical weathering. Our results suggest that the lacustrine CH4 emissions acted as a positive feedback to global warming and contributed to the demise of the Late Paleozoic Ice Age.
Similar content being viewed by others
Introduction
Methane (CH4) is a powerful greenhouse gas that has 25-times the global warming potential of carbon dioxide (CO2) over a centennial time-scale1. Global freshwater CH4 emissions, expressed as CO2 equivalents, offset at least 25% of the continental greenhouse gas sink2. Particularly, lakes are a considerable source of atmospheric CH4 and play an important role in the greenhouse gas balance2,3. Methane emissions from lakes were estimated as 23–142 Tg CH4 yr–1 (ref. 4; higher than oceanic emissions), accounting for 4–25% of total global emissions (576 Tg CH4 yr–1; ref. 5). Understanding the scale and dynamics of CH4 emissions from lacustrine ecosystems is therefore fundamental for predicting and reconstructing climate change. Current CH4 emissions from lakes have been well documented2,3,4,5,6,7. However, in geological records, lacustrine CH4 emissions are poorly understood, and linking lake-source CH4 to climate warming in the geological past remains a significant challenge, which substantially hinders our ability to understand the role of this key component of the carbon cycle in ancient climate change and biological systems. Specifically, large uncertainties remain on the metabolic activities of microbial CH4 production (methanogens) and consumption (methanotrophs) in large paleo-lakes, which controlled the net CH4 emissions to the atmosphere6,7,8,9.
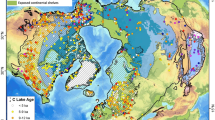
The recent discovery of a fossil record of methanogenic archaea in authigenic dolomite from the Permian lacustrine Lucaogou Formation in northwestern China10 provides an opportunity to examine geological CH4 emissions at the ecosystem level. The extent of the Permian lacustrine deposits in northern Xinjiang covers a total area of ~270,000 km2 (900 × 300 km; Fig. 1)11, more than three-fold the size of Lake Superior (82,100 km2), the largest modern freshwater lake in the world. This Permian lake (referred to as paleo-Lake Junggar in this study) represents one of the largest known Phanerozoic lakes, characterized by the world’s thickest organic-rich lacustrine source rock interval11,12,13.
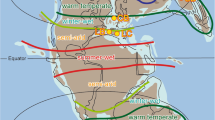
The map shows the locations of the Junggar, Turpan-Hami, and Santanghu basins in northwestern China (modified from ref. 71), with the maximum known extent of Permian lake deposits marked by the purple dashed line11. Yellow star represents the location of the Jingjingzigou section. The Early Permian paleogeographic map shows the position of Junggar (adapted after Ronald Blakey, https://deeptimemaps.com, source map © 2016 Colorado Plateau Geosystems Inc). Widespread ice sheet occurred in the Southern Hemisphere. Orange spots represent the locations of other Sakmarian–Artinskian lake systems in the North China block, Southern Arabian plate (SA), Cooper Basin (Australia; Au), Gondwana basins (India; In), East Africa (EA), North America (NA), and Western and Central Europe (Eu). See Supplementary Dataset 8 for associated essential information about these lakes.
In this study, high-precision U-Pb zircon geochronology by chemical abrasion–isotope dilution–thermal ionization mass spectrometry (CA-ID-TIMS) and organic carbon isotope (δ13Corg) chemostratigraphy are utilized to constrain the precise depositional age of the Lucaogou Formation. Our results assign this sequence to the Early Permian (Cisuralian), coincident with a critical episode of climate warming in the Artinskian14. This time period marks the end of the Late Paleozoic glacial maximum and a climate transition from a long-lived icehouse (340–290 million years ago; Ma)15 to a postglacial greenhouse state16,17,18,19,20, and it was accompanied by enhanced continental chemical weathering18,21, elevated atmospheric partial pressure of CO2 (pCO2)15,16, and conspicuous biotic replacement14. We further present detailed biogeochemical proxies to reconstruct microbial CH4 cycling in paleo-Lake Junggar during this critical climate transition and discuss its possible relevance to the rising global temperatures.
Results and discussion
Age and stratigraphic constraints
Samples were collected from the Lucaogou Formation at the Jingjingzigou section along the southern margin of the Junggar Basin (Fig. 1 and Supplementary Fig. 1) for zircon U-Pb and geochemical analyses. The Lucaogou Formation can be subdivided into two members. The lower member mainly consists of mudstone, shale, dolomitic siltstone, and dolomite, with minor amounts of gypsum in some layers. The upper member is composed of organic-rich shale interbedded with dolomite beds and nodules without evaporite minerals (Fig. 2a, c; Supplementary Figs. 1 and 2). This sequence reflects the evolution from a relatively shallow evaporative lake to a persistently deep brackish-to-freshwater lacustrine environment (see Supplementary Note 1). Despite decades of sedimentological and geochemical/hydrocarbon research, due to the economic importance of the Lucaogou Formation11,12,13,22,23, the succession lacks any reliable age constraints in the absence of datable volcanic ash beds and biostratigraphically useful fossils24,25. Previous detrital zircon U-Pb geochronology obtained by in situ laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) assigned broad maximum depositional ages of ca. 270–268 Ma24 or ca. 261 Ma26 to the Lucaogou Formation. However, limited accuracy due to reworked zircons and/or post-crystallization Pb loss can lead to statistically biased results. In addition, a previously published high-precision U-Pb CA-ID-TIMS age of 281.39 ± 0.10 Ma27 from the overlying Hongyanchi Formation from the southern Bogda Mountains (Figs. 1 and 2c) resulted in a contradictory stratigraphic framework.
a Outcrop photograph of organic-rich shale interbedded with dolomite beds and nodules from the upper member of the Lucaogou Formation. The arrow points to the location of the volcanic ash bed (sample VA-1) sampled for zircon geochronology (inset shows a close-up view of the ash bed). b Concordia plot and 206Pb/238U ages of zircons analyzed using the U-Pb CA-ID-TIMS method; excluded analysis z4 shown in gray. Vertical bars represent 2σ analytical uncertainty of individual zircon analyses. c Stratigraphic column of the southern Junggar Basin (modified from ref. 11). Arrows indicate stratigraphic positions of dated ash beds (bentonites) and tuffaceous siltstone (blue–published ages of ref. 27; red–new ages presented in this study).
Here we present high-precision U-Pb zircon age from a volcanic ash bed in the upper, organic-rich member of the Lucaogou Formation (Fig. 2a, b). The 4 cm-thick ash layer interbedded within shales occurs ~925 m above the base of the Lucaogou Formation (Figs. 2c and 3). The sample (VA-1) contains zircons that are small, equant or prismatic, and euhedral, with oscillatory zoning under cathodoluminescence (Supplementary Fig. 3a). The Th/U ratios of the zircon crystals vary from 0.26 to 1.27 (Supplementary Dataset 1). The U-Pb ages determined by LA-ICP-MS have an average 2σ uncertainty of ±5.63 million years (Myr) and are distributed around a well-defined peak, with a weighted mean 206Pb/238U age of 286.14 ± 0.65 Ma (2σ internal error only; mean-squared weighted deviation [MSWD] = 1.01, n = 53; Supplementary Fig. 3a). For further verification, four single zircon grains from this sample were analyzed independently by the CA-ID-TIMS method (average 2σ uncertainty of ±0.55 Myr), with the three youngest analyses constituting a coherent cluster with a weighted mean 206Pb/238U age of 286.39 ± 0.25/0.30/0.43 Ma (2σ; MSWD = 2.0; Fig. 2b, c and Supplementary Dataset 2). Furthermore, one tuffaceous siltstone (sample TS-1) from the uppermost part of the underlying Jingjingzigou Formation was analyzed using the LA-ICP-MS method. Ninety-four zircon analyses from this sample yielded a wide range of ages, with a weighted mean 206Pb/238U age of 294.1 ± 1.4 Ma (2σ; MSWD = 1.5; Fig. 2c and Supplementary Fig. 3b) based on 13 youngest analyses (YC2σ[3+]28), and this is interpreted as being the maximum constraint on the depositional age. The available radioisotope geochronology collectively places the lower and upper boundaries of the Lucaogou Formation at ca. 294 Ma and ca. 285 Ma, respectively, and constrains its upper shale member to the Artinskian Stage; therefore, the age is significantly older than previous estimates24,25,26.
The different colors in the stratigraphic column indicate changes in the lithology of the Lucaogou Formation. a Bulk organic matter δ13Corg record. b Dolomite δ13Ccarbonate record. c C29 and C30 αβ hopane δ13C values. Error bars denote one standard deviation between duplicate analyses. d Chemical index of alteration (CIA) and land surface temperature (LST) estimates. The curves in (a) and (d) represent the seven-point moving averages. e CIA trend from the glacial to postglacial transition succession in the Karoo Basin of South Africa21 with CA-ID-TIMS zircon age constraints19 and temporal variations in low-Mg-calcite oxygen isotope (δ18O) values from low- and high-latitudinal fossil shells50,51. f Documented glacial deposits20 and reconstructed global atmospheric partial pressure of CO2 (pCO2) curve (75% confidence interval)15 during the Early Permian. Timescale from an updated version (2022) of the International Chronostratigraphic Chart72.
Organic carbon isotope excursions (CIEs) have been proven to be an effective global stratigraphic correlation proxy29. Our results show that a prominent negative δ13Corg excursion occurs at the upper part of the succession, with a total CIE magnitude of ~3.5‰ (above ~725 m; Fig. 3a and Supplementary Dataset 3). The observed parallel δ13CTLE and δ13CAsph records from the total lipid extract (TLE) and asphaltene (Asph), after extraction and separation, also exhibit a largely similar negative CIE in shape and magnitude to that of the bulk δ13Corg record. In addition, all the δ13Cn-alkane records of short-chain n-C19, mid-chain n-C21, and long-chain n-C27 alkanes (Supplementary Dataset 4) display a negative CIE with a magnitude of ~4‰ (Supplementary Fig. 4a). It is considered unlikely that the thermal maturity (early oil window) and proportional changes in the organic matter resulted in the observed CIE in the Lucaogou shales (see Supplementary Note 2; Supplementary Fig. 5). Importantly, under our high-precision CA-ID-TIMS age constraint, the negative CIE is comparable to that recorded in coeval marine brachiopod shells (USA and Russia)30, carbonate (South China)31, and in coastal strata (North China;29 Supplementary Fig. 4). Therefore, the observed parallel CIE signatures in bulk δ13Corg and δ13Cn-alkane reflect a perturbation of the global carbon cycle during the Artinskian (see Supplementary Note 2).
Intensified ecosystem-level microbial CH4 cycling
Investigating microbial CH4 cycling in pre-Holocene environments is challenging owing to the scarcity of diagnostic proxy records; some lipid biomarkers (e.g., glycerol dialkyl glycerol tetraether and archaeol32,33) may be invalid with an increase in the thermal maturity of the organic matter. In this study, we present the distinctive δ13C records of authigenic dolomites and hopanes (bacterial-derived biomarkers) from the Lucaogou Formation, which provide new insights into the metabolic activities of methanogens and methanotrophs in the lake ecosystem during the Early Permian.
Dolomite beds and nodules in the upper member (Fig. 2a) have very positive δ13C values (+5.8 to +16.0‰) that are significantly higher than the δ13C values (+5.3 to +8.3‰) of the dolomite in the lower member (Fig. 3b and Supplementary Dataset 5). Several mechanisms have been proposed for 13C enrichment in inorganic C pools34,35,36. Of these, the Rayleigh distillation of volatile CO2 under highly evaporative conditions34 would not have been effective in paleo-Lake Junggar, as the upper member was not deposited in a hypersaline environment12,13. In addition, the photosynthetic fixation of CO2 during productivity blooms cannot explain the positive values, because this process usually only enriches δ13C values by +2 to +3‰35, and this Permian lake was not eutrophic13. Such positive δ13C signatures have been recently attributed to authigenic dolomite precipitation associated with microbial methanogenesis10, and it is likely that some dolomite samples with less positive δ13C and lower δ18O values in the upper member have been influenced by subsequent diagenesis36 (Supplementary Fig. 6). Microbial methanogenesis is geochemically characterized by significant C isotopic fractionation, generating 13C-depleted biogenic CH4 (δ13C as low as −60 to −110‰)37 and 13C-enriched CO2 (δ13C up to +15‰ or higher)36. Such isotopically heavy CO2 acted as a substantial C source and was incorporated into the authigenic dolomite. The closest modern analogues of these dolomites, commonly observed in organic carbon-rich continental margin sediments, have been documented in the Gulf of California38 and along the Peru Margin39, where methanogenesis is highly active in oceanic sediments. Thus, the 13C-enriched authigenic dolomites presented here are a fingerprint of biogenic CH4 production in lake sediments.
Putative methanogen microfossils have been found in these 13C-enriched dolomites from the adjacent Hongyanchi section, and their abundances show a positive correlation with δ13C values10. The elevated δ13C signature of the dolomites can therefore be used to trace changes in methanogenesis. In the current study, the dolomite δ13C values show an overall increasing trend from the bottom to the top within the succession (Fig. 3b). The dolomite beds and nodules that are marked by high δ13C values occur above ~610 m. Most importantly, the more abundant strongly 13C-enriched dolomites occur within the upper part of the Lucaogou Formation (Fig. 3b), indicating a higher methanogenic rate and/or an expanded methanogen community in the anoxic lake sediments during this period. In contrast, the absence of exceptionally 13C-depleted authigenic dolomite in the studied section (δ13Ccarbonate values typically <−30‰; C largely derived from biogenic CH4)40 suggests that the anaerobic oxidation of methane was not an important process occurring in the anoxic sediments at that time. Therefore, large amounts of CH4 produced in bottom sediments escaped substantial consumption and were emitted into the overlying water column; as such, they would have provided substrates for aerobic methanotrophs.
An earlier comprehensive study13 revealed that the saturated hydrocarbon fraction from Lucaogou shales was depleted in 13C, and this possibly indicates the presence of hopanes derived from methanotrophic bacteria. In this study, we conducted a compound-specific C isotope analysis of hopanes. Hopanoids are not exclusive to methanotrophs, but their stable C isotopic compositions can be used to assess specific methanotroph contributions33,41,42,43,44,45. Methanotrophic bacteria use biogenic CH4 as a carbon source for the biosynthesis of membrane lipids (e.g., hopanoids) that are highly 13C-depleted. Our results show that the hopanoids in Lucaogou shales are dominated by C30 17α,21β-hopane and C29 17α,21β-norhopane (Supplementary Fig. 7 and Supplementary Note 1). The hopane δ13C values remain low in all samples analyzed, ranging from −44.1 to −62.6‰ for C30 17α,21β-hopane and −41.6 to −53.6‰ for C29 17α,21β-hopane (Fig. 3c). The δ13C values in these two compounds yield a positive correlation (Supplementary Fig. 5d), indicating that they have a similar bacterial community source. Their corresponding 17β,21α(H) isomers are also characterized by similar low isotopic signatures (Supplementary Dataset 4). The δ13C values of the hopanes are markedly lower than those observed in the co-occurring bulk organic matter (−24.0 to −32.0‰; Fig. 3a, c) and n-alkanes (n-C21: −33.0 to −38.1‰). Such 13C-depleted hopanoids also appear in some modern/Holocene (e.g., Lake Rotsee, Switzerland33) and Eocene (e.g., Green River Formation, USA46) lake systems, where aerobic CH4 oxidation by methanotrophic bacteria was prevalent in the water column. Here we conducted a survey of hopanoid δ13C values from 19 lakes (283 data points; δ13Chopanoid ranging from −22.2 to −71.9‰; Supplementary Fig. 8 and Supplementary Dataset 6). The data compilation (see Supplementary Note 3 for data overview) suggests that hopanoid δ13C values below −40‰ are indicative of a pronounced aerobic methanotroph contribution to these compounds (>10–20%; calculated from a C isotopic mass-balance approach;33 see Methods).
Hopanoid δ13C values can be used to trace the temporal changes in aerobic CH4 oxidation33,42,44,45. In this study, the consistently low hopane δ13C values (<−40‰) throughout the section indicate that methanotrophic activity within the lake was sustained and vigorous, particularly during the late depositional stage of the Lucaogou Formation (Fig. 3c). Specifically, in the lower member, the suitably low hopane δ13C values (ca. −50‰) and the less positive dolomite C isotopic signatures (<+8.5‰; Fig. 3b) indicate mild-to-moderately active methanotrophy and methanogenesis. In the upper member, however, the coupling between highly 13C-depleted hopanes and 13C-enriched authigenic dolomites suggests that both methanotrophs and methanogens thrived in the lake biosphere. In particular, above ~750 m, there is a persistent and obvious decrease in hopane δ13C values of >10‰ (from ca. −46 to −63‰ for C30 hopane and from ca. −43 to −54‰ for C29 hopane; Fig. 3c). The lowest values within the uppermost stratigraphic interval are among the most 13C-depleted reported in the C30 and C29 hopanes for lacustrine systems (Supplementary Fig. 8). These isotopic signatures indicate that substantially intensified CH4 oxidation occurred in the water column, which closely coincided with elevated CH4 production in the sediments, as indicated by a temporal increase in dolomite δ13C values (Fig. 3b, c). The combined evidence from both authigenic dolomite and molecular fossil (hopane) suggests that an intensification of the microbial CH4 cycling occurred during the Artinskian age. Furthermore, active CH4 cycling had a wide geographical distribution in paleo-Lake Junggar, with evidence of similar 13C-depleted hopanes also documented in the Lucaogou shales from the adjacent Sangonghe section22 and the Santanghu Basin23, hundreds of kilometers from the studied area (Fig. 1). Based upon our estimated depositional duration (ca. 9 Myr) of the Lucaogou Formation, the intensified microbial CH4 cycling persisted for at least ca. 3–5 Myr. To our knowledge, such a long-term dynamic of lacustrine CH4 cycling in the Earth’s history has not been previously and directly revealed.
Positive feedback to Artinskian climate warming
To investigate the relationship between temperature and microbial CH4 cycling, we used the chemical index of alteration (CIA)47 to reconstruct changes in the land surface temperature (LST;18,48 see Methods). The collected samples were not affected by K-metasomatism, and their uniform Ti/Al ratios indicate no changes in provenance48 (Supplementary Fig. 9 and Supplementary Dataset 7), and they thus provide a reliable record of climate variation. The CIA profiles show an increase from 50–55 in the lower member to 65–75 in the upper member, suggesting a rapid rise in the estimated LSTs from ~4 °C (Sakmarian) to ~14 °C (Artinskian; Fig. 3d). Overall, the pronounced progression toward higher CIA values, combined with the alternative chemical index of weathering (CIW;49 Supplementary Fig. 9), indicates a shift toward warmer conditions14,18,48. This record is consistent (within age uncertainties) with an independently derived CIA trend in a contemporaneous succession from the Karoo Basin of South Africa19,21 (Fig. 3e). A cross-basin correlation revealed that a significant increase in CIA (temperature) globally began near the Sakmarian–Artinskian boundary (ca. 290 Ma)18. This major climate transition can be further corroborated by a coincident decrease in δ18O values from both low- and high-latitudinal fossil shells composed of low-Mg calcite50,51 (Fig. 3e). Therefore, the elevated continental weathering in this study reflects a global climate warming signal (i.e., the Artinskian Warming Event14), which developed contemporaneously with the intensification of CH4 cycling in paleo-Lake Junggar (Fig. 3).
Higher temperatures may have stimulated methanogenesis in lake sediments, supporting a temperature control on CH4 cycling at the ecosystem level8,42,52. It has been proposed that the metabolic responses of methanogens are particularly sensitive to increases in temperature8,52. Since the predominant microbial methanogenesis occurred in the shallow sediment columns53, it would be expected that the increase in atmospheric temperature warmed the sediments and subsequently facilitated methanogenic activity. Additionally, under global warming, enhanced continental weathering (Fig. 3d) may have increased riverine nutrient influx and aquatic productivity in lakes, thereby resulting in increased substrate (e.g., acetate and H2/CO2; ref. 6) availability for methanogenesis8. However, methanotrophy is known to have a more positive effect on substrate (i.e., CH4) availability than temperature8, and the intensified CH4 consumption observed in the top part of the Lucaogou Formation (mid-Artinskian) was almost certainly a response to an increased CH4 substrate supply for methanotrophs (Fig. 3).
The balance between methanogenesis and methanotrophy ultimately controlled the amount of CH4 released into the atmosphere6,8,9. Nonetheless, if a warming-induced increase in CH4 production exceeds the increase in CH4 oxidation, an increase in net CH4 emissions is expected, and this provides potential positive feedback to climate warming. Indeed, owing to the different temperature sensitivities of methanogens and methanotrophs8,9, warming would increase CH4 emissions, which has been extensively observed in both modern freshwater ecosystems7,52,54 and laboratory incubations8,9,52. For example, experimental warming of artificial ponds has suggested a disproportionate increase in methanogenesis over methanotrophy9. Although aerobic methanotrophs did oxidize more CH4, but not enough to offset the greater warming-induced CH4 production9. Methane fluxes from lake ecosystems exhibit a temperature dependence8,52,54. The prevailing paradigm of the exponential response of CH4 emissions to temperature7,8,52,54 can be extrapolated to ancient lake systems, and the total CH4 emissions from paleo-Lake Junggar could potentially have increased by several-fold in response to Artinskian climate warming. Applying the average CH4 flux (total 31.6 Tg CH4 yr−1 in areas spanning 1,330,264 km2; i.e., 65 mg CH4 m–2 d–1)2 from modern lakes at similar latitudes to paleo-Lake Junggar (paleolatitude of 39–43°N)13, the flux was roughly estimated as 6.4 Tg CH4 yr–1 (accounting for 5–28% of annual lake CH4 emissions in the modern world4), and a total amount of ~19,200 Gt CH4 was emitted from this Early Permian lake (~270,000 km2; ref. 11; herein conservatively calculated using 3 Myr).
Although there is only evidence for intensified CH4 cycling in paleo-Lake Junggar (Fig. 1), this still provides a useful analogue for similar environments having responses to Artinskian (Early Permian) climate warming. In this respect, several contemporaneous lake systems (see Fig. 1 and Supplementary Dataset 8 for the locations of these lakes and associated essential information) may also be CH4 emission hotspots. However, accurate assessments of global CH4 emissions require clear constraints relating to the contemporaneous lake area, distribution, and environmental factors, and these are beyond the scope of this study. Nonetheless, large-scale lacustrine CH4 emissions would have acted as a positive feedback to Artinskian global warming and a critical mechanism for deriving carbon cycle perturbations. During this time period (after 290 Ma)15, the demise of the Late Paleozoic Ice Age (LPIA) was supported by a 6-fold drop in documented glacial deposits (Fig. 3f)20 and the full deglaciation in south-central Gondwana by 282 Ma19, representing one of the most prominent and enigmatic climate transitions in the Earth’s Phanerozoic history. Previous studies have demonstrated that widespread deglaciation was synchronous with an increase in atmospheric pCO2 (Fig. 3f)15 derived from volcanic eruptions (e.g., Tarim, Panjal, and Zaduo large igneous provinces)15,29, and this provides an evidence regarding the strong link between CO2 and glaciation. In addition to the contribution of CO2 (refs. 15,16,17) and potential methane clathrate release55, our results suggest that the injection of the terrestrial greenhouse gas CH4 into the atmosphere may have facilitated the demise of the LPIA and played a direct role in forcing the turnover from a long-lived icehouse to a greenhouse world.
In summary, this study investigated currently unexplored lacustrine ecosystem-level microbial CH4 cycling records, including methanogenesis and methanotrophy, in pre-Cenozoic sedimentary archives. Our results suggest that sustained and intensified CH4 cycling, as a response to Artinskian (Early Permian) climate warming, occurred in paleo-Lake Junggar. The release of the greenhouse gas CH4 from large paleo-lakes to the atmosphere could have provided a direct positive feedback to ancient global warming, at least during the Early Permian, which should improve our understanding of its role in near-future climate change within a warming-but-glaciated world.
Methods
Samples
The samples analyzed in this study were collected from the Jingjingzigou section (43°47′30″ N, 87°45′12″ E) in the Urumqi area, Xinjiang, Northwest China (Fig. 1). The outcrop mainly occurs along road cuts, which are generally continuous (Supplementary Fig. 1). One volcanic ash sample from the upper Lucaogou Formation and one tuffaceous siltstone sample from the uppermost part of the underlying Jingjingzigou Formation were collected for zircon U-Pb dating. Additionally, 67 shale and 82 dolomitic rocks were collected for petrographic and geochemical analyses. The samples were visually examined to ensure that they were fresh.
Microscopic analysis
Polished thin-sections were examined using a Nikon Eclipse LV100N POL fluorescence microscope. Freshly broken chips coated with platinum were observed using a Carl Zeiss Supra 55 field-emission scanning electron microscope (FE-SEM), equipped with an energy dispersive X-Ray spectrometer, at the State Key Laboratory for Mineral Deposits Research, Nanjing University (MiDeR-NJU), China. Analyses were carried out at an acceleration voltage of 5 kV and a beam current of 10 nA.
Zircon U-Pb geochronology
Zircon grains were separated using standard density and magnetic separation techniques, mounted in epoxy, and polished to a half section. Cathodoluminescence (CL) images were obtained using a JEOL JSM-7000F SEM. Zircon U-Pb dating was performed on an Agilent 7900 ICP-MS, equipped with a Resolution SE 193 nm laser ablation (LA) system at Nanjing Hongchuang Exploration Technology Service Co., Ltd. The detailed tuning parameters were similar to those described in ref. 56. Analyses were conducted with a spot diameter of 30 μm and a repetition rate of 5 Hz. U-Pb fractionation was corrected using zircon standard 91,500, and the accuracy was controlled using zircon standard GEMOC GJ-1. Fifteen analyses of the GJ-1 zircon in this study yielded a weighted mean 206Pb/238U age of 602.2 ± 1.8 Ma (2σ; MSWD = 1.5), which is in good agreement (within a 2σ error) with the reference TIMS age (599.8 ± 1.7 Ma; 2σ)57. Exported data were reduced offline using the Iolite software package58 and diagrams were created with the ISOPLOT/Ex program (ver. 4.15)59. The 206Pb/238U dates for the zircon grains were selected for all grains <1000 Ma28. The maximum depositional age was estimated from the weighted mean age of the youngest cluster of more than three statistically overlapping analyses (YC2σ[3+])28.
U-Pb analyses were conducted on single zircons using CA-ID-TIMS method at the Massachusetts Institute of Technology Isotope Laboratory, following the general procedures described in ref. 60. Prior to dissolution and analysis, the selected zircon crystals were thermally annealed at 900 °C for 60 h and subsequently leached in 29 M HF inside high-pressure vessels at 210 °C for 12 h to minimize the effects of radiation-induced Pb loss in the crystals61. The chemically abraded grains were thoroughly rinsed and fluxed to remove the leachates before being spiked with the EARTHTIME ET535 mixed 205Pb-233U-235U trace solution62,63 and dissolved completely in 29 M HF at 210 °C for 48 h. Both U and Pb were isolated using an HCl-based anion exchange column chemistry procedure, deposited onto outgassed rhenium filaments with a silica gel emitter solution, and analyzed on an IsotopX X62 multi-collector thermal ionization mass spectrometer equipped with a Daly photomultiplier ion-counting system. Data reduction, age calculation and error propagation were carried out using the Tripoli and ET_Redux software64,65. The sample age was derived from the weighted mean 206Pb/238U date obtained from a coherent cluster of the youngest zircon after excluding older (detrital or xenocrystic) outliers and is reported at 95% confidence level. Uncertainty is reported as ± X/Y/Z Ma, where X is the internal uncertainty (2σ) in the absence of all external errors, Y incorporates X and the tracer calibration error, and Z includes Y as well as the decay constant errors of ref. 66.
Total organic C, total N, and Rock-Eval pyrolysis analyses
The powdered shale samples were treated with 2 M HCl for 24 h to remove carbonate, rinsed with distilled water to neutral pH, and then oven-dried at 60 °C for ~72 h. The total organic carbon (TOC) and total nitrogen (TN) contents were measured by an Elementar Vario MACRO CHNS elemental analyzer. Rock-Eval pyrolysis analysis was performed with ~100 mg powdered samples that were heated gradually in an inert atmosphere. The pyrolysis parameters included free hydrocarbon (S1), hydrocarbons cracked from kerogen (S2), CO2 released from organic matter (S3), and the temperature of the maximum pyrolyzate yield (Tmax).
Bulk organic C isotope
Approximately 0.2–2 mg (based on TOC values) of de-carbonated powdered sample was weighed into tin capsules for bulk δ13Corg analyses. The δ13Corg values were measured by a Thermo Scientific Flash 2000 Elemental Analyzer coupled to a Thermo Scientific MAT 253 isotope ratio mass spectrometer (IRMS) via a Conflo IV open split interface at MiDeR-NJU. The organic C isotopic compositions of TLE and Asph (after extraction and separation procedures) were also measured and were expressed as δ13CTLE and δ13CAsph, respectively. The reproducibility and accuracy of organic C isotopes were evaluated by measuring standards (USGS 40) between sample measurements. The isotopic ratios were reported as δ13C values relative to the V-PDB standard with a precision of ±0.2‰ or better.
Biomarker and compound-specific C isotope analyses
Approximately 50 g of powdered shale sample was Soxhlet extracted for 72 h using dichloromethane (DCM):methanol (93:7; v/v). The obtained TLE was concentrated using rotary evaporation and then de-asphalted with n-hexane, followed by filtration. The Asph-free fractions were then separated into three fractions using chromatographic columns pre-filled with alumina:silica gel (1:3; w/w; activated at 120 °C for 12 h) via elution with n-hexane (saturated fraction), DCM:n-hexane (2:1; v/v; aromatic fraction), and ethanol (polar fraction). The retrieved saturated fraction of each sample was further treated with a ZSM-5 molecular sieve to isolate the branched and cyclic alkanes, following the method described in ref. 43. The molecular sieve was activated at 120 °C for 2 h and added to a Pasteur pipette plugged with a small piece of pre-extracted cotton wool. Approximately 2 mg of the saturated fraction was dissolved in ~0.5 mL of cyclohexane and transferred to the ZSM-5-filled Pasteur column. Subsequently, the branched/cyclic hydrocarbon fraction was eluted with 3 column bed volumes of cyclohexane (~6 mL) and concentrated under a gentle nitrogen flow.
Both the original saturated and ZMS-5 treated branched/cyclic fractions were analyzed by gas chromatography–mass spectrometry (GC-MS) and gas chromatography–isotope ratio mass spectrometry (GC-IRMS) at the State Key Laboratory of Shale Oil and Gas Enrichment Mechanisms and Effective Development of SINOPEC, China. GC-MS analysis was conducted using an Agilent 7890B-5977A GC-MS equipped with a 60 m × 0.25 mm i.d. J&W DB-5MS capillary column (film thickness of 0.25 µm). Samples were injected into the injector held at 290 °C in splitless mode; helium was used as the carrier gas. The GC oven was initially held at 80 °C for 3 min, increased to 230 °C at 3 °C min–1, and finally programmed to 310 °C at 2 °C min–1, followed by a 20 min isothermal hold. The MS was operated at an ion source energy of 70 eV with full-scan mode.
The GC-IRMS analysis was performed on an Agilent 7890 GC interfaced to a Thermo Scientific Delta V Plus IRMS. The GC was fitted with a DB-5 capillary column (30 m × 0.25 mm i.d., film thickness of 0.25 μm). The initial temperature of the GC oven was 80 °C held for 5 min; it was then programmed to 320 °C at 2 °C min–1 with an isothermal hold of 15 min. Sample injection was conducted in splitless mode at 305 °C. The isotopic ratios were reported as δ13C values relative to the V-PDB standard. Samples were measured in duplicate with a reproducibility of typically <1–2‰. The instrument stability was monitored via a regular analysis of an in-house gas (CO2) standard with a known δ13C value, and the long-term precision was found to be better than ±0.5‰.
Carbonate C and O isotope analysis
The bulk-rock C and O isotopes of dolomite were measured using a Thermo Finnigan Delta V Plus continuous flow IRMS at MiDeR-NJU. Approximately 80–120 µg of each powdered sample was reacted with orthophosphoric acid at 70 °C for >2 h in a continuous flow sample preparation device (Gas Bench II) that was connected to the IRMS. The isotopic ratios were reported as δ13C and δ18O values relative to the V-PDB reference. The internal precision (1 SD) was less than ±0.1‰ and the external precision was better than ±0.5‰.
Shale elemental analysis
The carbonate-free powdered shale samples were prepared for whole-rock major elemental concentration analysis using a Thermo Scientific ARL 9900 X-Ray fluorescence spectrometer (XRF) at MiDeR-NJU. A mixture of 1 g of powdered sample and 11 g of co-solvent (Li2B4O7/LiBO2/LiBr: 49.75%/49.75%/0.50%) was melted to a glass disk in a platinum crucible at 1050 °C. The analyses were performed on tablets with an accelerating voltage of 40 kV, a beam current of 70 mA, and a count time of 20 s for each element. The analytical precision was better than ±1% for elemental concentrations of >1% and ±10% for elemental concentrations of <1%.
Estimating contribution of methanotrophic bacteria to hopanoids
To estimate the contribution of methanotrophs to hopanoids, we applied a simple C isotopic mass-balance approach described in ref. 33,
where \({f}_{{{{{{{\rm{CH}}}}}}}_{4}-{{{{{\rm{C}}}}}}}\) is the fraction of the CH4-carbon sources in the hopanoids (%; i.e., the fraction of the hopanoids originating from methanotrophic bacteria), \({{{\updelta }}}^{13}{{{{{{\rm{C}}}}}}}_{{{{{{\rm{hopanoid}}}}}}-{{{{{\rm{sample}}}}}}}\) is the measured δ13C values of the hopanoids in a given sample, \({{{\updelta }}}^{13}{{{{{{\rm{C}}}}}}}_{{{{{{\rm{non}}}}}}-{{{{{{\rm{CH}}}}}}}_{4}-{{{{{\rm{C}}}}}}}\) is the C isotopic composition of the non-CH4 carbon end member (i.e., the organic C of hopanoids that is 0% CH4 derived), \({{{\updelta }}}^{13}{{{{{{\rm{C}}}}}}}_{{{{{{{\rm{CH}}}}}}}_{4}-{{{{{\rm{C}}}}}}}\) is the C isotopic composition of the CH4 carbon end member (i.e., the organic C of hopanoids that is 100% CH4 derived). For end member values, a \({{{\updelta }}}^{13}{{{{{{\rm{C}}}}}}}_{{{{{{\rm{non}}}}}}-{{{{{{\rm{CH}}}}}}}_{4}-{{{{{\rm{C}}}}}}}\) value of –30‰ and a \({{{\updelta }}}^{13}{{{{{{\rm{C}}}}}}}_{{{{{{{\rm{CH}}}}}}}_{4}-{{{{{\rm{C}}}}}}}\) value of −73 to −109‰ were used33. According to Eq. (1), the proposed baseline of hopanoid δ13C values of −40‰ indicated that approximately 10–20% of these compounds were derived from methanotrophic bacteria. In addition, hopanoid δ13C values of −60‰ showed that approximately 40–70% of the hopanoids originated from methanotrophs (Supplementary Fig. 8).
Paleoweathering indices and paleotemperature estimation
The CIA47 has been widely used to evaluate the degree of chemical weathering. The CIA value was calculated using molecular proportions as follows,
where CaO* only represents the CaO in the silicate fractions47. The correction methods for CaO* followed those described in refs. 67, 68. High CIA values (i.e., those approaching 100) indicate a greater weathering intensity caused by the removal of readily soluble cations (e.g., Ca2+, Na+, and K+) relative to the stable residual cations (e.g., Al3+ and Ti4+) during chemical weathering69. In contrast, low CIA values reflect less chemical alteration; fresh crystalline rocks are characterized by CIA values of ca. 40–50 (ref. 68). In addition, K-metasomatism can influence the K2O contents, resulting in lower CIA values. The effects of K-metasomatism were assessed using A-CN-K (Al2O3-CaO* + Na2O-K2O) diagrams69.
To avoid the effects of K-metasomatism, an alternative chemical index of weathering (i.e., CIW49) was developed that eliminates K2O from the CIA equation. The CIW proxy has been tested in numerous studies48,68 and is expressed as follows,
where CaO* also represents silicate-bound CaO49,67. To assess the possible impacts that changes in the sediment provenance have on the chemical weathering indices, Ti/Al ratios were applied (wt.% presented in this study)48.
The chemical weathering of silicate rocks is closely related to climatic conditions. The CIA values of modern suspended particulate matters from large global rivers are particularly more sensitive to LSTs, the latitude of the river mouth, and the soil depth in the drainage basin than other factors70. Furthermore, a relationship between the CIA and LST as a paleothermometer was quantified as follows18,
which is robust over a temperature range of 3–25 °C and has an uncertainty of approximately ±5 °C, corresponding to a CIA range of approximately 50–90. This LST estimation has been applied to quantify paleotemperatures of several Permian and Triassic terrestrial successions18,48. In this study, we used the CIA and LST proxies to assess changes in chemical weathering and temperature.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The geochronological and geochemical data generated in this study are provided in the Supplementary Dataset files.
References
Forster, P. et al. in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon, S. et al.) 129–134 (Cambridge Univ. Press, 2007).
Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M. & Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 331, 50 (2011).
Bastviken, D., Cole, J., Pace, M. & Tranvik, L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles 18, GB4009 (2004).
Rosentreter, J. A. et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 14, 225–230 (2021).
Saunois, M. et al. The global methane budget 2000–2017. Earth Syst. Sci. Data 12, 1561–1623 (2020).
Borrel, G. et al. Production and consumption of methane in freshwater lake ecosystems. Res. Microbiol. 162, 832–847 (2011).
Aben, R. C. H. et al. Cross continental increase in methane ebullition under climate change. Nat. Commun. 8, 1682 (2017).
Sepulveda-Jauregui, A. et al. Eutrophication exacerbates the impact of climate warming on lake methane emission. Sci. Total Environ. 636, 411–419 (2018).
Zhu, Y. et al. Disproportionate increase in freshwater methane emissions induced by experimental warming. Nat. Clim. Change 10, 685–690 (2020).
Sun, F. et al. Methanogen microfossils and methanogenesis in Permian lake deposits. Geology 49, 13–18 (2021).
Carroll, A. R. & Wartes, M. A. Organic carbon burial by large Permian lakes, northwest China. Geol. Soc. Am. Spec. Pap. 370, 91–104 (2003).
Carroll, A. R., Brassell, S. C. & Graham, S. A. Upper Permian lacustrine oil shales, southern Junggar Basin, Northwest China. AAPG Bull. 76, 1874–1902 (1992).
Carroll, A. R. Upper Permian lacustrine organic facies evolution, Southern Junggar Basin, NW China. Org. Geochem. 28, 649–667 (1998).
Marchetti, L. et al. The Artinskian Warming Event: an Euramerican change in climate and the terrestrial biota during the early Permian. Earth-Sci. Rev. 226, 103922 (2022).
Richey, J. D. et al. Influence of temporally varying weatherability on CO2-climate coupling and ecosystem change in the late Paleozoic. Clim. Past 16, 1759–1775 (2020).
Montañez, I. P. et al. CO2-forced climate and vegetation instability during Late Paleozoic deglaciation. Science 315, 87–91 (2007).
Montañez, I. P. & Poulsen, C. J. The Late Paleozoic ice age: an evolving paradigm. Annu. Rev. Earth Planet. Sci. 41, 629–656 (2013).
Yang, J., Cawood, P. A., Du, Y., Feng, B. & Yan, J. Global continental weathering trends across the Early Permian glacial to postglacial transition: Correlating high- and low-paleolatitude sedimentary records. Geology 42, 835–838 (2014).
Griffis, N. P. et al. Coupled stratigraphic and U-Pb zircon age constraints on the late Paleozoic icehouse-to-greenhouse turnover in south-central Gondwana. Geology 47, 1146–1150 (2019).
Soreghan, G. S., Soreghan, M. J. & Heavens, N. G. Explosive volcanism as a key driver of the late Paleozoic ice age. Geology 47, 600–604 (2019).
Scheffler, K., Hoernes, S. & Schwark, L. Global changes during Carboniferous–Permian glaciation of Gondwana: Linking polar and equatorial climate evolution by geochemical proxies. Geology 31, 605–608 (2003).
Xie, X. et al. Intact microbial fossils in the Permian Lucaogou Formation oil shale, Junggar Basin, NW China. Int. J. Coal Geol. 146, 166–178 (2015).
Liu, B. et al. Depositional environment of oil shale within the second member of Permian Lucaogou Formation in the Santanghu Basin, Northwest China. Int. J. Coal Geol. 175, 10–25 (2017).
Gao, Y. et al. Paleoenvironmental setting, mechanism and consequence of massive organic carbon burial in the Permian Junggar Basin, NW China. J. Asian Earth Sci. 194, 104222 (2020).
Huang, H. et al. Astronomical forcing of Middle Permian terrestrial climate recorded in a large paleolake in northwestern China. Paleogeogr. Paleoclimatol. Paleoecol. 550, 109735 (2020).
Zhao, R. et al. Tectonic evolution of Tianshan-Bogda-Kelameili mountains, clastic wedge basin infill and chronostratigraphic divisions in the source-to-sink systems of Permian-Jurassic, southern Junggar Basin. Mar. Pet. Geol. 114, 104200 (2020).
Yang, W. et al. Depositional environments and cyclo- and chronostratigraphy of uppermost Carboniferous–Lower Triassic fluvial–lacustrine deposits, southern Bogda Mountains, NW China—A terrestrial paleoclimatic record of mid-latitude NE Pangea. Glob. Planet. Change 73, 15–113 (2010).
Dickinson, W. R. & Gehrels, G. E. Use of U–Pb ages of detrital zircons to infer maximum depositional ages of strata: A test against a Colorado Plateau Mesozoic database. Earth Planet. Sci. Lett. 288, 115–125 (2009).
Lu, J. et al. Records of organic carbon isotopic composition (δ13Corg) and volcanism linked to changes in atmospheric pCO2 and climate during the Late Paleozoic Icehouse. Glob. Planet. Change 207, 103654 (2021).
Grossman, E. L. et al. Glaciation, aridification, and carbon sequestration in the Permo-Carboniferous: The isotopic record from low latitudes. Paleogeogr. Paleoclimatol. Paleoecol. 268, 222–233 (2008).
Buggisch, W., Wang, X., Alekseev, A. S. & Joachimski, M. M. Carboniferous–Permian carbon isotope stratigraphy of successions from China (Yangtze platform), USA (Kansas) and Russia (Moscow Basin and Urals). Paleogeogr. Paleoclimatol. Paleoecol. 301, 18–38 (2011).
Pancost, R. D. et al. Archaeol as a methanogen biomarker in ombrotrophic bogs. Org. Geochem. 42, 1279–1287 (2011).
Naeher, S. et al. Tracing the methane cycle with lipid biomarkers in Lake Rotsee (Switzerland). Org. Geochem. 66, 174–181 (2014).
Stiller, M., Rounick, J. S. & Shasha, S. Extreme carbon-isotope enrichments in evaporating brines. Nature 316, 434–435 (1985).
Hollander, D. J. & McKenzie, J. A. CO2 control on carbon-isotope fractionation during aqueous photosynthesis: A paleo-pCO2 barometer. Geology 19, 929–932 (1991).
Irwin, H., Curtis, C. & Coleman, M. Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 269, 209–213 (1977).
Whiticar, M. J., Faber, E. & Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 50, 693–709 (1986).
Kelts, K. & McKenzie, J. A. Diagenetic dolomite formation in Quaternary anoxic diatomaceous muds of Deep Sea Drilling Project Leg 64, Gulf of California. Initial Rep. Deep Sea Drill. Proj. 64, 553–569 (1982).
Meister, P. et al. Dolomite formation within the methanogenic zone induced by tectonically driven fluids in the Peru accretionary prism. Geology 39, 563–566 (2011).
Peckmann, J. & Thiel, V. Carbon cycling at ancient methane–seeps. Chem. Geol. 205, 443–467 (2004).
Freeman, K. H., Hayes, J. M., Trendel, J.-M. & Albrecht, P. Evidence from carbon isotope measurements for diverse origins of sedimentary hydrocarbons. Nature 343, 254–256 (1990).
Pancost, R. D. et al. Increased terrestrial methane cycling at the Palaeocene–Eocene thermal maximum. Nature 449, 332–335 (2007).
Volkman, J. K., Zhang, Z., Xie, X., Qin, J. & Borjigin, T. Biomarker evidence for Botryococcus and a methane cycle in the Eocene Huadian oil shale, NE China. Org. Geochem. 78, 121–134 (2015).
Inglis, G. N. et al. Terrestrial methane cycle perturbations during the onset of the Paleocene–Eocene Thermal Maximum. Geology 49, 520–524 (2021).
Yao, Y. et al. Permafrost thaw induced abrupt changes in hydrology and carbon cycling in Lake Wudalianchi, northeastern China. Geology 49, 1117–1121 (2021).
Collister, J. W. & Wavrek, D. A. δ13C compositions of saturate and aromatic fractions of lacustrine oils and bitumens: evidence for water column stratification. Org. Geochem. 24, 913–920 (1996).
Nesbitt, H. W. & Young, G. M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 299, 715–717 (1982).
Frank, T. D. et al. Pace, magnitude, and nature of terrestrial climate change through the end-Permian extinction in southeastern Gondwana. Geology 49, 1089–1095 (2021).
Harnois, L. The CIW index: A new chemical index of weathering. Sediment. Geol. 55, 319–322 (1988).
Korte, C., Jasper, T., Kozur, H. W. & Veizer, J. δ18O and δ13C of Permian brachiopods: A record of seawater evolution and continental glaciation. Paleogeogr. Paleoclimatol. Paleoecol. 224, 333–351 (2005).
Korte, C., Jones, P. J., Brand, U., Mertmann, D. & Veizer, J. Oxygen isotope values from high-latitudes: Clues for Permian sea-surface temperature gradients and Late Palaeozoic deglaciation. Paleogeogr. Paleoclimatol. Paleoecol. 269, 1–16 (2008).
Yvon-Durocher, G. et al. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 507, 488–491 (2014).
Zeikus, J. G. & Winfrey, M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl. Environ. Microbiol. 31, 99–107 (1976).
Marotta, H. et al. Greenhouse gas production in low-latitude lake sediments responds strongly to warming. Nat. Clim. Change 4, 467–470 (2014).
Van de Wetering, N., Esterle, J. S., Golding, S. D., Rodrigues, S. & Götz, A. E. Carbon isotopic evidence for rapid methane clathrate release recorded in coals at the terminus of the Late Palaeozoic Ice Age. Sci. Rep. 9, 16544 (2019).
Thompson, J. M., Meffre, S. & Danyushevsky, L. Impact of air, laser pulse width and fluence on U–Pb dating of zircons by LA-ICPMS. J. Anal. Spectrom. 33, 221–230 (2018).
Jackson, S. E., Pearson, N. J., Griffin, W. L. & Belousova, E. A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem. Geol. 211, 47–69 (2004).
Paton, C. et al. Improved laser ablation U‐Pb zircon geochronology through robust downhole fractionation correction. Geochem. Geophys. Geosyst. 11, Q0AA06 (2010).
Ludwig, K. R. Isoplot v. 4.15: a geochronological toolkit for Microsoft Excel. Berkeley Geochron. Cent. Sp. Publ. 4, 75 (2011).
Ramezani, J. et al. High-precision U-Pb zircon geochronology of the Late Triassic Chinle Formation, Petrified Forest National Park (Arizona, USA): Temporal constraints on the early evolution of dinosaurs. Geol. Soc. Am. Bull. 123, 2142–2159 (2011).
Mattinson, J. M. Zircon U–Pb chemical abrasion (“CA-TIMS”) method: Combined annealing and multi-step partial dissolution analysis for improved precision and accuracy of zircon ages. Chem. Geol. 220, 47–66 (2005).
Condon, D. J., Schoene, B., McLean, N. M., Bowring, S. A. & Parrish, R. R. Metrology and traceability of U–Pb isotope dilution geochronology (EARTHTIME Tracer Calibration Part I). Geochim. Cosmochim. Acta 164, 464–480 (2015).
McLean, N. M., Condon, D. J., Schoene, B. & Bowring, S. A. Evaluating uncertainties in the calibration of isotopic reference materials and multi-element isotopic tracers (EARTHTIME Tracer Calibration Part II). Geochim. Cosmochim. Acta 164, 481–501 (2015).
Bowring, J. F., McLean, N. M. & Bowring, S. A. Engineering cyber infrastructure for U‐Pb geochronology: Tripoli and U‐Pb_Redux. Geochem. Geophys. Geosyst. 12, Q0AA19 (2011).
McLean, N. M., Bowring, J. F. & Bowring, S. A. An algorithm for U‐Pb isotope dilution data reduction and uncertainty propagation. Geochem. Geophys. Geosyst. 12, Q0AA18 (2011).
Jaffey, A. H., Flynn, K. F., Glendenin, L. E., Bentley, W. C. & Essling, A. M. Precision measurement of half-lives and specific activities of 235U and 238U. Phys. Rev. C 4, 1889–1906 (1971).
McLennan, S. M. Weathering and global denudation. J. Geol. 101, 295–303 (1993).
Wang, P. et al. The chemical index of alteration (CIA) as a proxy for climate change during glacial-interglacial transitions in Earth history. Earth-Sci. Rev. 201, 103032 (2020).
Fedo, C. M., Nesbitt, H. W. & Young, G. M. Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 23, 921–924 (1995).
Li, C. & Yang, S. Is chemical index of alteration (CIA) a reliable proxy for chemical weathering in global drainage basins? Am. J. Sci. 310, 111–127 (2010).
Wartes, M. A., Carroll, A. R. & Greene, T. J. Permian sedimentary record of the Turpan-Hami basin and adjacent regions, northwest China: Constraints on postamalgamation tectonic evolution. Geol. Soc. Am. Bull. 114, 131–152 (2002).
Cohen, K. M., Finney, S. C., Gibbard, P. L. & Fan, J.-X. The ICS International Chronostratigraphic Chart. Episodes 36, 199–204 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant 41830425 to W.H.) and the Second Tibetan Plateau Scientific Expedition and Research (grant 2019QZKK0706 to S.S.). We thank Shengchao Yang, Xun Kang, Wenjie Zhang and Yifan Yu for assistance in the field; Yan Wang and Jinchao Liu for assistance with TOC and Rock-Eval pyrolysis analyses; and Xianwei Wu for assistance with LA-ICP-MS analysis.
Author information
Authors and Affiliations
Contributions
F.S. and W.H. designed and conceived the study. F.S. collected the samples and performed the geochemical and U-Pb LA-ICP-MS analyses. J.R. performed the U-Pb CA-ID-TIMS analyses. F.S. and W.H. wrote the manuscript with contributions from J.C., X.W., Z.Z., J.R. and S.S. All authors contributed to the interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, F., Hu, W., Cao, J. et al. Sustained and intensified lacustrine methane cycling during Early Permian climate warming. Nat Commun 13, 4856 (2022). https://doi.org/10.1038/s41467-022-32438-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32438-2
This article is cited by
-
Seawater sulphate heritage governed early Late Miocene methane consumption in the long-lived Lake Pannon
Communications Earth & Environment (2023)
-
The past is key for the future of our freshwater systems
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.