Abstract
Toxin-antitoxin (TA) systems are ubiquitous genetic modules in bacteria and archaea. Here, we perform structural and biochemical characterization of the Legionella pneumophila effector Lpg2370, demonstrating that it is a Ser/Thr kinase. Together with two upstream genes, lpg2370 constitutes the tripartite HipBST TA. Notably, the toxin Lpg2370 (HipTLp) and the antitoxin Lpg2369 (HipSLp) correspond to the C-terminus and N-terminus of HipA from HipBA TA, respectively. By determining crystal structures of autophosphorylated HipTLp, its complex with AMP-PNP, and the structure of HipTLp-HipSLp complex, we identify residues in HipTLp critical for ATP binding and those contributing to its interactions with HipSLp. Structural analysis reveals that HipSLp binding induces a loop-to-helix shift in the P-loop of HipTLp, leading to the blockage of ATP binding and inhibition of the kinase activity. These findings establish the L. pneumophila effector Lpg2370 as the HipBST TA toxin and elucidate the molecular basis for HipT neutralization in HipBST TA.
Similar content being viewed by others
Introduction
Toxin–antitoxin (TA) systems are bacterial and archaeal genetic modules enriched in mobile genetic elements and chromosomes that comprise two or more closely linked genes encoding a toxin protein and its cognate antitoxin1. Since the discovery that the ccdB/ccdA TA system maintains stable inheritance of the mini-F plasmid in Escherichia coli2, the biological roles of TA systems have been demonstrated to include maintaining stabilization and fitness of mobile genetic elements such as plasmids3 and protection against phages4. Toxins are stable enzymes (e.g., RNases and kinases) or other proteins (e.g., gyrase inhibitors and pore-like toxins) that, in the absence of cognate antitoxin, interfere with vital cellular processes such as DNA replication and protein translation5. Antitoxins are unstable proteins or RNAs that counteract toxins. Based on the antitoxin nature and toxin-neutralization mechanism, TA systems can be divided into types I–VIII6,7.
In type II TA systems such as HipBA modules, toxin neutralization depends on direct binding of a proteinaceous antitoxin1,8. HipA from HipBA TA module of the E. coli strain K-12 is a 440-amino acid (aa) Ser/Thr kinase that phosphorylates the tRNAGlu-bound glutamate-tRNA ligase GltX at Ser239, thereby inhibiting protein translation9,10. The growth arrest induced by E. coli HipA can be counteracted by HipB, a cro/C1-type helix–turn–helix (HTH) domain-containing protein11. The structures of HipBA modules from E. coli (HipBAEc) and Shewanella onesidensis (HipBASo) reveal that both TA modules form a HipA2–HipB2 heterotetramer in which HipB binds far from the kinase catalytic center of HipA12. Such neutralization strategy differs from most of other type II TA systems, where antitoxins binding usually occludes the active site13 or mediate allosteric regulation of the toxin14,15,16.
Recent studies demonstrated that TA systems containing toxins homologous to the E. coli HipA are widely distributed in bacterial genomes17, suggesting the diversity of HipBA TA systems17,18. Among these TA systems, a tripartite system designated HipBST was recently identified and experimentally characterized in the enteropathogenic E. coli serotype O127:H618. HipT, which serves as the toxin in the HipBST system, phosphorylates TrpS at Ser197, and its toxicity can be counteracted by the small protein encoded by the adjacent gene hipS17. Importantly, the toxin HipT and the antitoxin HipS of the HipBST system were found to correspond to the N-terminal subdomain 1 and the core kinase domain of the E. coli HipA, respectively. The third protein of the HipBST module, HipB, is analogous to HipB of the HipBA system and appears to enhance the neutralization effect of HipS by binding to an already formed HipT–HipS heterodimer18. Recently, a preprint study reported the structure of HipBST heterotrimer from E. coli serotype O127:H6 and concluded that ATP binding in HipT is prevented by comparing its structure in the heterotrimer to the available structures of E. coli HipBA complex19. However, the general mechanism for toxin neutralization in HipBST TA systems is not fully elucidated.
Legionella pneumophila, the causative agent of Legionnaires’ disease, extensively modifies host signal transduction pathway, especially the post-translational modifications such as ubiquitination and phosphorylation, by translocating hundreds of effectors into the host cell via the Dot/Icm system20,21,22,23. One such effector is the recently identified Lpg237024, which was previously predicted to be an E3 ligase but has not been characterized25.
In this work, we find that Lpg2370 in fact shares sequence identity with the C-terminus of the E. coli K-12 HipA, the toxin of type II TA system HipBA, which is then confirmed by experimental validation and determining the crystal structure of autophosphorylated Lpg2370 (pLpg2370). Furthermore, gene locus analysis indicates that lpg2370 is grouped with lpg2369 and lpg2368 into a tricistronic operon, which we proceed to characterize respectively as the toxin HipTLp, the antitoxin HipSLp, and HipBLp, that constitute the tripartite TA system HipBST. We also determine high-resolution structures of the toxin pHipTLp in complex with the ATP analog AMP–PNP and the binary complex with the antitoxin HipSLp and identify key HipTLp residues involved in ATP binding and interactions with HipSLp. Lastly, a comparison of the three structures determined in this study allowed us to propose the mechanism of toxin neutralization in the type II TA system HipBST.
Results
The L. pneumophila effector Lpg2370 is a Ser/Thr kinase
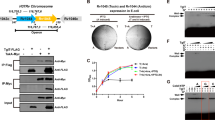
We first verified whether Lpg2370 is translocated into host cells via the type IV secretion system (T4SS) Dot/Icm as an effector protein. To this end, we performed TEM-1 β-lactamase translocation assay by infecting RAW264.7 macrophages with the fusion protein-expressing L. pneumophila cells grown to post-exponential phase. Vectors expressing TEM-1-RaIF (positive control), TEM-1-FabI (negative control) or TEM-1-Lpg2370 fusion proteins were introduced into the T4SS-competent L. pneumophila strain Lp02 or the Dot/Icm-deficient strain Lp03, which were then assessed for the delivery of the β-lactamase fusions into the host macrophage cells by visual inspection under a fluorescence microscope. Cells infected by Lp02 cells expressing the TEM-RalF fusion protein emitted blue fluorescence, whereas infection with the TEM-FabI-expressing cells did not result in any emission of blue fluorescence by host cells (Fig. 1a). In addition, none of the fusion proteins were detectably translocated upon infection with the Dot/Icm-deficient strain Lp03 (Fig. 1a). Consistent with previous studies24, the Lpg2370-overexpressing L. pneumophila strain Lp02 can be secreted into host cells, though at very low translocation efficiencies, suggesting that Lpg2370 is indeed a L. pneumophila effector protein.
a TEM-1 β-lactamase translocation assay demonstrates that Lpg2370 is a L. pneumophila effector protein. RAW264.7 cells were challenged with a T4SS-competent wild-type L. pneumophila strain Lp02 or the dotA-mutant deficient strain Lp03 carrying plasmids encoding TEM-1-RalF (positive control), TEM-1-FabI (negative control), or TEM-1- HipTLp. T4SS-mediated translocation of the fusion proteins into host cells was assessed 2 h after infection by the CCF4-AM-based fluorescence resonance energy transfer assay, scale bars 50 μm. b Schematic of Lpg2370 and the Ser/Thr kinase HipA from E. coli K-12 (HipAEc). Yellow and blue regions represent the approximate locations of the P-loop and activation motif of HipA, respectively, as well as the locations of corresponding sequences in Lpg2370. Sequence conservation of P-loop and the activation motif is presented as a weblog below the protein schematics. c Diagram depicting detection of thiophosphorylated Lpg2370 by thiophosphate labeling. PNBM: p-nitrobenzylmesylate. d Thiophosphate labeling assay with the purified 6×His-tagged Lpg2370. Thiophosphorylated Lpg2370 was visualized by immunoblotting. e Identification of the phosphorylated peptide by LC-MS/MS. The peptide SVQGVQK was observed at charge state 2+ in two forms differing by 70.97 Da in molecular mass.
Although previous studies implied that Lpg2370 is an E3 ubiquitin ligase based on sequence similarity with the RING-type E3 ubiquitin ligase FANCL25,26,27, we repeated primary sequence analysis and did not find any significant similarity between the two proteins. However, we found notable sequence identity (~20%) between Lpg2370 and the residues 64–440 of E. coli HipA, an atypical Ser/Thr kinase from the E. coli K-12 strain (Supplementary Fig. 1)28. The results particularly indicated conservation of the sequences corresponding to the P-loop (RISVAGAQ), the signature motif responsible for ATP binding, and the catalytic loop, which contains the catalytic residue D310 required for the kinase activity of E. coli HipA29,30,31 (Fig. 1b). Moreover, comparing the Lpg2370 sequence to the NCBI database using basic local alignment search tool (BLAST) identified Lpg2370 as a HipA-like Ser/Thr kinase. These findings led us to hypothesize that Lpg2370 could be a kinase.
Kinases frequently undergo autophosphorylation on an invariable Ser or Thr residue in the P-loop32. For instance, autophosphorylation of Ser150 on the P-loop has been observed in HipA kinases from E. coli (HipAEc) and the proteobacterium Shewanella oneidensis (HipASo)30,31. To investigate whether Lpg2370 is also autophosphorylated, we incubated purified recombinant Lpg2370 expressed in E. coli with the N6-benzyladenosine-5’-O-(3-thiotriphosphate) (N6-Bn-ATPγS) (Fig. 1c)33. Immunoblotting with anti-N6-Bn-ATPγS antibody detected a protein band corresponding to Lpg2370 (35 kDa) (Fig. 1d), which clearly indicated that Lpg2370 can be autophosphorylated. We next performed LC-MS/MS to identify the autophosphorylation site on Lpg2370. A mass shift of 79.97 Da (m/z = 684.85, z = 2) was consistently observed in the putative P-loop (53-MSVQGVQKK-61), revealing that the residue Ser54 within the P-loop is the autophosphorylation site (Fig. 1e). Taken together, these results suggest that Lpg2370 is a Ser/Thr kinase, though its substrates are currently unknown.
Lpg2370 adopts a Ser/Thr kinase-like fold
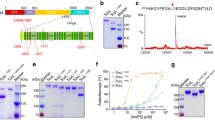
To gain deeper insight into the molecular function of Lpg2370, we set out to determine its crystal structure. Diffraction phases for the SeMet-labeled Lpg2370 were determined using the single-wavelength anomalous diffraction method and the final structural mode was refined at 1.46 Å (Table 1).
Like other members of the protein kinase superfamily, Lpg2370 has a globular kinase fold that can be further divided into N-lobe and C-lobe. The N-lobe, which contains the P-loop, is composed of sheets β1–5 sandwiched by helices α1 and α2, whereas the C-lobe is predominantly α-helical and consists of helix bundles α3–α6 and α8–α11 and a short β-sheet β6–8 (Fig. 2a). In line with the results of primary sequence analysis, there is no apparent structural similarity between Lpg2370 and the E3 ligase FANCL (Supplementary Fig. 2a), and residues 4–28 of Lpg2370, which are relatively well aligned with FANCL25, are a part of the typical kinase N-lobe. In agreement with our LC-MS/MS results, we can observe a phosphate group covalently attached to the Ser54 residue (Fig. 2b). However, unlike HipAEc and HipASo whose P-loops are disordered upon serine autophosphorylation29, the electron density of the Lpg2370 P-loop is well defined in the present structure (Fig. 2b). Interestingly, the P-loop of Lpg2370 differs from the counterparts of typical protein kinases in that it contains a single glycine residue34. The positioning of the phosphorylated Ser54 (pSer54) is stabilized by the positively charged side chains of K40, R131, and R134, as well as hydrogen bonds between side chain of Gln56 and side chains of Asp145 and Lys201 (Supplementary Fig. 2b).
a Cartoon representation of pLpg2370. The N- and C-termini of pLpg2370 and its secondary elements of are labeled correspondingly. The P-loop is colored yellow and the pSer54 is shown in stick representation. b Detailed view of the P-loop with the pSer54. The 2Fo-Fc omit map is contoured at the 1.0 σ level and the P-loop residues are labeled. Crystal structures of the unphosphorylated HipAEc (PDB ID: 3TPB) (c) and pHipAEc (PDB ID: 3TPE) (d). The P-loops are labeled on both structures and colored blue and white, respectively. The P-loop serine/phosphoserine is shown as a stick representation. e Superimposition of pLpg2370 with the structures of pHipA and HipA. f Detailed view of the P-loops from the three superimposed structures. Ser150 in HipAEc and phosphoserines in pHipASo and pLpg2370 are shown as spheres. Please note that the phosphorylated P-loop of Lpg2370 is bent toward N-lobe, similar to the unphosphorylated P-loop of HipA.
Dali search suggested that Lpg2370 shares the highest structural homology score with HipAEc and HipASo (Supplementary Table 1)35. Lpg2370 and HipAEc superimpose with a relatively large root-mean-square deviation (RMSD) value of 3.846 Å over 221 Cα atoms (Fig. 2c, d). In addition to lacking a counterpart to the N-terminal region of HipAEc (i.e., helices α1–α4 and strands β1–β3 of HipA), Lpg2370 differs from HipAEc mainly in the configuration of the N-terminal lobe (Fig. 2c). One prominent difference is that the Lpg2370 P-loop with phosphorylated Ser54 is exposed to solvent in an orientation similar to that of unphosphorylated P-loop of HipAEc, which upon serine autophosphorylation rotates by ~180° and bends away from the N-terminal lobe by 17.3 Å (Fig. 2e, f). Conversely, the six C-terminal α-helices of Lpg2370 and HipAEc, including the catalytic residues and some ATP-binding residues, are almost perfectly aligned (Fig. 2e).
Lpg2368–Lpg2369–Lpg2370 constitute the tripartite HipBST TA system of L. pneumophila
Further analysis of Lpg2370 showed that proteins containing the C-terminal domain of HipA are widespread in bacteria (Fig. 3a). Moreover, the structural similarity between Lpg2370 and E. coli HipA and the fact that E. coli HipA along with HipB from the same genomic locus composes a type II TA system prompted us to examine the locus of lpg2370. Indeed, we found that lpg2370 is preceded by open reading frames (ORFs) of lpg2368 and lpg2369. Analogously to TA systems such as HipBA28, lpg2368, and lpg2369 as well as lpg2369 and lpg2370 overlap by 4 bp. Further analysis suggested that lpg2369 encodes a 102-aa protein similar to the N-terminal region of E. coli HipA and that lpg2368 encodes a 72-aa protein homologous to the helix–turn–helix (HTH) domain of HipB (Fig. 3b and Supplementary Fig. 3a, b). Such locus organization is reminiscent of the HipBST TA module in E. coli O127:H617,18, suggesting that the lpg2368–lpg2369-lpg2370 locus is a potential tricistronic operon encoding component of a HipBST TA system.
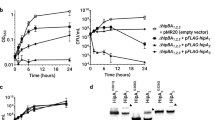
a The HipA-C-terminal domain-like proteins are found in bacteria, branches of the IQTree maximum likelihood phylogenetic tree of representative HipA-C-terminal sequences are colored by the taxonomic groupings as per the upper left panel. b Schematic of the lpg2368–lpg2369-lpg2370 operon in L. pneumophila and the HipBST operon from E. coli O127:H6 strain. In the genome of L. pneumophila, lpg2368, lpg2369, and lpg2370 (relative position of the conserved P-loop is highlighted in yellow) are located in the same operon and encode proteins that share homology with the HipBST TA system. c Comparison of elution profiles of Lpg2370 (11.3 mL), Lpg2370-Lpg2369 (10.9 mL), and Lpg2370-Lpg2369-Lpg2368 (9.6 mL) in size-exclusion chromatography (Superdex 75 increase column). The accompanying SDS-PAGE gel with peak fraction samples is provided on the right side. d Growth curve (upper panel) and the CFU count (bottom panel) of L. pneumophila ∆lpg2368-∆lpg2369-∆lpg2370 (∆3) expressing the kinase site active mutant H199A (H/A) and the wild-type Lpg2370, Lpg2370 causes cellular growth arrest upon induction, whereas the kinase site active mutant H199A (H/A) does not. e Growth curve (upper panel) and the CFU count (bottom panel) of overnight cultures L. pneumophila ∆lpg2368-∆lpg2369-∆lpg2370 (∆3) harboring pZL507 (pZL507:: lpg2370) or the empty pZL507 combined with pJL03 (pJL03:: lpg2368), pJL03 (pJL03:: lpg2369), pJL03 (pJL03:: lpg2368–lpg2369) or the empty low-copy-number pJL03 vector, as indicated, the tested bacterial strains were diluted in fresh AYE broth (supplemented with 10 μg/mL Gentamicin) to OD600 = 0.1 and split into 2-mL subcultures. 200 μM IPTG was added to induce the expression of Lpg2370, and 1% arabinose was added to induce the expression of Lpg2368, Lpg2369, or Lpg2368 + Lpg2369. In panels d and e, data shown are mean values ± SD (n = 3 independent experiments).
In the HipBST TA system of E. coli O127:H6, the toxin HipT (denoted HipTO127) can form a heterotrimeric complex with the antitoxin HipSO127 and the HTH domain protein HipBO12718. We therefore performed size-exclusion chromatography, pull-down assays and isothermal titration calorimetry (ITC) to analyze interactions between Lpg2370, Lpg2369, and Lpg2368. The co-expressed 6×His-tag Lpg2369 and untagged Lpg2370 were co-eluted using Ni affinity chromatography, and the size-exclusion chromatography analysis revealed that the peak is shifted forward by 0.4 mL compared to the peak of Lpg2370 alone, suggesting Lpg2369 can interact with Lpg2370 (Fig. 3c). Moreover, size-exclusion chromatography indicated that Lpg2368 co-elutes with the co-expressed 6×His-tagged Lpg2369–Lpg2370 complex and binds to the Lpg2369–Lpg2370 complex assembled in vitro (Fig. 3c), which was then further confirmed by the pull-down assays (Supplementary Fig. 4). These results suggest that Lpg2370 directly interacts with Lpg2369, whereas Lpg2368 binds to a stable Lpg2369–Lpg2370 complex. Moreover, the results of ITC assays demonstrated that the dissociation constants between Lpg2370 and Lpg2369 and Lpg2370-Lpg2369 complex and Lpg2368 are 42 nM and 1.5 µM, respectively (Supplementary Fig. 5a, b), which is in agreement with the previously published data on HipBSTO12718.
Given the established analogy between the L. pneumophila Lpg2368–Lpg2369–Lpg2370 operon and the HipBST TA system, we next aimed to functionally characterize Lpg2370 by investigating its potential toxicity to host bacteria. Heterogeneous expression of the recombinant Lpg2370 in E. coli had no observable effect on cell growth (Supplementary Fig. 6). The toxicity assays were performed in the L. pneumophila strain Lp02. To avoid undesirable effects from endogenous expression, we prepared deletion strain ∆lpg2368-∆lpg2369-∆lpg2370 (∆3) and examined bacterial growth upon overexpression of recombinant Lpg2370. Overexpression of Lpg2370 significantly inhibited the growth of L. pneumophila, both on plates and in liquid medium (Fig. 3d). Moreover, the catalytically inactive H199A (H/A) mutant failed to inhibit bacterial growth (Fig. 3d), indicating that the kinase activity of Lpg2370 is strictly required for its toxicity (Fig. 3d).
To assess the impacts of Lpg2368 and Lpg2369 on bacterial growth, we inserted lpg2370 into the low-copy-number IPTG-inducible vector pZL507, and lpg2368, lpg2369, or lpg2368–lpg2369 were separately inserted into the plasmid pJL03 with the arabinose-inducible pBAD promoter. Growth and viability of the L. pneumophila Δ3 strain carrying combinations of these plasmids was then monitored. Growth inhibition caused by the expression of Lpg2370 was counteracted by co-expression of Lpg2369, suggesting that Lpg2369 functions as the antitoxin (Fig. 3e). Co-expression of Lpg2368 and Lpg2369 was also found to counteract Lpg2370-dependent growth inhibition, whereas the expression of Lpg2368 without Lpg2369 could not prevent the growth inhibition (Fig. 3e). Taken together, these results are consistent with the findings on the E. coli O127:H6 HipBST module17,18 and demonstrate that Lpg2368, Lpg2369, and Lpg2370 from L. pneumophila constitute the tripartite HipBST TA system18 and will thus hereafter be referred to as HipTLp, HipSLp, and HipBLp, respectively17,18.
The kinase activity of HipTLp is likely independent of P-loop serine autophosphorylation
A comparison of the crystal structure of pHipTLp and the structures deposited in the PDB revealed that the autophosphorylated P-loop in HipTLp adopts an orientation similar to that of the P-loop in the crystal structure of E. coli HipA S150A mutant (Fig. 2f)30. This observation led us to speculate that pHipTLp can bind ATP. Thermal shift assays performed with the purified wild-type HipTLp revealed a 2.5 °C-increase in the melting temperature (Tm) in the presence of non-hydrolysable ATP analogue adenylyl-imidodiphosphate (AMP–PNP), suggesting that pHipTLp indeed binds ATP (Supplementary Fig. 7). Likewise, isothermal calorimetry determined that the dissociation constant between pHipTLp and AMP–PNP was about 70 µM (Fig. 4a), which is within the range of ATP-binding affinity expected for other kinases29.
a Binding of AMP–PNP to pHipTLp monitored by ITC, the presented data is from a single ITC experiment. b Left: cartoon representation of the pHipTLp–AMP–PNP complex. The N- and C-termini of pHipTLp are labeled. The bound AMP–PNP molecule and pSer54 are shown as sticks and colored red and yellow, respectively. Right: detailed view of the ATP-binding cavity of HipTLp and interactions formed with AMP–PNP. The distance between the interacting residues of HipTLp and AMP–PNP are in range of 2.7–3.3 Å, which was shown as sticks and hydrogen bonds are indicated with black dashed lines. c Sequence alignment of HipT variants from L. pneumophila, Escherichia coli O127:H6, Vibrio halioticoli and Haemophilus influenzae reveals conservation of the residues involved in ATP binding. The ATP-binding residues are encircled with black bold rectangles. d Growth curves of E. coli BL21(DE3) cells expressing recombinant wild-type HipTO127 or its mutant variants S57A, S57D, K64A, K86A, H212A, N215A, and D233A.
To elucidate how pHipTLp binds ATP, we determined the crystal structure of pHipTLp in complex with AMP–PNP at 1.36 Å resolution (Table 1). The structure of pHipTLp–ATP reveals that AMP–PNP is bound to the P-loop like in other representative kinases (Fig. 4b). The backbone of pHipTLp in the complex is virtually identical to the apo structure (RMSD = 0.35 Å), with the exception of P-loop that bends towards helix α2 to accommodate the AMP–PNP. ATP (AMP–PNP)-interacting residues appear to be conserved among the bacterial HipT toxins, implying a shared mechanism for ATP binding (Fig. 4c). In pHipTLp, the γ-phosphate of AMP–PNP is stabilized by V58, H199, and D219, the β-phosphate forms hydrogen bonds with Q59, K61, and K85, whereas the α-phosphate interacts with K85 and N202. The adenosine moiety interacts with the main chain of K130 and forms π-stacking interactions with the side chain of F132 (Fig. 4b).
A previous study demonstrated that the kinase activity of E. coli HipA is essential for the growth arrest of host cells36, and cell growth was inhibited when HipTO127 was expressed in E. coli BL21 (DE3) cells18. To confirm the role of the residues involved into the ATP binding in the HipT toxins in vivo, HipTO127 TA is used to perform the growth inhibition assays due to the easy manipulation of E. coli compared to L. pneumophila. To investigate whether the above-mentioned residues responsible for ATP binding are essential for the kinase activity of HipT, we performed in vivo toxicity assays with HipTO127 variants in which residues corresponding to the S54 and the highly conserved ATP-binding residues of HipTLp were substituted with alanine. Intriguingly, mutation on the residues corresponding to S54 of HipTLp (S57A and S57D HipTO127) remain toxic to E. coli cells, whereas substitutions of K64 (K61), K86 (K85), H212 (H199), N215 (N202), and D233 (D219) of HipTO127 (corresponding residues in HipTLp are indicated in parentheses) eliminated the toxic phenotype (Fig. 4d). Taken together, these results suggest that unlike in E. coli HipA, HipT retains the ATP-binding ability independent of the autophosphorylation on the conserved S54 in the P-loop and that HipT uses a universal mode for ATP recognition.
Structural basis for the toxin HipTLp recognition by the antitoxin HipSLp
Although the toxic activity of HipT in the HipBST TA system has been demonstrated to be counteracted by the antitoxin HipS17,18, the underlying molecular mechanism remains unknown. We therefore sought to determine the structure of the HipTLp–HipSLp complex. To express the HipTLp–HipSLp complex, a ribosomal-binding site (RBS, AGGAGA)37 was introduced between the stop codon of HipSLp and the start codon of HipTLp. The resultant HipSLp–RBS-HipTLp was cloned into pET21a (+) vector. The crystal structure of the SeMet-labeled HipTLp–HipSLp complex was determined and refined at 1.89 Å resolution (Table 1).
In the structure of HipTLp–HipSLp complex, a copy of HipTLp and HipSLp each were observed per crystal asymmetric unit. Residues belonging to helices α1 and α2 of HipTLp were not visible in the electron density map, whereas the density of the remaining residues was unambiguous (Fig. 5a). All 102 residues of HipSLp were successfully built into the model, showing that HipSLp is a small single-domain protein composed of five β-strands and three α-helices. The overall structure of the HipTLp-HipSLp complex is highly similar to E. coli HipA, with HipSLp and HipTLp aligning with the N- and C-terminal portions of E. coli HipA, respectively (Supplementary Fig. 8a). HipSLp superimposed with the N-terminus of E. coli HipA with an overall RMSD of 0.932 Å across 64 Cα. However, a notable difference can be observed on the β4–α2 loop of HipSLp, which is twisted and rotated by ~45° with respect to its counterpart in the N-terminus of E. coli HipA (Supplementary Fig. 8b).
a Cartoon representation of the HipTLp–HipSLp complex. HipTLp and HipSLp are colored cyan and green, respectively, and their N- and C-termini are labeled correspondingly. b Relative locations of three interacting regions formed between HipTLp and HipSLp. The two proteins are color-coded as in panel A, and HipTLp is shown in surface representation. Interacting residues of HipSLp are shown as sticks and the three regions involved in interaction with HipSLp are encircled with black, blue, and red rectangles, respectively. c–e Detailed view of the three interacting regions formed between HipTLp and HipSLp. Interacting residues are shown as sticks and hydrogen bonds are indicated with black dashed lines. f Pull-down assays performed with wild-type or mutant HipTLp carrying C-terminal 6×His-tag and untagged wild-type HipSLp.
In the structure of HipTLp–HipSLp complex, three α-helices of HipSLp form a helix bundle that sits above the cleft formed by the β-sheet in the N-terminal lobe of HipTLp, whereas the β-strands form a flank region in HipSLp (Fig. 5b). HipSLp binds the toxin HipTLp via hydrogen bonding in three main interacting regions, which constitute more than 1100 Å2 of total buried surface area (Fig. 5c–e). The intermolecular interactions are mainly formed between helices α1, α2 and α1–α2 loop of HipSLp and helix α3 and strand β5 of HipTLp. In the first interacting region, side chains of HipSLp E63 and HipTLp K157 form a salt bridge, side chain of HipSLp E58 engages in polar interactions with the main chain amide and side chain of HipTLp R154, and hydrogen bonds are additionally formed between main chain of HipSLp G59 and side chain of HipTLp K201 and main chains of HipSLp I65 and HipTLp G57 (Fig. 5c). The second interacting region includes a salt bridge between HipTLp D77 and HipSLp K73 and hydrogen bonds between (i) side chain of HipTLp D133 and HipTLp G94, (ii) side chain of HipTLp R154 and side chain of HipSLp N91 as well as main chain of HipSLp V92, (iii) side chain of HipSLp N91 and main chain of HipTLp Y79, and (iv) side chains of HipTLp Q78 and HipSLp Q90 (Fig. 5d). In the third interacting region, the side chain of HipTLp Q148 hydrogen bonds with the main chain of HipSLp F56, whereas the side chain of HipTLp E144 forms hydrogen bonds with the side chain of HipSLp S38 and the main chain of HipSLp L39 (Fig. 5e).
To verify the importance of these interactions for stable binding of HipSLp to HipTLp, we performed pull-down assays with untagged wild-type HipSLp and wild-type or mutant HipTLp carrying a N-terminal 6×His-tag. The HipTLp mutants D133A, R134A, and E144A completely lost their ability to bind HipSLp and the mutants R154A, K157A, K201A exhibited severely reduced HipSLp binding, suggesting that these residues form key interactions with HipSLp (Fig. 5f).
Molecular mechanism for toxin neutralization in the HipBST TA systems
One of the most striking features of the HipBST TA systems is that the role of antitoxin is taken by HipS which corresponds to the N-terminal portion of HipA toxin from the E. coli HipBA system. To better understand how the toxic activity of HipT is neutralized by HipS, we reinspected and compared the structures of apo pHipTLp, pHipTLp–AMP–PNP complex, and HipTLp–HipSLp complex. Apo pHipTLp and HipTLp from the HipTLp–HipSLp complex superimpose with RMSD of 0.464 Å over 215 Cα atoms. Notably, Ser54 of HipTLp is phosphorylated in the structure of apo pHipTLp but not in the HipTLp–HipSLp complex (please note that HipTLp–HipSLp was co-expressed in E. coli BL21) (Fig. 2a, b and Supplementary Fig. 8c). Since the residue S54 is phosphorylated when HipTLp is expressed alone, we wondered whether the phosphorylation on S54 influences the interaction between HipTLp-HipSLp and HipBLp. Size-exclusion chromatography revealed that the phosphorylation state of S54 does not appear to have a noticeable effect on interactions between HipTLp-HipSLp and HipBLp (Supplementary Fig. 9). Moreover, structural comparison suggests that the P-loop of HipTLp, which encircles ATP and is critical for catalytic activities in typical Ser/Thr kinases, underwent a conformational change from loop to helix upon HipSLp binding (Fig. 6a, b). Such allosteric regulation induced by the antitoxin binding has not been observed in E. coli HipBA TA system29. A conformational change similar to the loop-to-helix change of the HipTLp P-loop in HipTLp-HipSLp can also be observed in the recently released structure of HipBSTO127 trimer19 (Supplementary Fig. 10), suggesting a common mechanism of toxin neutralization.
a Overlay of apo pHipTLp and structure of HipTLp–HipSLp in cartoon representation. Apo pHipTLp is colored gray and its P-loop is colored yellow, whereas HipTLp from the binary complex is colored cyan and its P-loop is colored purple. HipSLp is colored green. b Close-up view of the overlay showing that HipSLp binding induces conformational change of the P-loop. c Overlay of the pHipTLp–AMP–PNP and the structure of HipTLp–HipSLp in cartoon representation. pHipTLp from the pHipTLp–AMP–PNP is colored white. The HipTLp–HipSLp complex is color-coded as in panel a. d Close-up view of the overlapping between HipTLp residues and AMP–PNP. AMP–PNP is shown as dotted surface mode and overlapping HipTLp residues are shown as sticks. e Crystal structure of HipBSTO127 (PDB:7AB5) HipTO127, HipSO127 and HipBO127 were colored cyan, green and yellow orange, respectively. f Structural comparison between the HipTLp–HipSLp and HipBSTO127. The P-loops of HipTLp and HipTO127 are colored red and blue, respectively. g ITC thermogram and binding curve demonstrated that the HipTLp–HipSLp complex does not display detectable affinity for AMP–PNP. h Proposed model for toxin-neutralization mechanism in the HipBST TA systems. The toxin HipT is a Ser/Thr kinase in which the P-loop motif is vital for ATP binding and subsequent substrate phosphorylation. Binding of the antitoxin HipS causes conformational changes in the P-loop, which blocks ATP binding and ultimately inhibits the kinase activity of HipT.
These observations led us to hypothesize that the loop-to-helix conformational transition induced upon HipSLp binding may obstruct the access of ATP to the kinase active site, resulting in inhibition of the HipTLp kinase activity. Superimposition of the structures of pHipTLp, pHipTLp–AMP–PNP, and the HipTLp–HipSLp further revealed that P-loop in the HipTLp–HipSLp complex overlaps with AMP–PNP in the pHipTLp–AMP–PNP complex (Fig. 6c, d and Supplementary Fig. 11). More specifically, the γ-phosphate and β-phosphate groups of AMP–PNP would clash with the side chains of Q59 and D219, respectively, whereas the α-phosphate would clash with the side chains of K61 and K85 (Fig. 6d). This may account for the unphosphorylated state of the P-loop in the HipTLp–HipSLp complex when they were co-expression (Supplementary Fig. 8c). Such conformational change also occurs in the P-loop of HipBSTO127, suggesting that a similar mechanism is utilized by HipBSTO12719 (Fig. 6e, f). To further verify whether ATP binding is abolished, we measured the binding affinity between the HipTLp–HipSLp complex and AMP–PNP with ITC and found that HipTLp completely lost the AMP–PNP binding affinity when binding with HipSLp (Fig. 6g). Consistent with these results, the thermal stability of the HipTLp-HipSLp complex did not change upon the addition of 4 mM AMP–PNP (Supplementary Fig. 11a). Considering that HipAEc in autophosphorylated form can bind ADP and AMP but not ATP30, we also investigated whether the HipTLp–HipSLp complex binds ADP and AMP. Again, the results of ITC experiments suggested that the HipTLp–HipSLp complex has no detectable affinity for ADP or AMP, even at concentrations of 1 mM (Supplementary Fig. 12). The allosteric regulation of the P-loop induced by the antitoxin binding was also observed in the HipBSTO12719. Together, these findings suggest that HipSLp binding induces conformational changes in the P-loop of HipTLp, which blocks ATP binding and consequently inhibits the HipTLp kinase activity (Fig. 6h).
Discussion
Although the biological functions of TA systems are often ambiguous and debatable, recent advances in the field and the discovery of numerous novel TA modules increasingly support their roles in viral defense or plasmid stability4,7 and interactions between hosts and their mobile genetic elements. Among known TA modules, the type II TA systems are most well understood. Type II TA systems typically consist of two components, though several tripartite type II TA modules have been identified, such as the Rv1955-Rv1956-Rv1957 TA-chaperone (TAC) system of Mycobacterium tuberculosis38,39, and the recently discovered E. coli HipA-like TA system HipBST of E. coli O127: H618.
Most type II antitoxins are composed of an N-terminal DNA-binding domain that regulates transcription from the TA locus through direct interaction with the promoter, and a C-terminal region responsible for the toxin binding and inhibition40. HipBA modules are representative type II TA systems and are ubiquitous in bacteria28. Based on the similarity searching using E. coli HipA against the sequenced microbial genomes, a three-component widespread HipBST TA was found and experimentally verified18. Later bioinformatic analysis has suggested the presence of several other HipBA-like TA in numerous bacterial species17. While the toxin HipTO127 of HipBSTO127 was found to exert its toxic function by phosphorylating TrpS, and in spite of the similarity between HipBO127 and E. coli HipB, HipBO127 however cannot neutralize the HipTO127 kinase. This task was taken over by HipS, a small protein with homology to the N-terminal part of E. coli HipA18,19. Our current study also experimentally identifies a tripartite HipBSTLp TA in which the antitoxin HipSLp instead of HipBLp restores the growth inhibition induced by HipTLp in human pathogen L. pneumophila, which is in agreement with the HipBSTO127 TA18.
Together with the preprint study in ref. 19, our study identifies and elucidates the toxin-neutralization mechanism in the tripartite HipBST TA systems of L. pneumophila. It is clear that the toxin neutralization mechanism in HipBST systems is notably different from the corresponding mechanism in HipBA TA system of E. coli18. The structural study by Bærentsen et al. on the E. coli O127:H6 HipBST found that HipT adopts an inactive conformation in the HipBST complex that prevents ATP binding19. However, it was unclear whether the blockage of ATP binding arises from the binding of the antitoxin HipS or autophosphorylation of the conserved P-loop serine19. Our structure of the HipTLp–HipSLp complex clearly shows the toxicity of HipTLp is neutralized directly upon HipSLp binding, leading to blocking of the ATP-binding site through steric hindrance. Although this study demonstrates that HipBLp forms a heterotrimer with the HipTLp–HipSLp complex, we failed to obtain crystals of the heterotrimer structure after extensive crystal screening. However, the available structure of E. coli HipBST shows that HipB binds to HipT but does not interact with the kinase active site19. In addition, overall architecture of HipBSTO127 system is reminiscent of the structure of HipBASo system in the HipBASo-DNA complex, implying that HipBST can also bind DNA19. Induction of HipBO127 was found to be required and sufficient for the transcriptional repression of the HipBST TA19. Thus, it seems that the toxin neutralization and the autoregulation of the HipBST TA are carried out by the antitoxin HipS and HipB, respectively19. Nevertheless, the exact role of HipB in the HipBST systems remains to be determined.
To the best of our knowledge, HipTLp is the only identified toxin of TA systems secreted into host cells via the type IV secretion system Dot/Icm. Nevertheless, the employment of TA toxins by the pathogenic bacteria during host cell infection is not unprecedented. For instance, the type III secretion system effector AvrRxo1-ORF1 from Xanthomonas oryzae pv. oryzicola constitutes a type II TA system with adjacent AvrRxo1-ORF241. AvrRxo1 can phosphorylate NAD in planta, leading to the suppression of the flg22-triggered ROS burst42. Moreover, previous investigation of fourteen effectors from Burkholderia gladioli revealed that the restriction endonuclease Tox-Rease-5 domain-containing effector TaseTBg can be employed to inhibit the growth of co-habiting bacterial species, and its activity is counteracted by the associated immunity protein TsiTBg43. Apart from the effectors LegK1–K5 and LegK7, HipTLp is the sixth effector known to function as a Ser/Thr kinase in L. pneumophila. LegK1 phosphorylates NF-κB to activate the genes expression involved in inflammation during infection44. Phosphorylation of Hsp70 at T495 by LegK445, leading to the inhibition of protein synthesis. LegK7 phosphorylates a conserved scaffold protein MoB1, hijacking the hippo pathway to promote its survival33,46. The exact substrate of HipTLp in its host remains to be identified in future studies. In summary, these results suggest that TA systems could serve as a reservoir for additional secreted effectors, which sheds light on the evolutionary links between the TA system and the effectors secreted by the pathogenic microorganisms.
Methods
Bacterial strains and growth conditions
The L. pneumophila strain Philadelphia-1 derivative Lp0247 was used as the progenitor of all derivative strains used in this study. To construct ∆lpg2368-∆lpg2369-∆lpg2370 triple-deletion mutant strain of L. pneumophila (termed ∆3), we first constructed deletion plasmid by cloning the upstream and downstream flanking regions into pSR47S. Briefly, the 1.2-kb fragment located upstream of lpg2368 and the 1.2-kb fragment located downstream of lpg2370 were obtained by PCR using ∆3 A1/∆3 A2 and ∆3 B1/∆3 B2 primer pairs and high-fidelity FastPfu DNA polymerase (TransGen) (Supplementary Table 2), respectively. The amplified PCR products were used as templates to produce a DNA fragment containing the flanking regions by fusion PCR with primers ∆3 A1/∆3 B2 and high-fidelity FastPfu DNA polymerase (TransGen). After digestion with BamHI and SalI, the DNA fragment was inserted into BamHI/SalI-digested pSR47S48. The deletion plasmid was then introduced into Lp02 by triparental mating, and conjugants were selected on CYET plates containing kanamycin (20 μg/mL) and streptomycin (50 μg/mL). Deletion mutants were verified by standard colony PCR techniques using primers ∆3 A1/∆3 B2 and 2×Taq master mix (Novoprotein) from colonies grown on CYET plates containing 5% sucrose49. Moreover, the lpg2370, lpg2368, lpg2369, and lpg2368–lpg2369 genes were inserted into pZL507 or pJL03 as BamHI/SalI fragments to construct pZL507-Lpg2370, pJL03-Lpg2368, pJL03-Lpg2369, and pJL03-Lpg2368-Lpg2369 plasmids, which were then introduced into the ∆lpg2368∆lpg2369∆lpg2370 strain. For effector translocation, fresh single colonies of Lp02 or Lp03 harboring expression plasmid for TEM-1-Lpg2370, TEM-1-RalF, or TEM-1-FabI fusion proteins were streaked onto BCYE plates 2 days before infection.
TEM-1 β-lactamase translocation assays
To test Dot/Icm-dependent transfer of the fusion proteins into host cells, L. pneumophila cells expressing the fusion proteins were grown in the presence of 0.5 mM IPTG to post-exponential phase and used to infect monolayers of RAW264.7 cells that were seeded in 96-well plates at an MOI of 20. The CCF4-AM substrates (Invitrogen, Carlsbad, CA) were mixed with medium in the wells two hours after infection. After further incubation for 1 hour at room temperature, infected cells were inspected under a Nikon IX-80 fluorescence microscope equipped with a β-lactamase FL-Cube (U-N41031, Chroma Technology Corp, Bellows Falls, VT). Images of infected cells were obtained using a DP-72 color fluorescence camera (Olympus). Translocation of the β-lactamase chimeras was assessed by the presence of cells emitting blue fluorescence signals. The percentage of infected cells was determined by counting the number of cells emitting blue fluorescence in specified areas of the wells. TEM-1-RalF and TEM-1-FabI fusion proteins were used as positive and negative controls, respectively. Experiments were performed in triplicate, and at least 300 cells were counted in each sample.
Protein expression
DNA fragments encoding full-length HipTLp, HipSLp, and HipBLp or their variants were inserted into pET21a (+) containing a N-terminal 6×His-tag, respectively. To obtain HipTLp–HipSLp complex, HipSLp–HipTLp was cloned in the pET21a (+) following previously described methods37,50 to express the 6×His HipTLp–HipSLp. The recombinant plasmids were transformed into E. coli BL21 (DE3) cells for protein expression. Overexpression was induced in log phase cultures (OD600 ≈ 0.6) by cooling cultures on ice for 20 min and adding IPTG to a final concentration of 0.4 mM, followed by overnight incubation at 16 °C and 220 rpm. The overexpression of the wild-type HipTLp did not lead to the growth arrest in E. coli. After target protein expression, cells were pelleted by centrifugation and resuspended in buffer A (50 mM Tris-HCl, pH 8.0, 100 mM NaCl). The cells were then lysed by ultrasonication and the lysate was centrifugated at 17,000×g and 4 °C for 30 min. The supernatant was loaded onto Ni2+-NTA column (Qiagen) for purification of target recombinant proteins. After washing with 100 mL of buffer A supplemented with 50 mM imidazole, the target proteins were eluted with buffer A supplemented with 250 mM imidazole. Fractions containing the target protein were pooled, concentrated to 0.5 mL and then purified with Superdex 75 increase column (GE Healthcare) equilibrated with buffer B (20 mM Tris-HCl, pH 8.0, 150 mM NaCl).
Selenomethionine (SeMet)-labeled HipTLp and HipTLp–HipSLp were expressed in M9 medium supplemented with 2 mM MgSO4, 0.1 mM CaCl2, 0.5% w/v glucose, 2 mg/L biotin, 2 mg/L thiamine, and 0.03 mg/L FeSO4. When bacterial cultures reached OD600 of 1.0, final concentrations of 100 mg/mL of phenylalanine, lysine, and threonine, and 50 mg/mL of isoleucine, leucine, valine, and of SeMet (Chemie Brunschwig) were added in form of solid powder, after which the cultures were incubated for 30 min. Protein expression was then induced with 0.2 mM IPTG, and the cultures were further incubated on a shaker at 16 °C for 20 h. Cells were collected at 5000×g for 15 min and 4 °C and resuspended in the lysis buffer (50 mM Tris pH 8.0, 100 mM NaCl, 5 mM β-mercaptoethanol). Protein purification was performed as described above.
In vitro kinase assays
Purified Lpg2370 (1 µg) was resolved by SDS-PAGE and transferred to the PVDF membrane using a Bio-Rad wet transfer system. The membrane was blocked with 5% milk for 1 h at room temperature. The membrane was incubated overnight at 4 °C with a primary antibody against thiophosphate ester (rabbit anti-thiophosphate ester (ab92570), Abcam, dilution 1:4000 v/v). The membrane was then washed three times with TBST before being incubated with a secondary antibody (HRP-conjugated AffiniPure goat anti-rabbit (Cat No. SA00001-2), Proteintech, dilution 1:5000 v/v) for 1 h at room temperature. The membrane was washed three times with TBST, and proteins were detected using an ECL detection reagent.
Liquid chromatography-mass spectrometry (LC-MS) analysis
LC-MS was used to analyze autophosphorylation of purified recombinant Lpg2370. After staining of gels with Coomassie blue, excised gel segments were subjected to in-gel trypsin digestion and dried. Electrospray ionization mass spectrometry (ESI-MS) was performed using an integrated HPLC/ESI-MS system (1260 Infinity, Agilent Technologies/amaZon SL, Bruker Corporation) equipped with a Luna 5 µm C18 column (100 Å, 250 × 4.60 mm, 5 µm). Peptides were dissolved in 10 µl 0.1% formic acid and were auto-sampled directly onto a homemade C18 column (35 cm × 75 μm i.d., 1.9 μm 100 Å). Samples were then eluted for 60 mins with linear gradients of 3–35% acetonitrile in 0.1% formic acid at a flow rate of 300 nl/min. The mass spectrometer was equipped with a CaptiveSpray source. Survey scans were recorded over 100–1700 m/z range and the mass spectra data were acquired with a timsTOF Pro mass spectrometer (Brucker) operated in PASEF mode. PASEF setting: 10 MS/MS scans (total cycle time 1.27 s), charge range 0–5, active exclusion for 0.4 min, Scheduling Target intensity 10000, Intensity threshold 2500, CID collision energy 42 eV.
The raw files generated from LC-MS/MS were analyzed by Peaks Studio X software (Bioinformatics Solutions Inc., Waterloo, ON, Canada) against the input of the Lpg2370 amino acid sequence. The following database search criteria were set to: enzyme, trypsin; variable modification, phosphorylation, precursor ion mass tolerance, 10 ppm; MS/MS fragment mass tolerance, 0.02 Da; tryptic enzyme specificity with two missed cleavages allowed. Identifications were filtered according to mass accuracy and 1% false discovery rate.
Protein crystallization and collection of crystallographic data
The purified target protein was concentrated to 0.5 mL and loaded onto a Superdex 75 increase column (GE Healthcare) Fractions containing purified proteins were then concentrated at 4000×g, 4 °C to ~15 mg/mL using an Amicon Ultra 30 K centrifugal filter. To obtain HipTLp–AMP–PNP complex, the purified HipTLp was incubated with AMP–PNP at a 1:1.2 molar ratio at 4 °C for 30 min and concentrated to about 12 mg/mL.
For crystallization of SeMet-labeled HipTLp, HipTLp–AMP–PNP complex, and SeMet-labeled HipTLp–HipSLp complex, the purified and concentrated protein samples were mixed with the reservoir solution at equal volumes and crystallized using the sitting drop vapor diffusion method at 16 °C. Initial crystals of SeMet-labeled HipTLp and HipTLp–AMP–PNP complex were obtained within three days in condition containing 8% Tacsimate (pH 6.0) and 20% w/v PEG 3350. Initial crystals of the SeMet-labeled HipTLp-HipSLp complex were obtained in the condition containing 0.1 M sodium acetate (pH 7.0) and 12% v/w PEG 3350. After extensive optimization, diffraction-quality crystals of SeMet-labeled HipTLp and the HipTLp–AMP–PNP complex were grown in the presence of 10% Tacsimate (pH 6.2) and 20% v/w PEG 3350. Diffraction-quality crystals of the HipTLp–HipSLp complex were grown in the presence of 0.1 M sodium acetate (pH 7.2) and 15% w/v PEG 3350. Harvested crystals were preserved in the respective reservoir solutions supplemented with cryoprotectant and flash-frozen in liquid nitrogen.
Structure determination and refinement
All X-ray diffraction data were collected at the BL-02U1 station of the Shanghai Synchrotron Radiation Facility (SSRF). Single-wavelength anomalous diffraction (SAD) datasets of SeMet-labeled HipTLp and SeMet-labeled HipTLp–HipSLp complex were obtained at high resolution and the data were processed with the HKL-2000 package51. Autosol program of PHENIX package was used for SAD phasing and initial model building. Residues 72–312 of HipTLp were auto-built, whereas the residues 1–71 residues were built by iterative manual building in Coot52. Structure refinement was carried out with PHENIX53. The HipTLp–AMP–PNP binary complex was determined using the molecular replacement method with the structure of HipTLp as the search model. Structure quality was analyzed during PHENIX refinements and later validated in the PDB validation server. Detailed crystallographic and structure refinement data are listed in Table 1. Structural images were generated using PyMol (Schrödinger, LLC).
Mutagenesis
Base substitutions in this study were introduced using two pairs of complementary primers (sense and antisense strand primers) containing the desired mutation. The primers used in this study were listed in Supplementary Table 2. All constructs were verified by DNA sequencing.
Isothermal titration calorimetry (ITC)
ITC experiments were performed in Nano ITC Low Volume (TA instruments). All samples were prepared in the buffer containing 20 mM HEPES (pH 8.0) and 150 mM NaCl. Typically, the titrant concentration in the syringe was 200–500 μM, and the titrand concentration in the reaction cell was 10–20 μM. Titration was conducted at 25 °C using multiple injection method with 150 s intervals. Obtained data were integrated, corrected, and analyzed using the NanoAnalyze software (TA Instruments) with a single-site binding model.
Pull-down assays
To perform pull-down assays, wild-type or mutant HipTLp carrying C-terminal 6×His-tag were incubated with Ni-agarose beads for 30 min and then washed twice with buffer containing 20 mM Tris-HCl (pH 8.0) and 150 mM NaCl. The beads were then incubated with untagged wild-type HipSLp (6×His-tag was previously cleaved with TEV protease) for 1 h and then washed twice. The proteins were eluted from beads using a buffer containing 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 250 mM imidazole. Eluted samples were analyzed using SDS-PAGE analysis.
In vivo toxicity assays
For in vivo toxicity assays of HipT from E. coli serotype O127:H6 (denoted HipTO127) to E. coli, which was performed by expressing the hipTO127 gene or its mutant variants in E. coli BL21(DE3). The gene encoding HipTO127 was synthesized and cloned into pET21a (+) vector. Plasmids encoding HipTO127 single-point mutants S57A, S57D, K64A, K86A, H212A, N215A, and D233A were prepared using site-specific mutagenesis, and the primers are listed in Supplementary Table 2. Transformed E. coli BL21 (DE3) cells were plated on agar and a single bacterial colony was transferred to 10 mL LB medium for culturing. Expression of wild-type HipTO127 and its mutant variants was induced with IPTG at 0.2 mM concertation when the bacterial cultures reached OD600 = 0.5, after which the bacterial growth curve was measured every 30 min for 6 h.
For the Lpg2370 in vivo toxicity assays in L. pneumophila, Lpg2370, Lpg2370-Lpg2369, Lpg2370-Lpg2368, Lpg2370-Lpg2369-Lpg2368 were overexpressed in L. pneumophila Lp02 or the ∆lpg2368∆lpg2369∆lpg2370 deletion strain. Overnight cultures of the tested bacterial strains were diluted in fresh AYE broth to OD600 = 0.1 and split into 2-mL subcultures into which different concentrations of arabinose or IPTG were added. The subcultures were then grown at 37 °C with constant rotation at 180 rpm. Cell viability was assessed by readout of OD600 value every 3 h and plotting the values on a log scale.
Thermal shift assays
Thermal shift assays were performed using 1 mg/mL phosphorylated HipTLp or its mutants incubated with ATP/AMP–PNP and varied concentrations of nucleotides (0–4 mM) in 50 mM Tris-HCl (pH 8.0) and 150 mM NaCl. Then the mixture was loaded in 96-well PCR plates, the fluorescence signals were recorded as a function of temperature using Prometheus NT.48 (NanoTemper Technologies) in FRET mode. Fluorescence intensity was measured at Ex/Em of 350/330 nm. The temperature gradient range was set as 20–95 °C with a 0.5 °C ramp over the course of 30 s. Control assays were conducted in the same buffer without ATP/AMP–PNP. The thermal unfolding value (Tm) for pHipTLp was calculated using the curve fitting software PR.ThermControl (NanoTemper Technologies).
Phylogenetic analysis
The sequence of Lpg2370 was blasted in the Uniprot (https://www.uniprot.org/) and all the hit sequences were used for sequence alignment in ClustalW, which was used to build the phylogenetic tree using MEGA software54. The phylogenetic tree was visualized using iTOL55.
Statistics and reproducibility
The TEM-1 β-lactamase translocation assays experiments in Fig. 1a, the western blotting experiment in Fig. 1d, the pull-down assays in Figs. 3c, 5f, Supplementary Fig. 4, and Supplementary Fig. 9 were performed at least triplicate at two independent times.
Data availability
The atomic coordinates and structure factors of the autophosphorylated toxin HipTLp, the complex of HipTLp with AMP–PNP, and HipTLp-HipSLp binary complex have been deposited in the Protein Data Bank under the accession codes 7VKC, 7WCF, and 7VKB. Source data are provided with this paper.
References
Pandey, D. P. & Gerdes, K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33, 966–976 (2005).
Ogura, T. & Hiraga, S. Mini-F plasmid genes that couple host cell division to plasmid proliferation. Proc. Natl Acad. Sci. USA 80, 4784–4788 (1983).
Ni, S. et al. Conjugative plasmid-encoded toxin-antitoxin system PrpT/PrpA directly controls plasmid copy number. Proc. Natl Acad. Sci. USA 118, https://doi.org/10.1073/pnas.2011577118 (2021).
Pecota, D. C. & Wood, T. K. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J. Bacteriol. 178, 2044–2050 (1996).
Van Melderen, L. et al. ATP-dependent degradation of CcdA by Lon protease. Effects of secondary structure and heterologous subunit interactions. J. Biol. Chem. 271, 27730–27738 (1996).
Wang, X., Yao, J., Sun, Y. C. & Wood, T. K. Type VII toxin/antitoxin classification system for antitoxins that enzymatically neutralize toxins. Trends Microbiol. https://doi.org/10.1016/j.tim.2020.12.001 (2020).
Jurenas, D., Fraikin, N., Goormaghtigh, F. & Van Melderen, L. Biology and evolution of bacterial toxin-antitoxin systems. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-021-00661-1 (2022).
De Bruyn, P., Girardin, Y. & Loris, R. Prokaryote toxin-antitoxin modules: complex regulation of an unclear function. Protein Sci. 30, 1103–1113 (2021).
Kaspy, I. et al. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat. Commun. 4, 3001 (2013).
Germain, E., Castro-Roa, D., Zenkin, N. & Gerdes, K. Molecular mechanism of bacterial persistence by HipA. Mol. Cell 52, 248–254 (2013).
Korch, S. B. & Hill, T. M. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J. Bacteriol. 188, 3826–3836 (2006).
Schumacher, M. A. et al. HipBA-promoter structures reveal the basis of heritable multidrug tolerance. Nature 524, 59–64 (2015).
Bertelsen, M. B. et al. Structural basis for toxin inhibition in the VapXD toxin-antitoxin system. Structure 29, 139–150 e133 (2021).
Kamada, K. & Hanaoka, F. Conformational change in the catalytic site of the ribonuclease YoeB toxin by YefM antitoxin. Mol. Cell 19, 497–509 (2005).
Hadzi, S. et al. Ribosome-dependent Vibrio cholerae mRNAse HigB2 is regulated by a beta-strand sliding mechanism. Nucleic Acids Res. 45, 4972–4983 (2017).
Brown, B. L. et al. Three dimensional structure of the MqsR:MqsA complex: a novel TA pair comprised of a toxin homologous to RelE and an antitoxin with unique properties. PLoS Pathog. 5, e1000706 (2009).
Gerdes, K., Baerentsen, R. & Brodersen, D. E. Phylogeny reveals novel HipA-homologous kinase families and toxin-antitoxin gene organizations. mBio 12, e0105821 (2021).
Vang Nielsen, S. et al. Serine-threonine kinases encoded by split hipA homologs inhibit tryptophanyl-tRNA synthetase. mBio 10 https://doi.org/10.1128/mBio.01138-19 (2019).
Bærentsen, R. L. et al. Structural basis for regulation of a tripartite toxin-antitoxin system by dual phosphorylation. Preprint at BioRxiv https://doi.org/10.1101/2022.01.28.478185 (2022).
Qiu, J. et al. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 533, 120–124 (2016).
Gan, N. et al. Regulation of phosphoribosyl ubiquitination by a calmodulin-dependent glutamylase. Nature 572, 387–391 (2019).
Ma, K. et al. The bacterial deubiquitinase Ceg23 regulates the association of Lys-63-linked polyubiquitin molecules on the Legionella phagosome. J. Biol. Chem. 295, 1646–1657 (2020).
Liu, S. et al. Interplay between bacterial deubiquitinase and ubiquitin E3 ligase regulates ubiquitin dynamics on Legionella phagosomes. eLife 9 https://doi.org/10.7554/eLife.58114 (2020).
Zhu, W. et al. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS ONE 6, e17638 (2011).
Lin, Y. H. et al. RavN is a member of a previously unrecognized group of Legionella pneumophila E3 ubiquitin ligases. PLoS Pathog. 14, e1006897 (2018).
Mondino, S., Schmidt, S. & Buchrieser, C. Molecular mimicry: a paradigm of host-microbe coevolution illustrated by Legionella. mBio 11, https://doi.org/10.1128/mBio.01201-20 (2020).
Pisano, A. et al. Revisiting bacterial ubiquitin ligase effectors: weapons for host exploitation. Int. J. Mol. Sci. 19 https://doi.org/10.3390/ijms19113576 (2018).
Moyed, H. S. & Bertrand, K. P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 155, 768–775 (1983).
Schumacher, M. A. et al. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science 323, 396–401 (2009).
Schumacher, M. A. et al. Role of unusual P loop ejection and autophosphorylation in HipA-mediated persistence and multidrug tolerance. Cell Rep. 2, 518–525 (2012).
Wen, Y. et al. The bacterial antitoxin HipB establishes a ternary complex with operator DNA and phosphorylated toxin HipA to regulate bacterial persistence. Nucleic Acids Res. 42, 10134–10147 (2014).
Pereira, S. F., Goss, L. & Dworkin, J. Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol Mol. Biol. Rev. 75, 192–212 (2011).
Lee, P. C. & Machner, M. P. The Legionella effector kinase LegK7 hijacks the host hippo pathway to promote infection. Cell Host Microbe 24, 429–438 e426 (2018).
Taylor, S. S. & Kornev, A. P. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36, 65–77 (2011).
Holm, L. & Rosenstrom, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–549 (2010).
Correia, F. F. et al. Kinase activity of overexpressed HipA is required for growth arrest and multidrug tolerance in Escherichia coli. J. Bacteriol. 188, 8360–8367 (2006).
Zhou, B. et al. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage. PLoS Pathog. 16, e1008394 (2020).
Bordes, P. et al. SecB-like chaperone controls a toxin-antitoxin stress-responsive system in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 108, 8438–8443 (2011).
Guillet, V. et al. Structural insights into chaperone addiction of toxin-antitoxin systems. Nat. Commun. 10, 782 (2019).
Harms, A., Brodersen, D. E., Mitarai, N. & Gerdes, K. Toxins, targets, and triggers: an overview of toxin-antitoxin biology. Mol. Cell 70, 768–784 (2018).
Han, Q. et al. Crystal structure of xanthomonas AvrRxo1-ORF1, a type III effector with a polynucleotide kinase domain, and its interactor AvrRxo1-ORF2. Structure 23, 1900–1909 (2015).
Shidore, T. et al. The effector AvrRxo1 phosphorylates NAD in planta. PLoS Pathog. 13, e1006442 (2017).
Yadav, S. K. et al. Immunity proteins of dual nuclease T6SS effectors function as transcriptional repressors. EMBO Rep. 22, e51857 (2021).
Ge, J. et al. A Legionella type IV effector activates the NF-kappaB pathway by phosphorylating the IkappaB family of inhibitors. Proc. Natl Acad. Sci. USA 106, 13725–13730 (2009).
Moss, S. M. et al. A Legionella pneumophila kinase phosphorylates the Hsp70 chaperone family to inhibit eukaryotic protein synthesis. Cell Host Microbe 25, 454–462 e456 (2019).
Lee, P. C. et al. The Legionella kinase LegK7 exploits the Hippo pathway scaffold protein MOB1A for allostery and substrate phosphorylation. Proc. Natl Acad. Sci. USA 117, 14433–14443 (2020).
Berger, K. H. & Isberg, R. R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19 (2010).
Merriam, J. J., Mathur, R., Maxfield-Boumil, R., Isberg, R. R. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immunity 65, 2497–2501 (1997).
Conover, G. M., Derré, I., Vogel, J. P. & Isberg, R. R. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48, 305–321 (2003).
Yao, J. et al. Novel polyadenylylation-dependent neutralization mechanism of the HEPN/MNT toxin/antitoxin system. Nucleic Acids Res. 48, 11054–11067 (2020).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol. Crystallogr 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Kumar, S., Nei, M., Dudley, J. & Tamura, K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9, 299–306 (2008).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFC2301403 to S.O), the National Nature Science Foundation of China grants (32170045 to X.K.Z., 82172287 to S.O., 31970134 and 32170182 to J.Z.Q.), the Fujian Provincial Department of Science and Technology (2020Y4007 to S.O.), and the High-level personnel introduction grant of Fujian Normal University (Z0210509 to S.O.). The diffraction data were collected at the beamline BL-02U1 of the Shanghai Synchrotron Radiation Facility (SSRF). We thank the support of the scientific research innovation program “Xiyuanjiang River Scholarship” of the College of Life Sciences, Fujian Normal University (22FSSK003 to X.K.Z.). We also thank our colleague Vanja Perčulija for the scientific and language editing of the manuscript.
Author information
Authors and Affiliations
Contributions
X.K.Z., S.O., and J.Z.Q. conceived the project and designed the experiments, X.K.Z. and Y.Y.W. performed crystallization and resolved the structures, J.L.G. performed the in vivo toxicity assays, J.Q.F., L.Y., N.N.L., Z.J.H., and Z.H.L. contributed to the protein expression and purification. X.K.Z. analyzed the structures, X.K.Z., S.O., Z.Q.L., and J.Z.Q. designed the biochemical experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Christina Bourne and the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhen, X., Wu, Y., Ge, J. et al. Molecular mechanism of toxin neutralization in the HipBST toxin-antitoxin system of Legionella pneumophila. Nat Commun 13, 4333 (2022). https://doi.org/10.1038/s41467-022-32049-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32049-x
This article is cited by
-
Engineered autonomous dynamic regulation of metabolic flux
Nature Reviews Bioengineering (2023)
-
Pan-kinome of Legionella expanded by a bioinformatics survey
Scientific Reports (2022)
-
A secreted effector with a dual role as a toxin and as a transcriptional factor
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.