Abstract
First identified in 1947, Zika virus took roughly 70 years to cause a pandemic unusually associated with virus-induced brain damage in newborns. Zika virus is transmitted by mosquitoes, mainly Aedes aegypti, and secondarily, Aedes albopictus, both colonizing a large strip encompassing tropical and temperate regions. As part of the international project ZIKAlliance initiated in 2016, 50 mosquito populations from six species collected in 12 countries were experimentally infected with different Zika viruses. Here, we show that Ae. aegypti is mainly responsible for Zika virus transmission having the highest susceptibility to viral infections. Other species play a secondary role in transmission while Culex mosquitoes are largely non-susceptible. Zika strain is expected to significantly modulate transmission efficiency with African strains being more likely to cause an outbreak. As the distribution of Ae. aegypti will doubtless expand with climate change and without new marketed vaccines, all the ingredients are in place to relive a new pandemic of Zika.
Similar content being viewed by others
Introduction
Viral pathogens with high epidemic potential have marked human history by massive losses of life and economic declines. Pandemics like the Spanish flu1 have left traces and the fear of new viral emergences materializes today with the Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) pandemic. Over the past decades, efforts invested into vaccination programs and development of antiviral treatments have led to major medical progress resulting in measles decline, smallpox eradication, appropriate treatments of Human Immunodeficiency virus and Hepatitis C virus, and improved control of SARS-CoV-2. As major viral pathogens, arthropod-borne viruses (arboviruses) are also on the rise. Arboviruses have developed a strategy to accomplish their transmission cycle via replication in both vertebrate and arthropod hosts. Their RNA genome contributes to the generation of large populations of genetically distinct variants permitting adaptations to environmental changes including host switching and transmission2. In the last 30 years, mosquito-borne viruses have dramatically expanded their distribution range leading to increasingly frequent and large epidemics3. In 2016, Zika was designated as a “Public Health Emergency of International Concern” by the World Health Organization, subsequently to the large spread of Zika virus (ZIKV) in the Pacific islands and the Americas with unusual notifications of microcephaly in newborns and different neurological disorders (e.g. Guillain-Barré syndrome); this happened 70 years after its first isolation from a human case in East Africa4. Local cases of Zika were detected in 2019 in Southern France additionally corroborating the role of the Asian tiger mosquito (Aedes albopictus) in ZIKV transmission in addition to chikungunya and dengue viruses5.
Aedes mosquitoes are important vectors of arboviruses, acquiring the virus through a blood meal from an infected host. Subsequently, the virus replicates within the mosquito and is transmitted to a new host. The virus must overcome two anatomical barriers (midgut and salivary glands) and circumvent major antiviral pathways before being delivered in saliva6. Geographic populations of a same mosquito species may not share the same immunological background, leading to different vector competences7. The outcome of infection depends on specific pairings of mosquito and pathogen genotypes described under genotype-by-genotype (G x G) interactions8. These interactions could structure viral populations through adaptations to their local vector populations8. Vector competence data have been used to derive risk maps informed with distribution of mosquito species, to help focus and strengthen vector control measures9. Nevertheless, other factors, non-genetic, biotic and abiotic, should be considered in the functioning of the vectorial system making it even more complex than initially thought10. A global vector competence analysis of potential Zika vectors (Aedes aegypti, Ae. albopictus, Aedes japonicus, Culex pipiens pipiens, Culex pipiens molestus, and Culex quinquefasciatus) was performed to assess the risk of Zika disease outbreaks. Once the vector is well established in a region, associated arboviruses have the potential to emerge through imported human cases. To this aim, we analyzed the vector competence of 50 mosquito populations from 15 locations and 12 countries (Brazil, Cambodia, Cameroon, Congo, Cuba, France, Gabon, Guadeloupe, Haiti, La Reunion, Madeira, The Netherlands, New Caledonia, Spain, and Switzerland) which were challenged with different ZIKV genotypes (West Africa, Asia, America) using standardized experimental protocols (mosquito infections, viral titrations, parameters of vector competence). In this work, we elaborated a vector competence data-driven prediction for ZIKV transmission to inform on the current risk of Zika transmission at a global scale. We show that Ae. aegypti populations tested are highly competent to transmit the different ZIKV genotypes and more specifically, the African genotype. Moreover, other mosquito species such as Ae. albopictus or Ae. japonicus can play a secondary role in transmission while Culex mosquitoes are definitively non-susceptible to ZIKV.
Results
Culex spp. mosquitoes were resistant to most ZIKV strains, successfully blocking infection with an overall infection rate (IR) of 0.2% (2 mosquitoes out of 1089 tested) and complete absence of both dissemination efficiency (DE) and transmission efficiency (TE). As such, they were not included in more complex vector competence analyses. Aedes spp. showed different patterns depending on species and ZIKV strain used for infection. For Ae. aegypti and Ae. albopictus, the experimental design required nested, within-country random effects. In the case of Ae. japonicus, the country and mosquito population effects were fully colinear, therefore analyses only relied on logistic regressions.
Differential susceptibility of Aedes spp. mosquitoes to ZIKV
Depending on mosquito species and ZIKV strain, we observed different patterns of viral progression within the vector’s organs. In Ae. aegypti infected with ZIKV strains, there were no notable differences between IR and DE at 21 days post-infection (dpi), with IR = 94.3% (95% CI: [92.1%–96.0%]) and 69.1% [64.0%–73.9%], and with DE = 89.4% [86.6%–91.8%] and 64.3% [59.1%–69.3%] for respectively, African and American strains. However, there was a large and significant decrease for TE = 71.9% [68.0%–75.6%] and 30.0% [25.3%–35.1%] for respectively, African and American strains as can be seen in Fig. 1. This is consistent with efficient viral progression through the midgut, and partial barriers to viral progression before reaching the salivary glands. In contrast, when infected with Asian ZIKV strains, both midguts and salivary glands mitigated progression of the virus: IR = 68.9% [64.3%–73.3%], DE = 56.7% [51.8%–61.5%], and TE = 28.9% [24.7%–33.5%].
Panels show the rates achieved by averaging over all sampled mosquitoes, i.e., by pooling together mosquitoes from all countries used in the study. Error bars are exact 95% binomial confidence intervals centered on the observed means of IR, DE and TE. (n = 30 biologically independent mosquitoes for each combination of species, country, mosquito population, days post-infection and ZIKV strain were studied, unless stated otherwise. See Supplementary Table 1 for complete break-down of sample sizes). Colors correspond to the studied outcome (IR, DE, TE).
In Ae. albopictus mosquitoes, viral progression was more efficient with the African ZIKV strain than with American or Asian ZIKV strains for all three outcomes. For the African ZIKV strain, a regular decrease was observed between IR (73.9%), DE (69.2%) and TE (55.8%), consistent with the absence of a marked barrier to viral progression through the midgut and into the salivary glands. In contrast, DE was significantly lower for American (15.6% [11.5–20.4%]) and Asian (11.2% [7.7–15.5%]) ZIKV strains, consistent with a lack of viral progression through the midgut.
Time since infection showed an increasing, approximately linear association with DE and TE in Ae. aegypti and Ae. albopictus mosquitoes, suggesting an accumulation pattern and an overall absence of viral clearance within the vector, while IR reached a peak value within 7 dpi and remained at comparable levels until 21 dpi. In contrast, Ae. japonicus infected by the African ZIKV strain showed that IR peaked at 14 dpi, with decreasing DE and TE values over time (TE7dpi = 15.0% [7.1–26.6%], TE14dpi = 15.0% [7.1–26.6%] and TE21dpi = 6.7% [18.5–16.2%]).
Transmission efficiency is higher with the African ZIKV
Although all species of Aedes studied were competent to transmit ZIKV, infected Ae. aegypti species were on average more prone to successful dissemination than Ae. albopictus and Ae. japonicus (TEaegypti = 34.0% [24.0–45.8%], TEalbopictus = 17.6% [11.4–26.0%], TEjaponicus = 7.2% [2.9–16.9%]). This was even more pronounced at 21 dpi when TE was significantly higher for Ae. aegypti than Ae. albopictus (46.7% [35.3–58.5%] vs. 25.3% [17.3–35.5%], p < 0.0001) and Ae. japonicus (46.7% [35.3–58.5%] vs. 10.8% [4.4–24.1%], p = 0.0002), the latter two not reaching statistical significance (p = 0.09). However, many combinations of ZIKV strains and mosquito populations were not available for Ae. japonicus for which results should be considered less robust.
The African ZIKV strain was the most efficient in infecting and disseminating, therefore posing a greater risk in case it was newly introduced in a region: the predicted expected marginal means 21 dpi were TE = 82.7% [78.5–86.1%] for Ae. aegypti and TE = 52.4% [45.4–59.3%] for Ae. albopictus (Fig. 2). American and Asian strains had comparable TE regardless of time since infection: (1) in Cameroon, Congo, and Gabon, TE was low (~10%), suggesting African mosquitoes are mostly susceptible to local ZIKV strains; (2) in Brazil, Haiti, Guadeloupe, Madeira, La Reunion and New Caledonia, TE reached moderate values ~20%. In Cambodia however, Asian and American ZIKV strains had much higher TE values, denoting mosquito populations highly susceptible to ZIKV.
TE predicted for (A) Ae. aegypti and (B) Ae. albopictus. Countries are ordered to reflect geographic proximity. Error bars show asymptotic 95% confidence interval from the mixed regression models centered on the average predictions of IR, DE and TE. (n = 30 biologically independent mosquitoes for each combination of species, country, mosquito population, days post-infection and ZIKV strain were studied, unless stated otherwise. See Supplementary Table 1 for complete break-down of sample sizes). Colors correspond to continent-aggregated ZIKV strains.
Breaking down variable importance in ZIKV susceptibility
We evaluated the extent to which each covariate of interest contributed to explanatory value of fitted models. For both Ae. aegypti and Ae. albopictus, TE showed low variability among mosquito populations originating from the same country (Fig. 3). Another striking result from Fig. 3 confirmed the higher susceptibility to African ZIKV strains among all tested mosquitoes, both within and across countries. This suggests that within a given country, mosquito populations were equally able to disseminate ZIKV regardless of other extrinsic factors. We found that 51.7% of variance in Ae. aegypti TE could be explained by a statistical model accounting for mosquito strain, ZIKV strain, and dpi (Table 1). The relative importance of these factors was assessed by estimating the proportion of variance explained solely by these factors. For Ae. aegypti TE, ZIKV strain was the most important factor explaining 33.5% of variance, followed by dpi (30.1%). Of the factors considered, mosquito strain had the least impact on TE, with only 6.6% of variance explained by the country of a sampled mosquito strain. An additional 2.6% of variance was explainable when we considered locations of strains within a country. For Ae. japonicus, no obvious pattern could be detected with only 5.2% of the observed variance explained by available covariates suggesting that mosquitoes were less susceptible to tested ZIKV strains.
Discussion
Our results confirmed that human-adapted Aedes mosquitoes prepare the field for new arbovirus emergences; in addition to other factors, some combinations of vector-virus are more prone to cause outbreaks, but globalization will smooth out differences leading most emergences to turn into pandemics owing to the large territories covered by vectors.
During the 2015–2016 Latin American and the Caribbean epidemic, the role of Culex as ZIKV vector has been the subject of major scientific debates which, if verified, would have revised the modus operandi of vector control11. As ZIKV has also been detected in field-collected Culex mosquitoes12,13, their susceptibility to ZIKV was then tested; from 36 studies on vector competence of Culex mosquitoes for ZIKV, seven studies showed that ZIKV was present in the salivary glands of Cx. restuans, Cx. quinquefasciatus, Cx. tarsalis, and Cx. Coronator. Asian and American genotypes of ZIKV may occasionally infect Culex mosquitoes but not African strains14. From our dataset15,16,17,18, we showed that ZIKV can accumulate in Cx. pipiens saliva only after forced infection by intrathoracic inoculation, short-circuiting the midgut barrier17; after oral infection, the virus was unlikely to disseminate in the mosquito ruling out the role of Culex mosquitoes in ZIKV transmission. Viral replication was not detected in Culex which was unable to trigger the RNAi pathway as no viral replication signatures such as 21 nt viral siRNAs were detected19. Even though vectors of the flavivirus West-Nile virus, a phylogenetically ZIKV-related arbovirus, mosquitoes of the Cx. pipiens complex are not competent for ZIKV transmission and are very unlikely to play a role of vector during a Zika outbreak. Thus, to control Zika, targeted interventions should focus largely on Aedes mosquitoes11,20.
Aedes aegypti is an African mosquito (named Ae. aegypti formosus) living primarily in rainforests colonizing natural breeding sites which in a subsequent step, has become adapted to living in the human environment (developing a human-biting behavior and colonizing human-made containers) before undergoing different waves of dissemination outside Africa21. The “domestication” of this species (named Ae. aegypti aegypti) allowed its world-wide dispersion coinciding with the first twentieth century pandemics caused by arboviruses, dengue22, and then chikungunya23 and Zika24. In addition to increased vector-host contacts, Ae. aegypti aegypti has developed an enhanced permissiveness to some arboviruses7. At the mosquito level, successful transmission implies that the virus passes through two anatomical barriers, midgut and salivary glands25. Ae. aegypti mosquitoes we tested were more prone to impose a strong bottleneck to the African ZIKV in the salivary glands leading to decrease ZIKV transmission. Nevertheless, all 11 Ae. aegypti populations transmitted at least 2–3 times more the African ZIKV than the American and Asian ZIKV strains underlining the high potential of African ZIKV to trigger an outbreak outside and inside Africa. It is now admitted that African ZIKV strains were more pathogenic than the Asian strains26,27. African Ae. aegypti (Cameroon, Congo, Gabon) were more resistant to American and Asian ZIKV than to African ZIKV suggesting a local adaptation between a ZIKV strain and a mosquito population. There is evidence today that Ae. aegypti formosus is found both in urban and forest habitats in sub-Saharan Africa28 and a mixture of Ae. aegypti aegypti and Ae. aegypti formosus is likely present in cities we sampled in Cameroon, Congo and Gabon29. As Ae. aegypti aegypti is more susceptible to ZIKV than Ae. aegypti formosus, an intermediate level of susceptibility can be expected7. Ae. aegypti formosus, which is predominant in Benoué, was previously found less susceptible to ZIKV compared to the other populations from Cameroon30. Nevertheless, mosquitoes were only identified according to morphological criteria31 and not based on their genotypes. As such, the observed local reduction in vector competence may also result from other inherited factors not detected through morphological examinations. Our experiments showed a decreased susceptibility within the country, with Ae. aegypti from Benoué having lower TE (34.4% [20.2–52.1%]) than those sampled elsewhere in Cameroon (ranging from 62.5 to 83.3%, Supplementary Table 2) except in Maroua, located close to Benoué. Intriguingly, human epidemics of ZIKV associated with Ae. aegypti have been observed in a limited number of countries in Asia or Africa following the 2015–2016 Latin American and the Caribbean epidemic24. The Asian Ae. aegypti population from Cambodia we tested, transmitted the most efficiently all three ZIKV strains, African, American and Asian, corroborating its good vector competence to ZIKV. Recent systematic screenings of patients revealed that ZIKV was circulating in Singapore, Thailand, Cambodia, Myanmar and Vietnam suggesting that ZIKV has been present in Asia at a low but sustained level for years or even decades32,33,34,35. Moreover, human population immunity could also modulate the risk of outbreaks36; a large population exposed to dengue viruses may develop neutralizing antibodies against dengue viruses which cross-react with ZIKV adjusting the outcome of ZIKV infection36. Thereby, Asian populations can be partially protected against ZIKV37. At an intra-country level, mosquito populations (in our study, e.g. five in Brazil18) share the same profiles of transmission with a highest transmission level with African ZIKV. Once introduced, the African ZIKV has the potential to cause an outbreak whatever the origin of Ae. aegypti populations. Our assessments are based on vector competence which is only one aspect of vectorial capacity; it results from complex interactions of multiple factors influencing pathogen transmission by a vector, including internal factors such as genetic, evolution, immunity, or interactions with other microorganisms, modulated by external factors such as abiotic (like climate or topography) and biotic (like nutrition or hosts)38.
In the tropical belt, Ae. aegypti and Ae. albopictus share the same ecological niche39. Ae. albopictus mosquitoes we tested were more susceptible to African ZIKV suggesting that the species can contribute to an epidemic like it happened in Gabon in 200740. A lower transmission of American and Asian ZIKV was presumably related to a strong mosquito midgut barrier30. Since its arrival in Central Africa in 2000, Ae. albopictus plays a major role in the transmission of different arboviruses41. Ae. albopictus tends to predominate over Ae. aegypti in habitats where both species are sympatric42 and will certainly promote arbovirus spillovers at the edge of their natural sylvatic cycle owing to its generalist behavior (biting both humans and animals, and living in rural areas)41. Ae. albopictus from Central Africa are likely originated from South America which themselves were introduced from North America in 198643. European Ae. albopictus are presumably a genetic mixture of populations from United States and La Reunion43, sharing similar profiles of ZIKV transmission with mosquitoes from La Reunion16,44,45. American ZIKV predominant during the 2015–2016 epidemic, was efficiently transmitted by Ae. albopictus, in Europe5 and Brazil46. In the absence of Ae. aegypti, Ae. albopictus is more likely to trigger ZIKV outbreaks when ZIKV comes from Africa.
Another invasive species, Ae. japonicus also experimentally transmits the African ZIKV47. Native to East Asia, Ae. japonicus arrived in Europe in 200041 and is established in 12 European countries up to now48. Its role in pathogen transmission is still unclear even if arboviral ZIKV RNAs have been detected in field-collected mosquitoes49. Its high ability to become infected, and to a lower extent, to ensure viral dissemination and transmission, concomitantly to its human-biting behavior gives Ae. japonicus the status of being a competent vector of ZIKV47,50. These findings are however subject to caution, as the experimental design may not allow for generalization of our observations: Ae. japonicus could only be sampled and bred in European countries (France (n = 90), Switzerland (n = 90), the Netherlands (n = 62)). To add to this limitation, only two ZIKV strains were tested against these mosquitoes and fully collinear with the country of mosquito sampling: French- and Swiss-sampled Ae. japonicus were infected with an African ZIKV strain, whereas mosquitoes from the Netherlands were infected with an American ZIKV strain (see data structure in Supplementary Fig. 1). Therefore, we cannot distinguish between mosquito susceptibility and ZIKV strain potency.
We conclude that from our comprehensive assessment of vector competence, invasive Aedes mosquitoes set the bed for pandemic arboviruses; in the absence of the historical vector of human arboviruses, Ae. aegypti, other species such as Ae. albopictus or Ae. japonicus can take over as ZIKV vectors and transmit even more efficiently when infected with the African ZIKV.
Methods
Experimental procedures
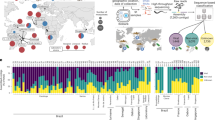
All partners of the ZIKAlliance project (https://zikalliance.tghn.org/) performed their own mosquito experimental infections using a common standardized protocol (Supplementary Fig. 2). Some deviations from this protocol could not be avoided (e.g. availability of blood source, feeding via blood droplets instead of via membrane). Fifty populations (Fig. 4 and Supplementary Table 1) were collected as immature stages and reared in insectaries under controlled conditions. Mosquito females were exposed to an infectious blood meal containing one third of viral suspension and two third of washed rabbit erythrocytes (Supplementary Fig. 3) at a final titer of ~107 TCID50/ml. After 1 h, engorged females were isolated in boxes (28°±1 °C and 80 ± 10% humidity) and fed with 10% sucrose until analysis.
The color code used represents the different mosquito species and numbers refer to the number of mosquito populations tested. In green, are the countries sampled. Colors correspond to mosquito species. The map was built using the open source map site https://cmap.comersis.com/cartes-Monde-WORLD.html.
Different geographic ZIKV strains were used (Supplementary Fig. 4): (1) Africa (Dakar; KU955592; isolated in 1984 from Aedes taylori in Senegal and passaged four times on BHK21 cells), (2) Asia (Cambodia; KU955593; isolated from a human case in 2010 and passaged three times on Vero cells and Malaysia; KX694533; isolated in 1966 from Aedes aegypti in Malaysia and passaged four times on Vero cells), and (3) America (Guadeloupe; LR792671.1; isolated from a human case in Guadeloupe in 2016 and passaged two times on Vero cells, Martinique; KU647676; isolated from a human case in 2015 and passaged three times on Vero cells, and Suriname; KU937936; isolated from a human serum in Suriname in 2016 and passaged 5–6 times on Vero cells). ZIKV Dakar, Malaysia, and Martinique provided by EVAg (https://www.european-virus-archive.com/) were used in most infections. ZIKV Cambodia was used for the mosquito populations Haiti, Funchal, Mont, Corse, ZIKV Suriname for Best, Amsterdam and Lelystad, and ZIKV Guadeloupe for HAV-PT, HAV-PRG.
At 7, 14, and 21 days post-infection (dpi), a batch of mosquitoes was processed to estimate if viral particles were present in the body (thorax and abdomen) as the marker for infection, head for dissemination and saliva for transmission; 0 refers to no viral particles and 1 to at least one viral particle detected. Presence of viral particles was asserted by cytopathic effects observed on a monolayer of Vero CCL-81 cells in 96-well plates45. Three parameters were measured: (1) IR corresponding to the proportion of mosquitoes with infected body (thorax and abdomen) among examined mosquitoes, (2) DE referring to the proportion of mosquitoes with virus detected in the head among examined mosquitoes, and (3) TE representing the proportion of mosquitoes with virus detected in saliva among examined mosquitoes. TE was used as the primary endpoint to evaluate vector competence with respect to ZIKV strain. It is important to note that, by definition, TE ≤ DE ≤ IR, the denominator of each being the total number of mosquitoes dissected.
Statistical analysis
Success for transmission, dissemination and infection were investigated as binomial outcomes to assess how their population-level counterpart (TE, DE, IR) were associated with available combinations of mosquito populations and ZIKV strains, according to time since infection. Time since infection was considered a categorical covariate. Prior to any modeling approach, all outcomes were visualized with heat maps similar to those presented in Supplementary Figs. 6, 8 and 9, for all combinations of ZIKV strain and mosquito population and at every value of time since infection. The resulting experimental design, consisting of combinations of country of study, mosquito species and populations as well as time since infection, was used to guide the development of generalized linear (mixed) models.
Owing to complex hierarchical experimental design, each mosquito species was modeled separately. When several mosquito populations were sampled at different locations within a given country, the variations resulting from the population effect was modeled by a random effect nested within the country, in a mixed regression framework. The contribution of each fixed-effect term was assessed by refitting univariable models and measuring the semi-partial R-squared value (as defined by Stoffel et al.51). Likewise, the contribution of random effects was measured as the adjusted intra-class correlation coefficient (as defined by Nakagawa et al.52). Analyses were conducted using the R software (v. 4.2.0)53 with the following packages: lme4 (v. 1.1-27.1)54, emmeans (v. 1.7.2), performance (v.0.8.0)55 and partR2 (v. 0.9.1).
The robustness of results was assessed using sensitivity analyses, where the same regression models were applied with data aggregated at larger geographical scales for covariates of interest. Three variations of the same analyses were conducted: (1) by aggregating country of study by continent (therefore assuming random variations across countries) and preserving individual ZIKV strains, (2) by aggregating ZIKV strains by continent (corresponding to relatedness between strains in the phylogenetic tree, see Supplementary Fig. 4) and preserving individual countries of study, and (3) aggregating both country and virus strain by continent. The results from aggregation detailed in point (2) are presented in the main text as they provide the most comprehensive set of results. The results corresponding to aggregation levels from points (1), (3) and (4) are presented in Supplementary Figs. 5 through 10.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are fully available in an electronic Supplementary table (Microsoft Excel XLSX format) at https://zenodo.org/record/6788898#.Ysgu0oTP2Uk as well as in the GitLab repository found in the Code availability section.
Code availability
All analyses conducted in this manuscript and associated code have been uploaded to a Git repository at https://gitlab.pasteur.fr/tobadia/17289-zikalliance and are readily available. The results of the present manuscript are based on commit 1477a8be1554c91d2a5ea038964407e88499ddf9.
References
Patterson, K. D. & Pyle, G. F. The geography and mortality of the 1918 influenza pandemic. Bull. Hist. Med. 65, 4–21 (1991).
Poirier, E. Z. & Vignuzzi, M. Virus population dynamics during infection. Curr. Opin. Virol. 23, 82–87 (2017).
Huang, Y.-J. S., Higgs, S. & Vanlandingham, D. L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 34, 104–109 (2019).
Dick, G. W. A., Kitchen, S. F. & Haddow, A. J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Tropical Med. Hyg. 46, 509–520 (1952).
Giron, S. et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Eurosurveillance 24, 1900655 (2019).
Sim, S., Jupatanakul, N. & Dimopoulos, G. Mosquito immunity against arboviruses. Viruses 6, 4479–4504 (2014).
Aubry, F. et al. Enhanced Zika virus susceptibility of globally invasive Aedes aegypti populations. Science 370, 991–996 (2020).
Lambrechts, L. et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evolut. Biol. 9, 160 (2009).
Mariconti, M. et al. Estimating the risk of arbovirus transmission in Southern Europe using vector competence data. Sci. Rep. 9, 17852 (2019).
Viglietta, M., Bellone, R., Blisnick, A. A. & Failloux, A.-B. Vector specificity of arbovirus transmission. Front. Microbiol. 12, 773211 (2021).
Roundy, C. M. et al. Lack of evidence for Zika virus transmission by Culex mosquitoes. Emerg. Microbes Infect. 6, 1–2 (2017).
Guo, X. et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg. Microbes Infect. 5, 1–5 (2016).
Guedes, D. R. et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes Infect. 6, 1–11 (2017).
Viveiros-Rosa, S. G., Regis, E. G. & Santos, W. C. Vector competence of Culex mosquitoes (Diptera: Culicidae) in Zika virus transmission: an integrative review. Rev. Panam. Salud Publica 44, e7 (2020).
Hery, L., Boullis, A., Delannay, C. & Vega-Rúa, A. Transmission potential of African, Asian and American Zika virus strains by Aedes aegypti and Culex quinquefasciatus from Guadeloupe (French West Indies). Emerg. Microbes Infect. 8, 699–706 (2019).
Gomard, Y., Lebon, C., Mavingui, P. & Atyame, C. M. Contrasted transmission efficiency of Zika virus strains by mosquito species Aedes aegypti, Aedes albopictus and Culex quinquefasciatus from Reunion Island. Parasites Vectors 13, 398 (2020).
Abbo, S. R. et al. Forced Zika virus infection of culex pipiens leads to limited virus accumulation in mosquito saliva. Viruses 12, 659 (2020).
Fernandes, R. S. et al. Vector competence of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus from Brazil and New Caledonia for three Zika virus lineages. Pathogens 9, 575 (2020).
Lourenço-de-Oliveira, R. et al. Culex quinquefasciatus mosquitoes do not support replication of Zika virus. J. Gen. Virol. 99, 258–264 (2018).
Lourenço-de-Oliveira, R. & Failloux, A.-B. Lessons learned on Zika virus vectors. PLoS Negl. Trop. Dis. 11, e0005511 (2017).
Gloria-Soria, A. et al. Global genetic diversity of Aedes aegypti. Mol. Ecol. 25, 5377–5395 (2016).
Guzman, M. G. & Harris, E. Dengue. Lancet 385, 453–465 (2015).
Weaver, S. C. & Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 372, 1231–1239 (2015).
Weaver, S. C., Charlier, C., Vasilakis, N. & Lecuit, M. Zika, Chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med 69, 395–408 (2018).
Kramer, L. D. & Ciota, A. T. Dissecting vectorial capacity for mosquito-borne viruses. Curr. Opin. Virol. 15, 112–118 (2015).
Aubry, F. et al. Recent African strains of Zika virus display higher transmissibility and fetal pathogenicity than Asian strains. Nat. Commun. 12, 916 (2021).
Smith, D. R. et al. African and Asian Zika virus isolates display phenotypic differences both in vitro and in vivo. Am. J. Trop. Med. Hyg. 98, 432–444 (2017).
Powell, J. R. Mosquitoes on the move. Science 354, 971–972 (2016).
Weetman, D. et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int. J. Environ. Res. Public Health 15, 220 (2018).
Kamgang, B. et al. Different populations of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Central Africa are susceptible to Zika virus infection. PLoS Negl. Trop. Dis. 14, e0008163 (2020).
McClelland, G. A. H. A worldwide survey of variation in scale pattern of the abdominal tergum of Aedes aegypti (L.) (Diptera: Culicidae). Trans. R. Entomol. Soc. Lond. 126, 239–259 (1974).
Ho, Z. J. M. et al. Outbreak of Zika virus infection in Singapore: an epidemiological, entomological, virological, and clinical analysis. Lancet Infect. Dis. 17, 813–821 (2017).
Ruchusatsawat, K. et al. Long-term circulation of Zika virus in Thailand: an observational study. Lancet Infect. Dis. 19, 439–446 (2019).
Tun, M. M. N. et al. Detection of Zika virus infection in Myanmar. Am. J. Trop. Med. Hyg. 98, 868–871 (2018).
Quyen, N. T. H. et al. Chikungunya and Zika virus cases detected against a backdrop of endemic Dengue transmission in Vietnam. Am. J. Trop. Med. Hyg. 97, 146–150 (2017).
Wen, J. & Shresta, S. Antigenic cross-reactivity between Zika and dengue viruses: is it time to develop a universal vaccine? Curr. Opin. Immunol. 59, 1–8 (2019).
Luo, X. S., Imai, N. & Dorigatti, I. Quantifying the risk of Zika virus spread in Asia during the 2015-16 epidemic in Latin America and the Caribbean: a modeling study. Travel Med. Infect. Dis. 33, 101562 (2020).
Lefèvre, T., Vantaux, A., Dabiré, K. R., Mouline, K. & Cohuet, A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 9, e1003365 (2013).
Lwande, O. W. et al. Globe-Trotting Aedes aegypti and Aedes albopictus: risk factors for arbovirus pandemics. Vector-Borne Zoonotic Dis. 20, 71–81 (2020).
Grard, G. et al. Zika Virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 8, e2681 (2014).
Ngoagouni, C., Kamgang, B., Nakouné, E., Paupy, C. & Kazanji, M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasites Vectors 8, 191 (2015).
Kamgang, B. et al. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl. Trop. Dis. 7, e2590 (2013).
Vega-Rúa, A. et al. Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun. Biol. 3, 1–13 (2020).
Nuñez, A. I. et al. Evidence of Zika virus horizontal and vertical transmission in Aedes albopictus from Spain but not infectious virus in saliva of the progeny. Emerg. Microbes Infect. 9, 2236–2244 (2020).
Vazeille, M. et al. Zika virus threshold determines transmission by European Aedes albopictus mosquitoes. Emerg. Microbes Infect. 8, 1668–1678 (2019).
Parra, M. C. P. et al. Detection of Zika RNA virus in Aedes aegypti and Aedes albopictus mosquitoes, São Paulo, Brazil. Infect., Genet. Evolution 98, 105226 (2022).
Glavinic, U. et al. Assessing the role of two populations of Aedes japonicus japonicus for Zika virus transmission under a constant and a fluctuating temperature regime. Parasites Vectors 13, 479 (2020).
Koban, M. B. et al. The Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in Europe, 17 years after its first detection, with a focus on monitoring methods. Parasites Vectors 12, 109 (2019).
Yang, F. et al. Cache valley virus in Aedes japonicus japonicus Mosquitoes, Appalachian Region, United States. Emerg. Infect. Dis. 24, https://doi.org/10.3201/eid2403.161275 (2018).
Abbo, S. R. et al. The invasive Asian bush mosquito Aedes japonicus found in the Netherlands can experimentally transmit Zika virus and Usutu virus. PLoS Negl. Trop. Dis. 14, e0008217 (2020).
Stoffel, M. A., Nakagawa, S. & Schielzeth, H. partR2: partitioning R2 in generalized linear mixed models. PeerJ 9, e11414 (2021).
Nakagawa, S., Johnson, P. C. D. & Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Lüdecke, D. et al. see: An R Package for Visualizing Statistical Models. https://doi.org/10.31234/osf.io/m4uax (2021).
Acknowledgements
We thank Richard Paul (Institut Pasteur) for correcting English. This study was supported by the European Union’s Horizon 2020 research and innovation program under ZIKAlliance grant agreement no. 734548. The funders had no role in study design, data collection and interpretation, or decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
T.O. performed the writing and editing, data analysis and interpretation. G.G.B., A.I.N., R.S.F., B.K., L.H., Y.G., S.R.A., D.J., U.G., M.V., A.Y., and C.J.M.K. did the investigation and data acquisition. V.D. did the investigation, methodology, and data acquisition. M.D.R., C.M.A., N.P., S.B., P.M., A.V.R., E.V., G.P.P., C.P., N.B., and R.L.O. contributed to supervision, data analysis and interpretation. C.D. and M.T.W. did the data analysis and interpretation. X.D.L. participated in funding acquisition and project administration. A.B.F. intervened in conceptualization, project administration, supervision, and writing original draft. All authors have read and agreed with the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sally Paulson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obadia, T., Gutierrez-Bugallo, G., Duong, V. et al. Zika vector competence data reveals risks of outbreaks: the contribution of the European ZIKAlliance project. Nat Commun 13, 4490 (2022). https://doi.org/10.1038/s41467-022-32234-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32234-y
This article is cited by
-
Evaluating vector competence for Yellow fever in the Caribbean
Nature Communications (2024)
-
Universal whole-genome Oxford nanopore sequencing of SARS-CoV-2 using tiled amplicons
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.