Abstract
Innate lymphoid cells (ILCs) exert important roles in host defense, tissue repair and inflammatory diseases. However, how ILC lineage specification is regulated remains largely elusive. Here we identify that circular RNA circTmem241 is highly expressed in group III innate lymphoid cells (ILC3s) and their progenitor cells. CircTmem241 deficiency impairs ILC3 commitment and attenuates anti-bacterial immunity. Mechanistically, circTmem241 interacts with Nono protein to recruit histone methyltransferase Ash1l onto Elk3 promoter in ILC progenitor cells (ILCPs). Ash1l-mediated histone modifications on Elk3 promoter enhance chromatin accessibility to initiate Elk3 transcription. Of note, circTmem241−/−, Nono−/− and Ash1l−/− ILCPs display impaired ILC3 differentiation, while Elk3 overexpression rescues ILC3 commitment ability. Finally, circTmem241−/−Elk3−/− mice show lower numbers of ILC3s and are more susceptible to bacterial infection. We reveal that the circTmem241-Nono-Ash1l-Elk3 axis is required for the ILCP differentiation into ILC3P and ILC3 maturation, which is important to manipulate this axis for ILC development on treatment of infectious diseases.
Similar content being viewed by others
Introduction
Innate lymphoid cells (ILCs) are heterogeneous populations of lymphocytes that react rapidly to environmental cues and exert critical roles in regulating mucosal immunity, lymphoid organogenesis, and tissue homeostasis1. ILC subsets mirror T helper (Th) cells based on transcription factors (TFs) and secreted cytokines. ILC1s express T-bet and secret IFN-γ to function in the immune response against intracellular pathogens2. ILC2s express Gata3 and secret Th2-like cytokines such as IL-5 and IL-13, which play an important role in allergic response and innate immunity to helminth3. ILC3s express RORγt and mainly produce IL-22 and/or IL-17 after activation4. All ILC subsets are developed from common lymphoid progenitors (CLPs), which give rise to common innate lymphoid progenitors (CILPs), then diverge into common helper innate lymphoid progenitors (CHILPs) and NK cell precursors (NKPs). CHILPs can then differentiate into lymphoid tissue inducer progenitors (LTiPs) and innate lymphoid cell precursors (ILCPs), which finally produce all groups of ILCs5,6. The commitment of ILCs is finely modulated by fate-decision TFs. Expression of inhibitor of DNA binding 2 (Id2) is required for establishing ILC cell fate from CLPs by repressing E-box protein activity7,8. Id2+ CHILPs give rise to more restricted ILCPs via expression of transcription factor PLZF (encoded by Zbtb16 gene) and possess the ability to produce all ILC subsets7,8. Recent studies have identified specific precursors of each ILC subsets9,10. ILC1 precursors (ILC1Ps, Lin−CD127+Eomes−CD49a+NK1.1+NKp46+) require T-bet, Nifl3 and Gata3 for ILC1 differentiation11. Transcription factors such as Bcl11b and Gata3 are involved in ILC2 commitment from ILC2 precursors (ILC2Ps, Lin−CD127+CD45+Flt3−CD117−Sca-1+CD25+Gata3+) to mature ILC2s9,12,13. Whereas ILC3 precursors (ILC3Ps, Lin−CD25−CD127+α4β7intGata3loBcl11b+Id2+RORγt+) drive ILC3-restricted lineage specification14. ILC3 commitment and function are finely regulated by various aspects. Extrinsic clues and TFs like Gata3 and Rorc are directly involved in ILC3 fate decision15,16,17. We previously showed that lncKdm2b activates Zfp292 expression to promote the maintenance and effector functions of ILC3s18. We also found that IL-7Rα glutamylation is required for ILC3 specification19.
Circular RNAs (circRNAs) are generated from precursor mRNA through back-splicing events, which are characterized by a covalent bond linking the 3′- and 5′-ends20. CircRNAs comprise exons, introns, or both. Compared to linear RNAs, circRNAs have a longer half-life and resist exonuclease degradation21,22. CircRNAs exist in various types of tissues and cells and exert critical roles in many biological processes, including development, stemness maintenance, and tumorigenesis. For example, circRNA Cdr1as regulates brain function via sponging miR-7 and miR-67123. Fusion circRNAs derived from transcribed exons of some genes promote tumorigenesis and enhance resistance against therapy24. A recent report showed that endogenous circRNAs may form imperfect RNA duplexes to suppress activation of PKR, resulting in inhibition of innate immunity25. We previously demonstrated that circRNA cia-cGAS is highly expressed in hematopoietic stem cells (HSCs) and maintains HSC homeostasis via blocking nuclear cGAS enzymatic activity26. We also showed that circRNA circKcnt2 facilitates colitis resolution by inhibiting ILC3 activation27. However, how circRNAs regulate ILC differentiation still remains elusive.
ETS domain-containing protein Elk3 (Elk3) belongs to the ETS-domain transcription factor family and the ternary complex factor (TCF) subfamily. Elk3 is well known to regulate the early response to growth factor stimulation in quiescent cells28. Elk3 is a strong transcriptional repressor and is involved in several biological processes such as angiogenesis and tumorigenesis29. A previous study showed that phosphorylation of Elk3 switches it to a transcriptional activator, leading to induction of VEGF expression and angiogenesis28. Elk3 can regulate chromatin landscape and initiate the progression of squamous cell carcinomas30. Importantly, Elk3 also plays an essential role in the development of neural cells31. Moreover, several studies revealed that Elk3 is a potential regulator of immune response32,33. Elk3 inhibits HO-1 and NOS2 expression under LPS or cytokine stimulation and regulates HO-1-mediated inflammatory response in macrophages32,34. In addition, Elk3 displays different expression patterns in CD4+ and CD8+ T cells that regulate immune response mediating tumorigenesis of colon cancer35. However, the role of Elk3 in ILC biology is still unclear. Here we identified an undefined circular RNA circTmem241 (derived from Tmem241 gene transcript, mmu_circ_0007131 in circBase) that is highly expressed in ILC3s and their progenitor cells CHILPs and ILCPs. CircTmem241 regulates ILC3 specification at the ILCP stage, and the circTmem241-Nono-Ash1l-Elk3 axis is required for ILC3 differentiation.
Results
CircTmem241 knockout reduces ILC3 numbers
We previously performed circRNA microarray analysis of ILC3s from Rag1−/− mice and identified differentially expressed circRNAs in ILC3s related to innate colitis27. To explore the role of circRNAs in ILC3 commitment, we selected the top 10 highly expressed circRNAs in Rag1−/− ILC3s that were conserved between mice and humans, whose circular characteristics were validated by PCR (Supplementary Fig. 1a, b), and Sanger sequencing (Supplementary Fig. 1c). These ten circRNAs were resistant to RNase R digestion (Supplementary Fig. 1d). We next knocked them down in CHILPs using lentivirus (Supplementary Fig. 1e) and conducted in vitro differentiation assay. Knockdown of circRNAs did not affect their expression levels of their parental genes (Supplementary Fig. 1f). Of these ten circRNAs, circTmem241 (circBase ID: mmu_circ_0007131) knockdown most significantly impaired ILC3 differentiation (Fig. 1a). CircTmem241 is generated by back-splicing of Tmem241 transcript from exon 8 to exon 14 and conserved across various species (Supplementary Fig. 2a, b). Human ortholog circTMEM241 was validated in human ILC3 by DNA sequencing (Supplementary Fig. 2c), indicating circTmem241 was highly conserved between mice and humans. We observed that circTmem241 was highly expressed in some tissues, especially in the bone marrow and digestive track (Fig. 1b). Among lymphocyte progenitors and immune cells tested, circTmem241 was most highly expressed in CHILPs, ILCPs, and intestinal ILC3s (Fig. 1c), which was further confirmed by fluorescence in situ hybridization (FISH) assay (Supplementary Fig. 2d). In addition, circTmem241 was mainly located in the nuclei of CHILPs, ILCPs, and ILC3s (Supplementary Fig. 2e).
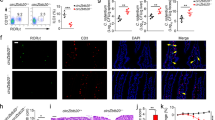
a Top 10 highly expressed circRNAs were knocked down in CHILPs, followed by in vitro differentiation assay. ILC3s (CD3−CD19−CD127+CD45lo RORγt+) were analyzed by FACS. n = 5 for each group. b CircTmem241 expression levels of indicated tissues were detected by Northern blot using biotin-labeled probes. 18 S RNA was used as a loading control. c Relative expression of circTmem241 was measured in indicated hematopoietic progenitors and mature cells by qPCR. Fold changes were normalized to endogenous 18 S. HSC (Lin−c-Kit+Sca-1+CD150+CD48−), MPP (Lin−c-Kit+Sca-1+CD150−CD48+), CLPs (Lin−CD127+c-KitintSca-1intFlt3+α4β7−), CHILPs (Lin−CD25−CD127+Flt3−α4β7+Id2GFP) and ILCPs (Lin−CD127+Flt3−c-Kit+α4β7+PLZFGFP) were isolated from BM cells. ILC1s (CD3−CD19−CD45+CD127+NK1.1+NKp46+), ILC2s (Lin−CD127+CD90+KLRG1+Sca1+) and ILC3s (CD3−CD19−CD127+CD45lo CD90hi) were isolated from small intestinal lamina propria. T cells (CD3+CD45+) were isolated from thymus. B cells (NK1.1−CD19+CD45+) and NK cells (NK1.1+CD19−CD45+) were isolated from spleen. n = 3 biological independent experiments. d–f ILC1s (CD3−CD19−CD45+CD127+ gated), ILC2s (CD3−CD19−CD127+CD90+ gated), and ILC3s (CD3−CD19−CD45low gated) were analyzed in small intestines of circTmem241+/+ and circTmem241−/− mice by FACS. n = 5 for each group. g Numbers of indicated ILCs in d–f were calculated. n = 5 for each group. h Immunofluorescence staining of ILC3s in small intestines from circTmem241+/+ and circTmem241−/− mice. Yellow arrows indicate ILC3s. Scale bar, 100 μm. i Lamina propria lymphocytes (LPLs) from circTmem241+/+ and circTmem241−/− mouse intestines were sorted and stimulated by IL-23 for 4 h, followed by IL-22+ ILC3 detection with FACS. n = 5 for each group. j IL-22 levels secreted by ILC3s from circTmem241+/+ and circTmem241−/− intestines were assayed by ELISA after stimulation by IL-23 for 24 h. n = 3 for each group. k Body weight changes of circTmem241+/+ and circTmem241−/− mice were analyzed after C. rodentium infection. n = 5 for each group. l Bacterial counts (CFUs) in feces of circTmem241+/+ and circTmem241−/− mice were measured after infection with C. rodentium for 6 days. n = 5 for each group. m Colon tissues from circTmem241+/+ and circTmem241−/− mice were analyzed by H&E staining. Scale bars, 100 μm. n Histopathological scores of colons from circTmem241+/+ and circTmem241−/− mice over C. rodentium infection. n = 5 for each group. **P < 0.01 and ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
To further determine the physiological role of circTmem241 in the regulation of ILC3 commitment and function, we sought to generate circTmem241-deficient mice. The complementary elements flanking circRNA sequences are essential for their generation21. We screened out the complementary sequences in the introns flanking circTmem241 and constructed plasmids for minigene assay (Supplementary Fig. 3a). We next generated circTmem241 knockout (circTmem241−/−) mice by deleting the downstream complementary element in the genome through CRISPR/Cas9 technology (Supplementary Fig. 3b). CircTmem241 deletion was validated in circTmem241−/− mice via PCR and qPCR analyses (Supplementary Fig. 3c, d). Of note, circTmem241 deletion did not affect the expression of its maternal gene Tmem241 (Supplementary Fig. 3d, e). We found that circTmem241−/− mice displayed comparable numbers of ILC1s and ILC2s in isolated intestinal lamina propria lymphocytes compared to circTmem241+/+ littermate control mice (Fig. 1d, e). However, circTmem241 deletion decreased intestinal ILC3 percentages and numbers (Fig. 1f, g), which was further verified by in situ immunofluorescence (IF) staining (Fig. 1h). Moreover, all three subsets of ILC3s displayed decreased numbers in circTmem241-deficient mice (Supplementary Fig. 3f). Consistently, circTmem241-deficient mice showed impaired development of gut-associated lymphoid tissues (Supplementary Fig. 3g). ILC3s are essential for gut homeostasis and bacterial defense via secreting IL-221. As expected, circTmem241 deletion decreased IL-22 positive ILC3s after Citrobacter rodentium infection and IL-22 production after IL-23 stimulation (Fig. 1i, j). Moreover, circTmem241−/− mice were accompanied by greater feces bacteria load, more significant body weight loss, and severer Intestinal injury after C. rodentium infection than circTmem241+/+ littermates (Fig. 1k–n). Taken together, circTmem241 is highly expressed in ILC3s and their progenitors and its deficiency reduces ILC3 numbers but not ILC1s or ILC2s.
CircTmem241 drives ILC3 specification from the ILCP progenitor stage
The reduced number of ILC3s might be caused by increased cell death and/or decreased development of cell populations. We then assessed the turnover rate of ILC3s in vivo. BrdU was intraperitoneally injected into mice at 100 mg/kg for 18 h, followed by detection of BrdU uptake by FACS. We found that ILC3s derived from circTmem241−/− mice showed comparable BrdU uptake to those from littermate WT control mice (Supplementary Fig. 4a). Consistently, percentages of Ki67+ ILC3s in circTmem241−/− mice displayed similar levels compared with those of circTmem241+/+ mice (Supplementary Fig. 4b). In addition, circTmem241 deficiency didn’t affect death rates of ILC3s (Supplementary Fig. 4c), indicating that circTmem241 does not affect cell proliferation and survival of ILC3s. To further determine the role of circTmem241 deficiency on ILC3 reduction in vivo, we compared hematopoietic progenitor cells by FACS in circTmem241−/− mice versus circTmem241+/+ littermate control mice. We noted that circTmem241 deficiency did not affect the percentage and number of ILC progenitors, including CLPs, α4β7+ CLPs, CHILPs, and ILCPs (Fig. 2a–d). ILCPs diversify into specific precursors of different ILC subsets before maturation36,37. We observed that circTmem241 deficiency caused decreased percentages and numbers in ILC3Ps but not ILC1Ps or ILC2Ps (Fig. 2e–h). We next isolated CHILPs or ILCPs for in vitro differentiation assay and found that circTmem241 knockout impaired ILC3P and ILC3 differentiation but not ILC1 or ILC2 (Fig. 2i, j and Supplementary Fig. 4d, e). Ectopic expression of circTmem241 in circTmem241−/− CHILPs or ILCPs was able to rescue the differentiation ability of CHILPs or ILCPs toward ILC3Ps and ILC3s (Fig. 2i, j). Altogether, circTmem241 is required for ILC lineage commitment from ILCPs to ILC3Ps.
a–c FACS analysis of circTmem241+/+ and circTmem241−/− ILC lineage progenitors. Plots displayed frequencies of CLPs (Lin−CD127+c-KitintSca-1int gated), α4β7+CLPs (Lin−CD127+c-KitintSca-1int gated), CHILPs (Lin−CD25−CD127+Flt3− gated) and ILCPs (Lin−CD127+Flt3−c-Kit+ gated). n = 5 for each group. ns, no significance. d Numbers of indicated progenitor cells in a–c were calculated. n = 5 for each group. ns, no significance. e–g Precursor cells ILC1P (Lin−CD127+Eomes−CD49a+ gated), ILC2P (Lin−CD127+CD45+Flt3−CD117−Sca-1+ gated) and ILC3P (Lin−CD25−CD127+α4β7intGata3lowBcl11b+ gated) were analyzed in circTmem241+/+ and circTmem241−/− mice by FACS. n = 5 for each group. h Numbers of indicated ILC precursor cells in e–g were calculated. n = 5 for each group. ns, no significance. i–j CHILPs (Lin−CD25−CD127+Flt3−α4β7+) or ILCPs (Lin−CD25−CD127+Flt3−α4β7+c-Kit+PD-1+) were sorted from circTmem241+/+ and circTmem241−/− mice and cultured under in vitro differentiation condition for 7 or 14 days. Flow cytometric analysis of indicated cells was performed and absolute numbers were calculated. n = 5 for each group. **P < 0.01 and ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
CircTmem241 regulates ILC3 commitment in an intrinsic manner
To further determine whether the role of circTmem241 in ILC3 commitment is intrinsic or extrinsic, we conducted two groups of bone marrow transplantation experiments (Fig. 3a). For non-competitive bone marrow transplantation, we transplanted 5 × 106 CD45.2+ circTmem241−/− or circTmem241+/+ mouse BM cells into lethally irradiated CD45.1+ recipients. After 8 weeks, we found that recipients reconstituted with CD45.2+ circTmem241−/− BM cells decreased ILC3 numbers (Fig. 3b). For competitive bone marrow transplantation assays, we transferred 1:1 ratio mixture of CD45.1+ WT and CD45.2+ circTmem241+/+ or circTmem241−/− BM cells into lethally irradiated recipient mice for 8 weeks. Recipients reconstituted with circTmem241−/− BM cells showed reduced ILC3 numbers compared to those reconstituted with circTmem241+/+ BM cells (Fig. 3c). Consequently, circTmem241−/− BM cell reconstituted recipients were also susceptible to C. rodentium infection (Fig. 3d–f). These data indicate that circTmem241 regulates ILC3 commitment and anti-bacterial immunity in an intrinsic manner.
a Schematic diagram of bone marrow transplantation. b 5 × 106 CD45.2+ BM cells from circTmem241+/+ or circTmem241−/− mice were transferred into lethally irradiated CD45.1+ recipients. 8 weeks later, ILC3s (Lin−CD45.2+CD127+RORγt+) were analyzed. n = 5 for each group. c 1:1 mixture of CD45.1+ wild-type and CD45.2+ circTmem241+/+ or circTmem241−/− BM cells were transferred into lethally irradiated CD45.1+ recipient mice for 8 weeks. Then ratios of CD45.1+ to CD45.2+ ILC3s in chimeras were analyzed by gating on CD45.2+Lin−RORγt+ (circTmem241+/+ or circTmem241−/−) and CD45.1+Lin−RORγt+ (WT). n = 5 for each group. d Irradiated recipient mice were reconstructed with circTmem241+/+ or circTmem241−/− BM cells as indicated in a (left). Colons isolated from reconstructed mice infected with C. rodentium were subjected to HE staining. Scale bars, 100 μm. e Histological scores of colons from reconstructed mice in d. n = 5 for each group. f Reconstituted mice, as indicated in d, were infected with C. rodentium and survival rates were measured. N = 10 for each group. **P < 0.01 and ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
CircTmem241 promotes Elk3 expression
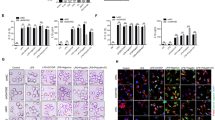
To explore the molecular mechanism of circTmem241 in the regulation of ILC3 commitment, we crossed circTmem241−/− mice with PLZFGFP mice and isolated ILCPs from PLZFGFP or PLZFGFPcircTmem241−/− mice for transcriptome analysis. We found that circTmem241 deletion caused downregulation of many TFs (Fig. 4a). Among the top 10 downregulated TFs, Elk3 was most downregulated (Fig. 4b). Furthermore, knockdown of Elk3 significantly decreased percentages of ILC3s by in vitro differentiation assay (Fig. 4c and Supplementary Fig. 4f). Elk3 downregulation in circTmem241−/− ILCPs was also confirmed by Western blot (Fig. 4d). Elk3 overexpression could rescue the reduction of ILC3s by in vitro differentiation assay, indicating Elk3 played a critical role in circTmem241-mediated ILC3 commitment (Supplementary Fig. 4g, h). Through chromatin isolation by RNA purification (CHIRP) assay, we observed that circTmem241 was enriched on the promoter region (−200~0) of Elk3 (Fig. 4e). We also found that circTmem241 bound to the segment (−200~0) of Elk3 promoter via hybridization with biotinylated circTmem241 RNA probe (Fig. 4f). This result was further validated by luciferase assay (Fig. 4g). These data suggest that circTmem241 binds to Elk3 promoter to regulate its transcription.
a Heatmap of top ten downregulated TFs in circTmem241−/− ILCPs versus circTmem241+/+ ILCPs by transcriptome analysis. b Relative expression levels of top 10 TFs in circTmem241+/+ and circTmem241−/− ILCPs were analyzed by qRT-PCR. Fold changes were normalized to endogenous Gapdh. n = 3 biological independent experiments. c Top 10 downregulated TFs were knocked down in ILCPs were analyzed by FACS. n = 3 biological independent experiments. d Elk3 protein levels in circTmem241+/+ or circTmem241−/− ILCPs by western blotting. e Enrichment of circTmem241 on Elk3 gene promoter was analyzed by CHIRP assay. CircTmem241 probe was biotin labeled. n = 4 for each group. f Enrichment of circTmem241 on Elk3 gene promoter was further validated by dot blot. g Luciferase reporter assay was performed to validate circTmem241 function on Elk3 transcription activation. FL, full-length (represent −2000~0 region upstream Elk3 transcriptional start site). n = 4 for each group. h, i CircTmem241 hairpin region (HR) sequence mutations were incubated with Elk3 promoter regions, followed by dot blot. j DNase I assay of chromatin accessibility in Elk3 promoters of circTmem241+/+ or circTmem241−/− ILCPs by qRT-PCR. n = 3 for each group. k Transcription activities of Elk3 in circTmem241+/+ or circTmem241−/− ILCPs were measured by nuclear run-on assay. n = 4 for each group. l ILCPs from circTmem241+/+ or circTmem241−/− mice were infected with virus expressing circTmem241-WT or circTmem241-mut, followed by in vitro differentiation assay. ILC3s (CD3−CD19−CD127+CD45loRORγt+) were analyzed by FACS. n = 5 for each group. WT, wild-type circTmem241; mut, circTmem241 mutation. *P < 0.05, **P < 0.01, and ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
Previous studies have revealed that circRNA loops are involved in the interaction of circular RNAs20. We predicted the secondary structure of circTmem241 with a bioinformatics tool and identified four loop regions of circTmem241 transcript (Supplementary Fig. 5a). To determine which loop (HR1 (hairpin region #1, HR1), HR2, HR3, and HR4) was required for the association between circTmem241 transcript and Elk3 promoter, we constructed several circTmem241 mutations to abrogate loop structures (Supplementary Table 1). We found that HR1 was required for their interaction (Fig. 4h). Through sequence alignment, we noticed that pairing complementary bases existed in the Elk3 promoter (−200~0) versus the HR1 of circTmem241 (Supplementary Fig. 5b). We generated the mutations of circTmem241 transcript to delete their base pairing bases (circTmem241-mut), followed by a hybridization assay. We found that circTmem241-mut really abolished the interaction between circTmem241 transcript and Elk3 promoter (Fig. 4i). In addition, substitution mutation in Elk3 promoter pairing region failed to activate luciferase activity by circTmem241 overexpression (Supplementary Fig. 5c). Through DNase I accessibility assay, we found that Elk3 promoter in circTmem241−/− ILCPs was less susceptible to DNase I digestion (Fig. 4j). Consistently, circTmem241 deficiency caused attenuated transcription of Elk3 mRNA by nuclear run-on assay (Fig. 4k). To further confirm the important role of the pairing bases, we conducted in vitro differentiation assay using ILCPs from circTmem241+/+ or circTmem241−/− mice. We observed that only overexpression of circTmem241-WT was able to rescue ILC3 differentiation (Fig. 4l). Altogether, circTmem241 binds to Elk3 promoter to promote its expression, leading to the commitment of ILC3s.
CircTmem241 associates with Nono in ILCPs
To further explore how circTmem241 regulates Elk3 transcription in ILCPs, we conducted RNA-pulldown assay using biotin-labeled circTmem241 transcripts to pulldown their interacting components from bone marrow cells, followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), silver staining, and mass spectrometry. We found that circTmem241 bound to Nono from bone marrow lysates (Fig. 5a and Supplementary Fig. 5d). We further validated the interaction of circTmem241 with Nono via CHIRP assay and immunoblotting analysis (Fig. 5b, c). In addition, circTmem241 was enriched by anti-Nono antibody through RNA immunoprecipitation assay (Fig. 5d). Moreover, the interaction between circTmem241 and Nono was further validated by electrophoretic mobility shift assay (EMSA) assay (Fig. 5e). Consistently, circTmem241 was co-localized with Nono in ILCP cells (Fig. 5f).
a BM cells from WT mice were lysed and incubated with in vitro transcribed linear biotin-labeled circTmem241 transcripts, antisense, or scramble control. Pulldown components were separated by SDS-PAGE and followed by silver staining. Binding proteins of circTmem241 were identified via mass spectrometry. b BM cell lysates were incubated with circTmem241 probes or controls, followed by Western blotting. c Association between circTmem241 and Nono was validated by CHIRP assay, followed by immunoblotting. d RNA immunoprecipitation (RIP) assay was conducted using anti-Nono antibody or IgG through BM cell lysates and circTmem241 enrichment was detected by qRT-PCR. n = 3 for each group. e EMSA was performed using biotin-labeled circTmem241 transcripts and recombinant Nono proteins with or without anti-Nono antibody. f CircTmem241 was colocalized with Nono in ILCPs by immunofluorescence staining and confocal imaging. Scale bar, 10 μm. g RNA-pulldown assay was performed using indicated truncations of linear biotin-labeled circTmem241 RNAs with BM cell lysates. Interaction of Nono with indicated circTmem241 mutants was analyzed by western blotting. h Validation of binding ability of circTmem241 with indicated Nono truncations by RNA-pulldown assay, followed by western blotting. i Luciferase reporter assay was conducted to validate Nono function on Elk3 transcription activation. n = 3 for each group. j Transcription activity of Elk3 in Nono+/+ or Nono−/− ILCPs was measured by nuclear run-on assay. n = 3 for each group. ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
To further determine which region of circTmem241 transcript was required for its interaction with Nono, we performed RNA-pulldown assay using biotin-labeled truncated circTmem241 transcripts. We observed that the 161–240 region truncation of circTmem241 transcript (Δ161–240) abolished the association between circTmem241 and Nono (Fig. 5g). In addition, the N-terminal RNA recognition motif (RRM) of Nono protein was responsible for its interaction with circTmem241 (Fig. 5h). Nono overexpression significantly enhanced luciferase activity of Elk3 promoter (Fig. 5i). Moreover, Elk3 transcription was remarkably attenuated after Nono knockout (Fig. 5j and Supplementary Fig. 5e, f). Collectively, circTmem241 directly interacts with Nono protein in ILCPs.
CircTmen241 recruits histone methyltransferase Ash1l onto Elk3 promoter to trigger its transcription
We next sought to explore how circTmem241 regulated Elk3 transcription. We used anti-Nono antibody to go through bone marrow cell lysates for immunoprecipitation assay. Precipitated candidates were separated by SDS-PAGE, followed by silver staining and mass spectrometry. A major differential band was identified to be the histone methyltransferase Ash1l (Fig. 6a and Supplementary Fig. 6a). The interaction of Nono with Ash1l was validated by co-IP assay (Fig. 6b). By RNA-pulldown assay, circTmem241 failed to pulldown Ash1l from Nono deleted bone marrow lysates (Fig. 6c). Consistently, circTmem241 didn’t interact with Ash1l directly by in vitro binding assay (Fig. 6d), suggesting that Nono acted as an adaptor protein for the association of circTmem241 with Ash1l. Of note, Nono co-localized with Elk3 promoter in WT ILCPs but not in circTmem241−/− ILCPs (Fig. 6e). Moreover, with cross-linking treatment, Ash1l was co-eluted with Elk3 promoter in circTmem241+/+ bone marrow cell lysates, but not in circTmem241−/− cell lysates (Fig. 6f).
a Immunoprecipitation assay was performed using BM cells from WT mice with anti-Nono antibody or IgG. Eluted fractions were resolved by SDS-PAGE, followed by silver staining and mass spectrometry. b BM cell lysates were incubated with anti-Nono antibody or IgG, followed by western blotting. c RNA-pulldown assay using biotin-labeled circTmem241 transcripts with lysates from Nono+/+ and Nono−/− bone marrow cells. d Interaction between circTmem241 and Ash1l was measured by in vitro binding assay. e DNA FISH showed that Elk3 promoter co-localized with Nono and circTmem241. Scale bar, 2 μm. f BM cells from circTmem241+/+ and circTmem241−/− mice were lysed and treated with 1% formaldehyde for cross-linking. Anti-Nono antibody was incubated with treated lysates for ChIP assays, followed by size fractionation with sucrose gradient ultracentrifugation. Eluate gradients were examined by Western blotting and PCR assay. g Enrichment of Ash1l on Elk3 gene promoter was analyzed by ChIP assay with anti-Ash1l antibody. n = 3 for each group. h, i Enrichment of indicated histone modifications on Elk3 promoter was analyzed by ChIP assay. n = 3 for each group. j Enrichment of H3K4me3 on the Elk3 promoter in indicated ILC progenitor cells (Lin−CD127+c-KitintSca-1intα4β7+) was examined. n = 3 for each group. k WT, Nono-deficient, or Nono- and circTmem241-deficient ILCPs were subjected to nuclear run-on assay, followed by RT-PCR analysis. n = 3 for each group. l, m Relative mRNA l and protein m levels of Elk3 in indicated ILCPs were analyzed. n = 3 for each group in l. ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
Ash1l is mainly responsible for H3K4me3 and H3K36me3 modifications that promote chromatin accessibility for gene transcription38. Through Chromatin immunoprecipitation (ChIP) assay with indicated antibodies, Ash1l was enriched on Elk3 promoter region (−200~0) and circTmem241 knockout decreased H3K4me3 and H3K36me3 modification levels (Fig. 6g–i). These data suggest that circTmem241 recruits Ash1l onto Elk3 promoter that facilitates its histone methylation modifications in ILCPs. To further test whether Nono and Ash1l regulated Elk3 transcription, we deleted Ash1l via AAV delivery of guide RNAs in ILCPs from Vav-Cre;Cas9-KI mice (Supplementary Fig. 6b, c). We found that Elk3 promoter was less enriched with H3K4me3 and H3K36me3 modifications in Nono−/− and Nono−/−circTmem241−/− ILCPs (Fig. 6j and Supplementary Fig. 6d). In addition, Elk3 mRNA was less transcribed in Nono−/− and Nono−/−circTmem241−/− ILCP cells by nuclear run-on assay (Fig. 6k). Consequently, Elk3 expression was remarkably inhibited in Nono−/− and Nono−/−circTmem241−/− ILCPs (Fig. 6l, m). In parallel, Ash1l knockout also exhibited less enriched histone modifications and dramatically attenuated Elk3 expression (Fig. 6j–m and Supplementary Fig. 6d). Taken together, circTmem241 recruits the histone methyltransferase Ash1l to initiate Elk3 transcription in a Nono-dependent manner.
Elk3 deficiency impairs ILC3 specification and anti-bacterial immunity
We next established Elk3-deficient mice via a CRISPR/Cas9 approach (Supplementary Fig. 6e–g). We performed a series of in vitro differentiation assays to verify the circTmem241-Nono-Ash1l-Elk3 axis in ILC3 commitment. We found that Elk3−/− ILCPs showed decreased ILC3P and ILC3 numbers (Fig. 7a, b). By contrast, Elk3 overexpression in Elk3−/− ILCPs was able to rescue ILC3 numbers (Fig. 7b). Of note, deletion of Nono or Ash1l in ILCPs suppressed the differentiation of ILC3s, which could be rescued by Nono, Ash1l or Elk3 overexpression (Fig. 7c). Moreover, ILCPs of circTmem241−/− or circTmem241−/− Elk3−/− mice showed significantly attenuated ability to differentiate into ILC3s, which could be partially rescued by Elk3 overexpression (Fig. 7d). Notably, circTmem241 overexpression in circTmem241−/−Elk3−/− ILCPs failed to restore ILC3 numbers, while overexpression of both circTmem241 and Elk3 almost fully rescued ILC3 differentiation ability (Fig. 7d). Taken together, the circTmem241-Nono-Ash1l-Elk3 axis is required for ILC3 differentiation.
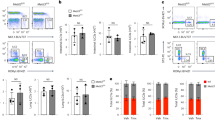
a–d Indicated ILCPs were isolated and conducted in vitro differentiation assay with OP9-DL1 feeder cells. ILC3Ps were analyzed 7 days later and ILC3s were analyzed 14 days later by FACS. n = 5 for each group. e–g ILC1s (CD3−CD19−CD45+CD127+ gated), ILC2s (CD3−CD19−CD127+CD90+ gated) and ILC3s (CD3−CD19−CD45lo gated) were analyzed in small intestines of Elk3+/+ and Elk3−/− mice by FACS. n = 5 for each group. h Numbers of indicated ILCs in e–g were calculated. n = 5 for each group. i LPLs from Elk3+/+ and Elk3−/− mouse intestines were stimulated by IL-23 for 4 h, followed by IL-22+ ILC3 detection with FACS. j ILC3Ps and ILC3s from Elk3+/+, Elk3−/−, and circTmem241−/−Elk3−/− mice were analyzed by FACS, and indicated cell numbers were calculated. n = 5 for each group. k CFUs in feces of Elk3+/+, Elk3−/− and circTmem241−/−Elk3−/− mice were measured after C. rodentium infection for 6 days. n = 5 for each group. l Colon tissues from Elk3+/+, Elk3−/− and circTmem241−/−Elk3−/− mice were analyzed by H&E staining. Scale bars, 100 μm. m Survival rates of indicated mice were measured after infection with C. rodentium. n = 10 for each group. **P < 0.01 and ***P < 0.001. Data were analyzed by an unpaired two-side Student’s t test and shown as means ± SD. Data are representative of at least three independent experiments. Source data are provided as a Source Data file.
As expected, Elk3 deletion markedly decreased ILC3 numbers but did not affect the numbers of ILC1s or ILC2s in vivo (Fig. 7e–h). In addition, the development of gut-associated lymphoid tissues was also impaired in Elk3-deficient mice (Supplementary Fig. 6h). Consistently, numbers of IL-22+ ILC3s were also dramatically reduced in Elk3−/− mice (Fig. 7i). Importantly, circTmem241−/−Elk3−/− mice displayed much lower numbers of ILCPs and ILC3s (Fig. 7j) as well as were much more susceptible to bacterial infection than their littermate WT or Elk3−/− mice (Fig. 7k–m). Collectively, Elk3 regulates circTmem241-mediated ILC3 differentiation that exerts anti-bacterial immunity.
Discussion
ILC3s play important roles in the early innate immune response against pathogens and maintaining tissue homeostasis4. Abnormity of ILC3 development causes severe intestinal diseases after infection. Here we identified an undefined circular RNA circTmem241 that is highly expressed in ILC3s and their progenitor cells. CircTmem241 deficiency impairs ILC3 commitment and consequently attenuates anti-bacterial immunity against C. rodentium infection. Mechanistically, circTmem241 interacts with Nono protein to recruit histone methyltransferase Ash1l onto Elk3 promoter in ILCP cells. Ash1l-mediated histone modifications on Elk3 promoter enhance chromatin accessibility to initiate Elk3 transcription. Of note, circTmem241−/−, Nono−/−, and Ash1l−/− ILCPs display impaired ILC3 differentiation, while Elk3 overexpression rescues ILC3 commitment ability. Finally, circTmem241−/−Elk3−/− mice show much lower numbers of ILC3s and are much more susceptible to bacterial infection. Therefore, we conclude that the circTmem241-Nono-Ash1l-Elk3 axis is required for ILC3 differentiation.
Many circRNAs contain miRNA-binding elements that act as miRNA sponges to exert their functions20. Instead, circRNAs without miRNA sponge potential utilize alternative mechanisms to exert their roles. Some circRNAs work as scaffolds for their interactions with proteins, DNAs, RNAs, or other molecules26. For instance, circBIRC6 interacts with AGO2 to maintain stem cell pluripotency39. We previously demonstrated that circKcnt2 associates with Mbd3 to inhibit Batf transcription, leading to ILC3 inactivation27. CircPan3 binds Il13ra1 mRNA in the intestinal stem cells (ISCs) to increase its stability, which results in the expression of IL-13Rα1 in ISCs to sustain their self-renewal40. cia-cGAS binds DNA sensor cGAS in the nucleus of HSCs to block its synthase activity, thereby protecting dormant HSCs from cGAS-mediated exhaustion27. CircTmem241 is derived of Tmem241 transcripts from exon 8 to exon 14, whose formation depends on upstream and downstream complementary sequences of the Tmem241 transcripts. We show that circTmem241 is highly expressed in ILC progenitors and regulates ILC3 specification from the ILCP stage. CircTmem241 knockout does not affect the mRNA levels of its parental gene Tmem241 and other neighbor genes. CircTmem241 associates with Nono protein to recruit Ash1l to initiate Elk3 transcription, which drives ILC3 specification from the ILCP stage. We also found that circTmem241 was highly expressed in the intestine and mature ILC3s. Tmem241 is a predicted sugar transport protein, raising the possibility of nutrient component regulation on circTmem241 formation and function of ILC3s. Previous studies showed that ILC3s-mediated immune response can be modulated by nutrients-derived microenvironments41,42,43. For instance, Vitamin A deficiency results in impaired ILC3 effector function and dramatic ILC2 amplification, which represents an adaption of local immune system against ongoing mucosal barrier challenges42. However, the formation and decay of circTmem241 in ILCPs and ILC3s needs to be further investigated.
Cell fate decision is a sophisticated process that is strictly regulated by both genetic and epigenetic factors44,45. Histone modifications remodel chromatin status to modulate global genome accessibility for gene transcription initiation. Different kinds of histone modifications (called ‘histone code’), including methylation, acetylation, phosphorylation, and ubiquitylation, work separately or together to regulate gene expression46,47. Accumulating evidence shows that epigenetic modifications play important roles in ILC commitment and functions6,48,49. We previously showed that Yeats4 drives ILC lineage commitment via recruitment of Dot1l to activate Lmo4 expression50. We also showed that lnckdm2b recruits Satb1 and the NURF complex to initiate Zfp292 transcription, contributing to the maintenance of ILC3 homeostasis18. In this study, we showed that circTmem241 directly interacts with Nono to recruit Ash1l onto Elk3 promoter in ILCPs. Ash1l as a histone methyltransferase catalyzes histone methylation modifications at H3K4 and H3K36 sites, which is tightly associated with active gene transcription38. Ash1l contributes to the enrichment of H3K4me3 and H3K36me3 on Elk3 promoter, which triggers Elk3 transcription to drive ILC3 commitment. Thus, circTmem241-mediated ILC3 commitment is in an epigenetic manner.
ILC specification is a multi-step and finely controlled process. Several ILC progenitors, including EILPs, CHILPs, and ILCPs, have been identified and their major transcription factors regulating their downstream differentiation have also been defined. ILCPs are characterized by the expression of transcription factor PLZF, which drives differentiation of all groups of ILCs51. However, how the precursors downstream of ILCPs generate is still elusive. A recent scRNA-seq study defined several new subpopulations of ILC progenitors in bone marrow14. They clustered one subpopulation as the ILC3P cells that harbor the potential to specifically differentiate into ILC3s. However, how ILCPs differentiate into ILC3Ps and ILC3s remains unclear. Herein we identified an undefined circular RNA circTmem241 highly expressed in ILCPs that drives ILC3 specification from the ILCP to ILC3P stage. We reveal that the circTmem241-Nono-Ash1l-Elk3 axis is required for the ILCP differentiation into ILC3P and ILC3 maturation, which is important to manipulate this axis for ILC development on treatment of infectious diseases.
Methods
All experiments in this article comply with all relevant ethical regulations and are approved by the Institutional Committee of Institute of Biophysics, Chinese Academy of Sciences. The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Institute of Biophysics, Chinese Academy of Sciences.
Antibodies and reagents
Anti-Nono (Cat# 11058-1-AP and 66361-1-Ig) was from Proteintech. Anti-Elk3 (Cat# NBP1-83960) was from Novus Biologicals. Anti-Tmem241 (Cat# 203644-T32) was from Sinobiological. Anti-Lineage cocktail (Cat# 88-7772-72), Anti-CD127 (A7R34), anti-Sca-1 (D7), anti-Flt3 (A2F10), anti-α4β7 (DATK32), anti-Id2 (ILCID2), anti-PLZF (Mags.21F7), anti-Eomes (Dan11mag), anti-NKp46 (29A1.4), anti-NK1.1 (PK136), anti-CD45 (30-F11), anti-CD25 (PC61.5), anti-Gata3 (TWAJ), anti-RORγt (AFKJS-9), anti-Bcl11b (1F8H9), anti-CD3 (17A2), anti-CD19 (1D3), anti-KLRG1 (2F1), anti-CD90 (HIS51), anti-IL-22 (IL22JOP), anti-Ki67 (SolA15), anti-CD45.2 (104), anti-BrdU (BU20A), anti-PD-1 (J43) and anti-CD45.1 (A20) were purchased from eBiosciences (San Diego, USA). Anti-c-Kit (2B8), anti-CD150 (TC15-12F12.2), anti-CD48 (HM48-1), and anti-CD49a (HMα1) were purchased from Biolegend (California, USA). Anti-β-actin (Cat# RM2001) was purchased from Beijing Ray Antibody Biotech. Paraformaldehyde (PFA) and 4’,6-diamidino-2-phenylindole (DAPI) were from Sigma. IL-22 ELISA kit was purchased from Neobioscience.
Generation of knockout mice and cells by CRISPR/Cas9 technology
For a generation of circTmem241−/− and Elk3−/− mice, CRISPR-mediated single-stranded oligodecxynucleotides donors were synthesized as previously described52. Zygotes from C57BL/6 mice were injected with sgRNAs and subsequently transferred to the uterus of pseudo-pregnant females from which viable founder mice were obtained. For a generation of Nono−/− and Ash1l−/− cells, sgRNAs targeting Nono or Ash1l were synthesized and cloned into AAV-delivering vectors and infected CHILPs or ILCPs isolated from Vav-Cre;Cas9-KI mice. Genomic DNA mutation was identified by PCR screening and DNA sequencing, followed by Western blotting or Northern blotting. sgRNA sequences were listed in Supplementary Table 2. Vav-Cre, Cas9-KI, and PLZFGFPcre mice were purchased from the Jackson Laboratory. All the mouse strains were C57BL/6 background and maintained under specific pathogen-free conditions with approval by the Institutional Committee of the Institute of Biophysics, Chinese Academy of Sciences. This study is compliant with all relevant ethical regulations regarding animal research.
In vitro differentiation assay
ILC in vitro differentiation assay was performed as previously described53. Briefly, cells from femurs were flushed out using phosphate-buffered saline (PBS) containing 5% FBS and filtered through 70 μm strainers. Collected cells were treated with RBC lysis buffer (Tiangen, Beijing) to exclude red cells. CHILPs (Lin−CD25−CD127+Flt3−α4β7+) or ILCPs (Lin−CD25−CD127+ Flt3−α4β7+c-Kit+ PD-1+) were sorted by FACSAria III instrument. OP9-DL1 cells were maintained in complete αMEM medium (supplemented with 10% FBS, 1% streptomycin, 1% penicillin). Before seeding of progenitor cells, OP9-DL1 cells were treated with 4 μg/ml mitomycin for 2 h to inhibit cell division. Then, cells were digested and plated at the density of 1 × 106 cells per 24-well plate. After OP9-DL1 cells were adhered, indicated progenitor cells were inoculated on OP9-DL1 feeder cells in a complete RIPM1640 medium (Supplemented with 10% FBS, 1% streptomycin, 1% penicillin, 20 ng/ml IL-7, 20 ng/ml SCF). Progenitor cells were detected after culture for 7 days while differentiated ILCs were analyzed 14 days later by FACS.
Intestinal lymphocyte separation
Intestinal lymphocytes were separated as described before27. Briefly, intestines were cut open longitudinally and washed using PBS several times to clean intestinal contents. Then, intestines were cut into 2–5 mm pieces, washed with a solution I buffer (10 mM HEPES and 5 mM EDTA in HBSS) five times to remove epithelial cells, followed by lamina propria digestion with solution II buffer (DNase I, 5% FBS, 0.5 mg/ml collagenase II and collagenase III) three times. Finally, the digested lamina propria lymphocytes (LPLs) were filtered through 70 μm strainers and used for downstream experiments.
Flow cytometry
For flow cytometric analysis, HSC (Lin−Sca-1+c-Kit+CD150+CD48−), MPP (Lin−Sca-1+c-Kit+CD150−CD48+), CLP (Lin−CD127+c-KitintSca-1intFlt3+α4β7−), α4β7+ CLP (Lin−CD127+c-KitintSca-1intFlt3+α4β7+), CHILP (Lin−CD25−CD127+Flt3−α4β7+Id2+), ILCP (Lin−CD127+Flt3−c-Kit+α4β7+PLZFGFP), ILC1P (Lin−CD127+Eomes−CD49a+NK1.1+NKp46+), ILC2P (Lin−CD127+CD45+Flt3−CD117−Sca-1+CD25+Gata3+), ILC3P (Lin−CD25−CD127+α4β7intGata3loBcl11b+Id2+RORγt+), siILC1 (CD3−CD19−CD127+CD45+NK1.1+NKp46+), siILC2 (CD3−CD19−CD127+CD90+KLRG1+Sca-1+), siILC3 (Lin−CD127+CD45+RORγt+), NK1.1+ NK, CD19+ B and CD3+ T populations were analyzed or sorted with a FACSAria III instrument (BD Biosciences). PLZFGFPcre mice were used for ILCP (Lin−CD127+α4β7+PLZFGFP) isolation and Id2+/GFP mice were used for CHILP (Lin−CD25−CD127+Flt3−α4β7+Id2GFP) isolation by FACS. Data were collected with FACSAria IIIu instrument and analyzed by FlowJo Version 10.0 software.
IF staining
Intestine tissues were collected and fixed using 4% PFA (Sigma-Aldrich) for one day at room temperature followed by frozen sectioning. Intestine sections were further fixed in 4% PFA for 1 hour at room temperature and permeabilized with PBS containing 1% TritonX-100 for 1 hour, blocked with 10% donkey serum for 1 h at room temperature, incubated with appropriate primary antibodies at 4 °C overnight, and then incubated with fluorescence-conjugated secondary antibodies. For ILC3 staining, anti- RORγt (host species: rat) was used at a dilution of 1:100, and Alexa-Flour 594 donkey anti-rat secondary antibody at 1:500. Anti-CD127/IL-7R (host species: rabbit) was used at 1:200 and Alexa-Flour 647 donkey anti-rabbit secondary antibody at 1:500. FTIC-conjugated anti-CD3 was used at 1:300 directly. For ILCP staining, anti-Nono (host species: mouse) was used at a dilution of 1:200 and Alexa-Flour 594 donkey anti-mouse secondary antibody at 1:500. DAPI was used for nucleus staining. Sections were visualized with Nikon A1R+ confocal microscopy.
Fluorescence in situ hybridization
CHILPs (Lin−CD25−CD127+Flt3−α4β7+), ILCPs (Lin−CD25−CD127+Flt3−α4β7+c-Kit+PD-1+) or ILC3s (Lin−CD127+CD90highCD45lo) were isolated and spread on high-adherent slides. Cells were fixed using 4% PFA (Sigma-Aldrich) for 20 min at room temperature, permeabilized with PBS containing 1% TritonX-100 for 20 min, prehybridized with hybridization buffer (50% formamide, 5× SSC, 500 ng/μl yeast tRNA, 1× Dehardt’s solution, 500 ng/μl sperm DNA, 50 ng/μl Heparin, 2.5 mM EDTA, 0.1% Tween-20, 0.25% CHAPS) for 1 h at 45 °C, incubated with biotinylated or cy5-labeled probes at 45 °C for 2 hours, and then washed three times with SSC washing buffer. After blocking with 10% donkey serum, cells were subjected to IF staining and visualized with Nikon A1R + confocal microscopy.
DNA in situ hybridization
DNA in situ hybridization was conducted according to a previous study with minor modification54. In Brief, ILCPs were spread on adherent slides and fixed by 4% paraformaldehyde (PFA, Sigma-Aldrich), and quenched in 0.1 M Tris-HCl (pH 7.4). After premetallized with 0.1% TritonX-100, slides were incubated in PBS containing 20% glycerol for 20 min. Then slides were subjected to 3 freeze/thawing cycles in liquid nitrogen. after which cells were equilibrated in hybridization buffer (50% formamide, 2× SSC) for 10 min. Biotin-labeled probe against Elk3 promoter was pipetted onto slides and incubated at 78 °C for exact 2 min, followed by incubation at 37 °C overnight. Cells were subjected to IF staining with indicated antibodies the next day. DAPI was used for nucleus staining. Cells were visualized with Nikon A1R + confocal microscopy.
Real-time quantitative PCR
Total RNA was isolated through a RNA Miniprep Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. cDNA was synthesized using 5× All-In-one RT Mastermix (Abm, Vancouver, Canada) and analyzed on QuantStudio1 qPCR system using specific primer pairs listed in Supplementary Table 3. The relative expression level was calculated and normalized to endogenous 18 S or Gapdh. CircRNAs were analyzed using specific divergent and convergent primers (Supplementary Table 4).
CHIRP assay
CHIRP assay was performed according to previous research55. ILCPs were cross-linked with 1% glutaraldehyde at room temperature for 10 min and quenched by 0.1 M glycine for 5 min. Then cells were washed twice with PBS, lysed with Lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris, supplemented with PMSF, proteinase inhibitor cocktails, and RNase inhibitor) and sonicated to produce 200~500 bp DNA fragments. Lysates were incubated with biotinylated antisense probes against circTmem241 junction site for 4 hours at 37 °C. Streptavidin agarose beads were then added to isolate probe binding complex. After washing steps, DNA, RNA, or proteins were eluted from beads and subjected to downstream analysis.
ChIP assay
ILCPs were cross-linked with 1% formaldehyde at 37 °C for 10 min. Then cells were washed twice with PBS, lysed with SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris), and sonicated to make 200 to 500 bp DNA fragments. Lysates were pre-cleared with Protein A Agarose/Salmon Sperm DNA (50% Slurry) and then incubated with 4 μg indicated antibodies overnight at 4 °C. After washing steps, DNA was eluted from beads and purified. DNA fragments were analyzed using primer pairs listed in Supplementary Table 5.
Luciferase reporter assay
For luciferase reporter assay, the full-length promoter of Elk3 (from −2000 to 0 upstream from transcriptional start site) or truncated Elk3 promoter was cloned into pGL3 vector. For mutation of Elk3 promoter, the pairing region was substituted with mutated sequence (5’-AGAAGTTCCATAAT-3’) in full-length promoter. Exon region of circTmem241 with flanking complementary elements was constructed into pcDNA4 vector for overexpression. 293 T cells were seeded in 24-well plate one day before transfection. For transfection of each well, 100 ng pGL3 plasmid, 1 ng pRL-TK and 500 ng pcDNA4 plasmid were used. Cells were transferred to 96-well assay plate at 10000/well 24 h later. After cells were attached, luminescence signals were analyzed using ONE-Glo Luciferase Assay System (Promega, Madison) according to the manufacturer’s protocol.
C. rodentium infection
Mice were fasted for 8 h and then infected with 5 × 109 C. rodentium by intragastric gavage. C. rodentium was a gift from B. Ge (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences). After infection for seven days, mice were sacrificed and colons were collected for pathological analysis. Feces were analyzed for bacterial loads. LPLs were isolated for analyses of ILC3s and IL-22 production by FACS and enzyme-linked immunosorbent assay (ELISA).
Histopathological analysis
Colons from indicated mice were cut open longitudinally and fixed in 4% PFA followed by paraffin sectioning and H&E staining. The histopathological score was evaluated and analyzed according to a previous study56. Briefly, sections were assessed for epithelial hyperplasia (calculated based on percentage change above control, 0 for no change, 1 for 1–50%, 2 for 51-100%, 3 for more than 100%), epithelial integrity (0 for no change, 1 for <10 epithelia shedding per lesion, 2 for 11–20 shedding per lesion, 3 for epithelia ulceration, 4 for sever crypt destruction), granulocyte mononuclear cell infiltration (0 for none, 1 for mild, 2 for moderate, 3 for severe), depletion of goblet cells (goblet cells from ×400 magnification filed were calculated, 0 for >50, 1 for 25–50, 2 for 10–25, 3 for <10) and submucosal oedema (0 for no change, 1 for mild, 2 for moderate, 3 for severe).
Bone marrow transplantation
For non-competitive transplantation, 5 × 106 CD45.2+ BM donor cells from indicated mice were injected into lethally irradiated CD45.1+ recipient mice. Eight weeks after transplantation, CD45.2+ ILC3s derived from donor cells were analyzed by cytometric analysis. For competitive transplantation, 1 × 106 CD45.2+ BM cells from indicated mice and 1 × 106 wild-type CD45.1+ BM cells were mixed and transplanted into lethally irradiated CD45.1+ recipient mice. Eight weeks after transplantation, the ratio of CD45.2+ ILC3s to CD45.1+ ILC3s were examined.
Plasmids construction and virus preparation
RNA interference was performed as previously described27. And target sequences were listed in Supplementary Table 6. For circTmem241 overexpression, genomic exon region of circTmem241 was constructed into pcDNA4 vector flanked by the upstream and downstream complementary elements. Lentiviral vector (pSIN-EF2-GFP) was co-transfected with packaging plasmids pMD2G and psPAX2 into HEK293T cells for 48 h, followed by culture medium collection and ultracentrifugation at 25,000×g for 2 h. Pellets were resuspended in DMEM medium and viral titers were determined by infecting HEK293T cells with diluted viruses. Cells were incubated with lentiviruses and centrifuged at 500×g for 2 h in the presence of 8 μg/ml polybrene. Cells were cultured for 24 h to allow GFP expression, followed by sorting of GFP positive cells through a flow cytometer. HEK293T cells were from ATCC (CRL-11268) and tested negative for mycoplasma contamination.
RNA-pulldown assay
Bone marrow cells were lysed and supernatants were incubated with biotin-labeled circTmem241 or antisense control probe. Then precipitated components were separated with SDS-PAGE and silver staining. Differential bands enriched by circTmem241 were analyzed by mass spectrometry or western blot.
Transcriptome analysis
For transcriptome RNA-seq analysis, ILCPs were isolated from circTmem241+/+ or circTmem241−/− mice. Then RNAs were isolated using Trizol reagent (Invitrogen) and subjected to RNA sequencing by Beijing Genomics Institute. Subsequent data analysis was conducted by R studio and GSEA software.
Electrophoretic mobility shift assay
For analysis of the interaction between circTmem241 and Nono, biotin-labeled linearized circTmem241 was synthesized by in vitro transcription and Nono was expressed and purified by GST-tag. CircTmem241 and Nono were incubated in EMSA reaction buffer and mobility shift assay was performed using a Light Shift Chemiluminescent RNA EMSA Kit (Thermo Scientific) according to the manufacturer’s protocol.
Nuclear run-on assay
Nuclear run-on assay was performed as previously described27. ILCPs isolated from indicated mice were suspended in nuclear extraction buffer (10 mM Tris-HCl, 150 mMKCl, 4 mM MgOAc, pH 7.4), followed by centrifugation to collect cell pellets. Then, pellets were lysed by lysis buffer (nuclear extraction buffer supplemented with 0.5% NP-40), followed by sucrose density gradient centrifugation to prepare transcriptional active crude nuclei components. Crude nuclei were incubated with a biotin labeling mix (Roche, Basel, Swiss) and RNase inhibitor at 28 °C for 5 min. RNAs were extracted using TRIzol reagent according to the manufacturer’s instructions. DNA was digested by DNase I for 15 min at room temperature. RNA transcripts were enriched with streptavidin affinity beads, followed by reverse transcription and RT-PCR analysis.
Enzyme-linked immunosorbent assay
LPLs or ILC3s were isolated and cultured for 24 h with indicated cytokines. Then supernatants were collected and cytokines were detected using ELISA kit (Neobioscience) according to the manufacturer’s instructions.
Statistical and Reproducibility
For statistical evaluation, an unpaired Student’s t test was applied for calculating statistical probabilities in this study. For all panels, at least three independent experiments were performed with similar results, and representative experiments are shown. Data were analyzed by using Microsoft Excel 2016 or GraphPad Prism Version 8.3. P values ≤0.05 were regarded as statistical significance.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The source data for Figs. 1–7 and Supplementary Figs. 1–6 are provided as a Source Data file. The RNA sequencing data generated in this study have been deposited in the GEO database under accession code GSE201065. All other data are included in the supplemental information or available from the authors upon reasonable requests. Source data are provided with this paper.
References
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity 38, 769–781 (2013).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. USA 107, 11489–11494 (2010).
Zeng, B. et al. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 10, 315 (2019).
Kaye, J. ILC development: TCF-1 reporting. Nat. Immunol. 16, 1011–1012 (2015).
Seillet, C. et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 17, 436–447 (2016).
Harly, C., Cam, M., Kaye, J. & Bhandoola, A. Development and differentiation of early innate lymphoid progenitors. J. Exp. Med. 215, 249–262 (2018).
Diefenbach, A., Colonna, M. & Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41, 354–365 (2014).
Yu, Y. et al. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J. Exp. Med. 212, 865–874 (2015).
Willinger, T. Metabolic control of innate lymphoid cell migration. Front. Immunol. 10, 2010 (2019).
Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Hoyler, T. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012).
Wang, L. et al. TGF-beta induces ST2 and programs ILC2 development. Nat. Commun. 11, 35 (2020).
Walker, J. A. et al. Polychromic reporter mice reveal unappreciated innate lymphoid cell progenitor heterogeneity and elusive ILC3 progenitors in bone marrow. Immunity 51, 104–118.e107 (2019).
Scoville, S. D. et al. A progenitor cell expressing transcription factor rorgammat generates all human innate lymphoid cell subsets. Immunity 44, 1140–1150 (2016).
Pokrovskii, M. et al. Characterization of transcriptional regulatory networks that promote and restrict identities and functions of intestinal innate lymphoid cells. Immunity 51, 185-+ (2019).
Serafini, N. et al. Gata3 drives development of RORgammat+ group 3 innate lymphoid cells. J. Exp. Med. 211, 199–208 (2014).
Liu, B. et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat. Immunol. 18, 499–508 (2017).
Liu, B. et al. IL-7Ralpha glutamylation and activation of transcription factor Sall3 promote group 3 ILC development. Nat. Commun. 8, 231 (2017).
Patop, I. L., Wust, S. & Kadener, S. Past, present, and future of circRNAs. EMBO J. 38, e100836 (2019).
Kristensen, L. S. et al. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691 (2019).
Li, X., Yang, L. & Chen, L. L. The biogenesis, functions, and challenges of circular RNAs. Mol. Cell 71, 428–442 (2018).
Piwecka, M. et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357 eaam8526 (2017).
Guarnerio, J. et al. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell 165, 289–302 (2016).
Liu, C. X. et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell 177, 865–880.e821 (2019).
Xia, P. et al. A circular RNA protects dormant hematopoietic stem cells from DNA sensor cGAS-mediated exhaustion. Immunity 48, 688–701.e687 (2018).
Liu, B. et al. An inducible circular RNA circKcnt2 inhibits ILC3 activation to facilitate colitis resolution. Nat. Commun. 11, 4076 (2020).
Zheng, H. et al. The transcription factor Net regulates the angiogenic switch. Genes Dev. 17, 2283–2297 (2003).
Criqui-Filipe, P., Ducret, C., Maira, S. M. & Wasylyk, B. Net, a negative Ras-switchable TCF, contains a second inhibition domain, the CID, that mediates repression through interactions with CtBP and de-acetylation. EMBO J. 18, 3392–3403 (1999).
Yang, H. et al. ETS family transcriptional regulators drive chromatin dynamics and malignancy in squamous cell carcinomas. Elife 4, e10870 (2015).
Rogers, C. D., Phillips, J. L. & Bronner, M. E. Elk3 is essential for the progression from progenitor to definitive neural crest cell. Dev. Biol. 374, 255–263 (2013).
Tsoyi, K. et al. Elk-3 is a KLF4-regulated gene that modulates the phagocytosis of bacteria by macrophages. J. Leukoc. Biol. 97, 171–180 (2015).
Meng, L., Xing, Z., Guo, Z. & Liu, Z. LINC01106 post-transcriptionally regulates ELK3 and HOXD8 to promote bladder cancer progression. Cell Death Dis. 11, 1063 (2020).
Chen, Y. H. et al. Elk-3 is a transcriptional repressor of nitric-oxide synthase 2. J. Biol. Chem. 278, 39572–39577 (2003).
Yang, X. et al. Immune-related genes in tumor-specific CD4(+) and CD8(+) T cells in colon cancer. BMC Cancer 20, 585 (2020).
Liu, Q., Kim, M.H., Friesen, L. & Kim, C.H. BATF regulates innate lymphoid cell hematopoiesis and homeostasis. Sci. Immunol. 5 eaaz8154 (2020).
Xu, W. et al. An Id2(RFP)-reporter mouse redefines innate lymphoid cell precursor potentials. Immunity 50, 1054–1068.e1053 (2019).
Zhang, T., Cooper, S. & Brockdorff, N. The interplay of histone modifications - writers that read. EMBO Rep. 16, 1467–1481 (2015).
Yu, C. Y. et al. The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat. Commun. 8, 1149 (2017).
Zhu, P. et al. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat. Immunol. 20, 183–194 (2019).
Shi, Z., Ohno, H. & Satoh-Takayama, N. Dietary derived micronutrients modulate immune responses through innate lymphoid cells. Front Immunol. 12, 670632 (2021).
Spencer, S. P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Hou, Q., Huang, J., Xiong, X., Guo, Y. & Zhang, B. Role of nutrient-sensing receptor GPRC6A in regulating colonic group 3 innate lymphoid cells and inflamed mucosal healing. J. Crohns Colitis 3, jjac020 (2022).
Ding, Y., Liu, Z. & Liu, F. Transcriptional and epigenetic control of hematopoietic stem cell fate decisions in vertebrates. Dev. Biol. 475, 156–164 (2021).
Moris, N., Pina, C. & Arias, A. M. Transition states and cell fate decisions in epigenetic landscapes. Nat. Rev. Genet. 17, 693–703 (2016).
Zhou, V. W., Goren, A. & Bernstein, B. E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 12, 7–18 (2011).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Verma, M. et al. The molecular and epigenetic mechanisms of innate lymphoid cell (ILC) memory and its relevance for asthma. J Exp Med. 218, e20201354 (2021).
Lim, A. I. et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 168, 1086–1100.e1010 (2017).
Liu, B. et al. Yeats4 drives ILC lineage commitment via activation of Lmo4 transcription. J. Exp. Med. 216, 2653–2668 (2019).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Zhu, X. et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep. 4, 6420 (2014).
Holmes, R. & Zuniga-Pflucker, J. C. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb. Protoc. 2009, prot5156 (2009).
Bolland, D.J., King, M.R., Reik, W., Corcoran, A.E. & Krueger, C. Robust 3D DNA FISH using directly labeled probes. J. Vis. Exp. 15, 50587 (2013).
Chu, C., Quinn, J. & Chang, H.Y. Chromatin isolation by RNA purification (ChIRP). J. Vis. Exp. 25, 3912 (2012).
Gibson, D. L. et al. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol. 10, 388–403 (2008).
Acknowledgements
We thank Jiajia Hou, Zixin Zhao, Yihui Xu, and Junying Jia for technical support. We thank Jing Li (Cnkingbio Company Ltd, Beijing, China) for his technical support. We also thank professor Liu (Academy of Military Medical Sciences) for providing the OP9-DL1 cell line. This work was supported by the National Key R&D Program of China (2019YFA0508501, 2020YFA0803501, 2021YFA1302000), National Natural Science Foundation of China (31930036, 82130088, 81921003, 92042302, 32070533, 91940305, 81772646, 32170874, 31870883), Strategic Priority Research Programs of the Chinese Academy of Sciences (XDB19030203).
Author information
Authors and Affiliations
Contributions
N.L., J.H., and D.F. performed experiments; N.L. designed the project, analyzed the data, and wrote the paper; D.F. and X.Z. constructed genetic mouse strains. N.L., J.H., Y.G., J.W., H.L., and Y.D. analyzed data; Y.T. generated animal models and analyzed data; Z.F. and B. L. initiated the study, organized, designed, and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kyung-Soon Park and Tim Willinger for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, N., He, J., Fan, D. et al. Circular RNA circTmem241 drives group III innate lymphoid cell differentiation via initiation of Elk3 transcription. Nat Commun 13, 4711 (2022). https://doi.org/10.1038/s41467-022-32322-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32322-z
This article is cited by
-
Regulation of noncoding RNAs in innate lymphoid cells
Cellular & Molecular Immunology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.