Abstract
SARS-CoV-2 Omicron BA.1 and BA.2 subvariants are genetically divergent. We conducted a matched, test-negative, case-control study to estimate duration of protection of the second and third/booster doses of mRNA COVID-19 vaccines against BA.1 and BA.2 infections in Qatar. BNT162b2 effectiveness was highest at 46.6% (95% CI: 33.4–57.2%) against symptomatic BA.1 and at 51.7% (95% CI: 43.2–58.9%) against symptomatic BA.2 infections in the first three months after the second dose, but declined to ~10% or below thereafter. Effectiveness rebounded to 59.9% (95% CI: 51.2–67.0%) and 43.7% (95% CI: 36.5–50.0%), respectively, in the first month after the booster dose, before declining again. Effectiveness against COVID-19 hospitalization and death was 70–80% after the second dose and >90% after the booster dose. mRNA-1273 vaccine protection showed similar patterns. mRNA vaccines provide comparable, moderate, and short-lived protection against symptomatic BA.1 and BA.2 Omicron infections, but strong and durable protection against COVID-19 hospitalization and death.
Similar content being viewed by others
Introduction
Qatar endured a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron (B.1.1.529)1 wave that started on December 19, 2021 and peaked in mid-January, 20222,3,4,5. The wave was first dominated by the BA.1 Omicron subvariant, but within a few days, the BA.2 subvariant predominated (Fig. 1). While BA.1 and BA.2 remain classified as subvariants of the Omicron variant, there is considerable genetic distance between them6. Accordingly, we investigated duration of protection of BNT162b2 (Pfizer-BioNTech)7 and mRNA-1273 (Moderna)8 mRNA coronavirus disease 2019 (COVID-19) vaccines, after the second dose and after the third/booster dose, against symptomatic BA.1 and BA.2 infections, between December 23, 2021 and February 28, 2022. Duration of vaccine protection was also investigated against any severe (acute-care hospitalization)9, critical (intensive-care-unit hospitalization)9, or fatal10 infection due to either Omicron subvariant.
Vaccine effectiveness was estimated using the test-negative, case–control study design11,12, applying methodology that was developed earlier to assess duration of protection of the BNT162b213 and mRNA-127314 vaccines in the same population during pre-Omicron SARS-CoV-2 infection waves (Methods). Cases (persons infected with BA.1, BA.2, or any-Omicron-subvariant) and controls (uninfected persons) were exact-matched by sex, 10-year-age group, nationality, and calendar week of polymerase chain reaction (PCR) test to control for established differences in the risk of exposure to SARS-CoV-2 infection in Qatar15,16,17,18,19.
Results
Main analyses
By February 28, 2022 (end of study), 1,308,926 individuals received 2 or more BNT162b2 doses, and 355,979 of these received a booster dose. Meanwhile, 894,142 individuals received 2 or more mRNA-1273 doses, and 146,961 of these received a booster dose. The median dates of first, second, and third doses were May 3, 2021, May 24, 2021, and December 27, 2021 for BNT162b2; and May 28, 2021, June 27, 2021, and January 16, 2022 for mRNA-1273, respectively. The median time between the first and second doses was 21 days (interquartile range (IQR), 21–22 days) for BNT162b2 and 28 days (IQR, 28–30 days) for mRNA-1273. The median time between the second and booster doses was 251 days (IQR, 233–275 days) for BNT162b2 and 236 days (IQR, 213–261 days) for mRNA-1273.
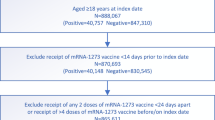
The process used to select the study populations is shown in Fig. 2. Demographic characteristics of the study populations are presented in Tables 1, 2. The study was conducted based on the total population of Qatar. The study populations are therefore representative of the internationally diverse, but predominantly young and male population of Qatar.
BNT162b2 effectiveness against symptomatic BA.1 infection was highest at 46.6% (95% confidence interval (CI): 33.4–57.2%) in the first 3 months after the second dose, but then declined to ~10% or below thereafter (Fig. 3a and Table 3). Effectiveness rapidly rebounded to 59.9% (95% CI: 51.2–67.0%) in the first month after the booster dose, but then declined to 40.5% (95% CI: 30.8–48.8%) in the second month and thereafter. A similar pattern was observed for mRNA-1273 effectiveness (Fig. 3b and Table 4).
Effectiveness (a) of the BNT162b2 vaccine and (b) of the mRNA-1273 vaccine. a Includes 7022 and 12,278 biologically independent samples for cases and controls, respectively, in the BA.1 analysis and 21,541 biologically independent samples for each of cases and controls in the BA.2 analysis in the BNT162b2 vaccine study. b includes 3574 and 6176 biologically independent samples for cases and controls, respectively, in the BA.1 analysis and 13,537 biologically independent samples for each of cases and controls in the BA.2 analysis in the mRNA-1273 vaccine study. Data are presented as effectiveness point estimates. Error bars indicate the corresponding 95% confidence intervals.
BNT162b2 effectiveness against symptomatic BA.2 infection was highest at 51.7% (95% CI: 43.2–58.9%) in the first 3 months after the second dose, but then declined to ~10% or below thereafter (Fig. 3a and Table 3). Effectiveness rapidly rebounded to 43.7% (95% CI: 36.5–50.0%) in the first month after the booster dose and was 40.2% (95% CI: 34.2–45.7%) in the second month and thereafter. A similar pattern was observed for mRNA-1273 effectiveness (Fig. 3b and Table 4).
BNT162b2 effectiveness against any symptomatic Omicron infection, regardless of subvariant, was highest at 47.8% (95% CI: 40.8–53.9%) in the first 3 months after the second dose, but then declined to ~15% or below thereafter (Fig. 4a and Table 3). Effectiveness rapidly rebounded to 55.5% (95% CI: 49.3–61.0%) in the second and third weeks after the booster dose, but then gradually declined to 21.9% (95% CI: 7.7–33.9%) from the fourteenth week and thereafter. A similar pattern was observed for mRNA-1273 effectiveness (Fig. 4b and Table 4).
Effectiveness of the BNT162b2 and mRNA-1273 vaccines against any symptomatic SARS-CoV-2 Omicron infection regardless of subvariant (panels a and b, respectively) and against any severe9, critical9, or fatal10 COVID-19 due to an Omicron infection (c, d, respectively). a Includes 39,855 and 23,814 biologically independent samples for cases and controls, respectively, (b) includes 21,810 and 13,288 biologically independent samples for cases and controls, respectively, (c) includes 268 and 692 biologically independent samples for cases and controls, respectively, and (d) includes 164 and 404 biologically independent samples for cases and controls, respectively. Data are presented as effectiveness point estimates. Error bars indicate the corresponding 95% confidence intervals.
Effectiveness against any severe, critical, or fatal COVID-19 due to an Omicron infection, regardless of subvariant, was in the range of 70–80% at any time after the second dose for both the BNT162b2 and mRNA-1273 vaccines (Fig. 4c, d and Table 5). However, BNT162b2 effectiveness against any severe, critical, or fatal COVID-19 after the booster dose was greater than 90%. 95% CIs around estimates of mRNA-1273 effectiveness against any severe, critical, or fatal COVID-19 after the booster dose lacked adequate statistical precision—there were too few hospitalized COVID-19 cases among mRNA-1273 vaccinated persons (Table 5).
Additional analyses
Sensitivity analyses adjusting for documented prior infection and healthcare worker status yielded similar findings to the main analyses (Supplementary Tables 1, 2). This is not unexpected as a strength of the test-negative design is its ability to disentangle one form of immunity from another, as validated through mathematical modeling analyses20. Sensitivity analyses to assess the impact of excluding children <12 years of age (Supplementary Tables 3, 4), or individuals <20 years of age (Supplementary Tables 5, 6), also yielded similar findings to the main analyses. A case-only analysis to examine differential waning for BA.1 versus BA.2 by comparing odds of BA.2 infection to odds of BA.1 infection among those vaccinated, with exposure being time-since vaccination, showed no evidence for a difference in the pattern of waning over time between the two subvariants (Supplementary Table 7).
Discussion
No discernable differences were observed in the duration of mRNA vaccine protection against BA.1 versus BA.2 symptomatic infection. For each of these subvariants, vaccine effectiveness against symptomatic infection was ~50% in the first 3 months after the second dose, but declined to negligible levels thereafter. Effectiveness rapidly rebounded after the booster dose to reach similar levels to those seen right after the second dose, but waned again thereafter. There were also no discernable differences in effectiveness of BNT162b2 vaccine versus mRNA-1273 vaccine. Notably, the rapid waning in vaccine effectiveness against Omicron infections contrasts with the more durable protection for prior infection against Omicron reinfection21,22.
Despite only moderate and rapidly waning protection against symptomatic infection, mRNA vaccine effectiveness against COVID-19 hospitalization and death due to Omicron infections was strong at greater than 70% after the second dose. It was also higher after the booster dose at greater than 90%. These findings support the durability of vaccine protection against COVID-19 hospitalization and death for at least several months after receiving the second dose,13,14,23 but also demonstrate the importance of booster vaccination in achieving robust protection against any hospitalization and death due to Omicron infections. These findings suggest the need to consider rapid implementation of booster vaccination campaigns coincident with the emergence of a new wave or variant, at least to those most vulnerable to COVID-19 hospitalization and death.
This study has limitations. With the lower severity of Omicron infections24,25 and the young population of Qatar15,26, case numbers were insufficient to estimate the duration of protection against COVID-19 hospitalization and death for each subvariant separately. BA.1 and BA.2 ascertainment was based on proxy criteria, presence or absence of an S-gene “target failure” using the TaqPath PCR assay (Methods), but this method of ascertainment is well established not only for Omicron subvariants, but also for other variants such as Alpha27,28,29. Some Omicron infections may have been misclassified Delta infections, but this is not likely, as Delta incidence was limited during the study duration (Methods).
While nearly all third doses were administered as booster doses, few hundreds of them were administered as third primary-series doses for the immunocompromised population. However, this is not likely to affect our estimates as the number of immunocompromised individuals is very small in Qatar30, compared to the number of individuals who received the third dose as a booster dose. Vaccine protection was assessed for only several months after the second dose, and only several weeks after the booster dose. Longer-term protection against symptomatic infection and COVID-19 hospitalization and death remain uncertain. Vaccine effectiveness reached small but statistically significant negative values at 7 months or more after the second dose. Negative estimated effectiveness likely reflects an effect of bias and not true negative biological effectiveness. This bias may have risen from vaccinated persons having a higher social contact rate or adhering less to safety measures than unvaccinated persons31,32,33. With the high vaccine coverage among adults in Qatar (>85%)13, this bias may have also risen because the reference group of unvaccinated individuals included mainly children or young persons; therefore, it may not be representative of the wider population. However, sensitivity analyses excluding children and young persons confirmed the same study findings (Supplementary Tables 3–6). Unvaccinated adults are a small minority that may not be truly immune-naïve due to undocumented prior SARS-CoV-2 infections, especially now that we are two years into this pandemic. Earlier seroprevalence studies conducted in the same population have shown that a considerable proportion of infections went undocumented17,18,19. Bias due to depletion of the susceptible population may lead to underestimation of vaccine effectiveness34, even in the test-negative, case–control, study design, which is less prone to effect of this bias13.
While matching was done for sex, age, and nationality, this was not possible for other factors, such as comorbidities, as such data are not available. However, matching by these factors provided demonstrable control of bias in studies of different epidemiologic designs and that used control groups in Qatar13,14,35,36,37. Effectiveness was assessed using an observational, test-negative, case–control, study design11,12, rather than a design in which cohorts of vaccinated and unvaccinated individuals were followed up. However, the cohort study design applied earlier to the same population of Qatar yielded findings similar to those of the test-negative case–control design36,38,39, supporting the validity of this standard approach in assessing vaccine effectiveness11,12,36,40. Moreover, our recent study of the effectiveness of booster vaccination against any symptomatic Omicron infection, relative to that of the primary series, used a cohort study design5 and its results are consistent with results generated in the present study using the test-negative, case–control study design.
Nonetheless, one cannot exclude the possibility that in real-world data, bias could arise in unexpected ways, or from unknown sources, such as subtle differences in test-seeking behavior or changes in the pattern of testing with introduction of other testing modalities, such as rapid-antigen testing (RAT). For example, with the large Omicron wave in Qatar, use of RAT was expanded to supplement PCR testing starting from January 5, 2022. However, RAT was broadly implemented in the population and probably did not differentially affect PCR testing to introduce bias. With only 9% of Qatar’s population ≥50 years of age15,41, our findings may not be generalizable to other countries in which elderly citizens constitute a larger proportion of the total population.
Notwithstanding these limitations, consistent findings were reached, indicating rapid waning of vaccine protection against symptomatic Omicron infection that are consistent with findings of other studies for effectiveness against Omicron infection (with no BA.1/BA.2 subvariant specified)42,43,44,45,46,47,48. Moreover, with the mass scale of PCR testing in Qatar13, the likelihood of bias is perhaps minimized. Extensive sensitivity and additional analyses were conducted to investigate effects of potential bias in our earlier studies for the BNT162b213 and mRNA-127314 vaccines, which used the same methodology used here. These included different adjustments in the analysis, different approaches for factoring prior infection in the analysis, and different study inclusion and exclusion criteria to investigate whether effectiveness estimates could have been biased13,14. These analyses showed consistent findings13,14.
In conclusion, mRNA vaccines provide only moderate protection against symptomatic BA.1 and BA.2 Omicron infections, with no discernable differences in protection against either BA.1 or BA.2. Protection also wanes rapidly to negligible levels, starting 4 months after the second dose. Vaccine protection rebounds after booster vaccination, but also wanes thereafter. Meanwhile, vaccine protection against COVID-19 hospitalization and death is strong and durable after the second dose, and is most robust after a booster dose.
Methods
Oversight
Hamad Medical Corporation and Weill Cornell Medicine—Qatar Institutional Review Boards approved this retrospective study with waiver of informed consent. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The STROBE checklist is found in Supplementary Table 8.
Study population and data sources
This study was conducted in the resident population of Qatar, applying methodology that was developed earlier to assess duration of protection of the BNT162b213 and mRNA-127314 coronavirus disease 2019 (COVID-19) vaccines in the same population during earlier acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection waves. COVID-19 laboratory testing, vaccination, clinical infection data, and demographic details were extracted from the national, federated SARS-CoV-2 databases that include, with no missing information, all polymerase chain reaction (PCR) testing, COVID-19 vaccinations, and COVID-19 hospitalizations and deaths in Qatar since the start of the pandemic.
Every PCR test conducted in Qatar is categorized based on symptoms and the reason for testing. Qatar has young, international demographics. Only 9% of Qatar’s population is ≥50 years of age and 89% are international expatriates from over 150 countries15,41. The vast majority of individuals were vaccinated in Qatar, but if vaccinated elsewhere, those vaccinations were still registered in the health system at the port of entry upon arrival in Qatar.
Study design
Vaccine effectiveness against symptomatic SARS-CoV-2 Omicron (B.1.1.529)1 infection during the large Omicron wave in Qatar, between December 23, 2021 and February 28, 2022, was estimated using the test-negative, case–control study design, a standard design for assessing vaccine effectiveness11,12,36,40. A symptomatic Omicron infection was defined as a nasopharyngeal PCR-positive swab collected during the Omicron wave because of clinical suspicion of infection, i.e., symptoms indicative of a respiratory tract infection. Cases (BA.1, BA.2, or any-Omicron-subvariant infected persons) and controls (uninfected persons) were exact-matched by sex, 10-year age group, nationality, and calendar week of PCR test. The ratio of matching in each analysis was determined based on available cases and controls (Fig. 2). Matching was implemented to control for established differences in the risk of exposure to SARS-CoV-2 infection in Qatar15,16,17,18,19.
Only the first PCR-positive test during the study was included for each case, whereas all PCR-negative tests during the study were included for each control. Controls included individuals with no record of a positive PCR or rapid-antigen test (RAT) during the study period. Only PCR tests conducted because of clinical suspicion of infection, i.e., symptoms indicative of a respiratory tract infection, were included in the analysis for cases and controls. All persons who received a vaccine other than BNT162b2 or mRNA-1273, or who received a different mix of vaccines, were excluded. These inclusion and exclusion criteria were implemented to minimize different types of potential bias based on earlier analyses in the same population13,14. Every case (or control) that met the inclusion criteria and that could be matched to a control (case) was included in the analysis. COVID-19 vaccination status was ascertained at the time of the PCR test. The age range for those with two and three BNT162b2 vaccine doses was 12–100 years and 13–97 years, respectively, among cases and 12–95 years and 12–97 years, respectively, among controls. The age range for those with two and three mRNA-1273 vaccine doses was 17–101 years and 20–81 years, respectively, among cases and 17–94 years and 18–92 years, respectively, among controls.
Vaccine effectiveness was also estimated against any severe, critical, or fatal COVID-19 infection due to Omicron, using the same methodology. Classification of COVID-19 case severity (acute-care hospitalizations)9, criticality (intensive-care-unit hospitalizations)9, and fatality10 followed World Health Organization (WHO) guidelines, and assessments were made by trained medical personnel using individual chart reviews (detailed description below). Each person who had a PCR-positive test result and COVID-19 hospital admission was subject to an infection severity assessment every three days until discharge or death, regardless of the hospital stay length or the time between the PCR-positive test and the final disease outcome. Individuals who progressed to severe9, critical9, or fatal10 COVID-19 between the PCR-positive test result and the end of the study were classified based on their worst outcome, starting with death, followed by critical disease, and then severe disease.
COVID-19 severity, criticality, and fatality classification
WHO defines severe COVID-19 as a SARS-CoV-2 infected individual with “oxygen saturation of <90% on room air, and/or respiratory rate of >30 breaths/min in adults and children >5 years old (or ≥60 breaths/min in children <2 months old or ≥50 breaths/min in children 2–11 months old or ≥40 breaths/min in children 1–5 years old), and/or signs of severe respiratory distress (accessory muscle use and inability to complete full sentences, and, in children, very severe chest wall indrawing, grunting, central cyanosis, or presence of any other general danger signs)”9. Detailed criteria are in the WHO technical report9.
Critical COVID-19 is defined as a SARS-CoV-2 infected individual with “acute respiratory distress syndrome, sepsis, septic shock, or other conditions that would normally require the provision of life sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy”9. Detailed criteria are in the WHO technical report9.
COVID-19 death is defined as “a death resulting from a clinically compatible illness, in a probable or confirmed COVID-19 case, unless there is a clear alternative cause of death that cannot be related to COVID-19 disease (e.g., trauma). There should be no period of complete recovery from COVID-19 between illness and death. A death due to COVID-19 may not be attributed to another disease (e.g., cancer) and should be counted independently of preexisting conditions that are suspected of triggering a severe course of COVID-19”. Detailed criteria are in the WHO technical report10.
Laboratory methods and subvariant ascertainment
Real-time reverse-transcription polymerase chain reaction testing
Nasopharyngeal and/or oropharyngeal swabs were collected for PCR testing and placed in Universal Transport Medium (UTM). Aliquots of UTM were: 1) extracted on KingFisher Flex (Thermo Fisher Scientific, USA), MGISP-960 (MGI, China), or ExiPrep 96 Lite (Bioneer, South Korea) followed by testing with real-time reverse-transcription PCR (RT-qPCR) using TaqPath COVID-19 Combo Kits (Thermo Fisher Scientific, USA) on an ABI 7500 FAST (Thermo Fisher Scientific, USA); 2) tested directly on the Cepheid GeneXpert system using the Xpert Xpress SARS-CoV-2 (Cepheid, USA); or 3) loaded directly into a Roche cobas 6800 system and assayed with the cobas SARS-CoV-2 Test (Roche, Switzerland). The first assay targets the viral S, N, and ORF1ab gene regions. The second targets the viral N and E-gene regions, and the third targets the ORF1ab and E-gene regions.
All PCR testing was conducted at the Hamad Medical Corporation Central Laboratory or at the Sidra Medicine Laboratory, following standardized protocols.
Classification of infections by subvariant
Surveillance for SARS-CoV-2 variants in Qatar is mainly based on viral genome sequencing and multiplex RT-qPCR variant screening49 of random positive clinical samples2,13,36,38,50,51, complemented by deep sequencing of wastewater samples2,52.
A total of 315 random SARS-CoV-2-positive specimens collected between December 19, 2021 and January 22, 2022 were viral whole-genome sequenced on a Nanopore GridION sequencing device. Of these, 300 (95.2%) were confirmed as Omicron infections and 15 (4.8%) as Delta (B.1.617.2)1 infections2,4,5. Of 286 Omicron infections with confirmed subvariant status, 68 (23.8%) were BA.1 cases and 218 (76.2%) were BA.2 cases.
Additionally, a total of 8811 random SARS-CoV-2-positive specimens collected between December 22, 2021 and February 28, 2022 were RT-qPCR genotyped. The RT-qPCR genotyping identified 470 B.1.617.2-like Delta case, 1017 BA.1-like Omicron cases, 4429 BA.2-like Omicron cases, and 2895 were undetermined cases where the genotype could not be assigned due to weak PCR Ct values.
The accuracy of the RT-qPCR genotyping was verified against either Sanger sequencing of the receptor-binding domain (RBD) of SARS-CoV-2 surface glycoprotein (S) gene, or by viral whole-genome sequencing on a Nanopore GridION sequencing device. From 147 random SARS-CoV-2-positive specimens all collected in December of 2021, RT-qPCR genotyping was able to assign a genotype in 129 samples. The agreement between RT-qPCR genotyping and sequencing was 100% for Delta (n = 82), 100% for Omicron BA.1 (n = 18), and 100% for Omicron BA.2 (n = 29). Of the remaining 18 specimens: 10 failed PCR amplification and sequencing, 8 could not be assigned a genotype by RT-qPCR (4 of 8 were B.1.617.2 by sequencing, and the remaining 4 failed sequencing). All the variant RT-qPCR genotyping was conducted at the Sidra Medicine Laboratory following standardized protocols.
The large Omicron-wave exponential-growth phase in Qatar started on December 19, 2021 and peaked in mid-January, 20222,3,4,5. The study duration coincided with the intense Omicron wave where Delta incidence was limited. Accordingly, any PCR-positive test during the study duration, between December 23, 2021 and February 28, 2022, was assumed to be an Omicron infection. Of note that the study duration started on December 23, 2021, and not on December 19, 2021, to minimize the occurrence of residual Delta incidence during the first few days of the Omicron wave.
Informed by the viral genome sequencing and the RT-qPCR genotyping, a SARS-CoV-2 infection with the BA.1 subvariant was proxied as an S-gene “target failure” (SGTF) case using the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific, USA53) that tests for the S-gene and is affected by the del69/70 mutation in the S-gene27. A SARS-CoV-2 infection with the BA.2 subvariant was proxied as a non-SGTF case using this TaqPath Kit. While all PCR-confirmed infections were included in this study, subvariant status was only available for the 70.5% of PCR tests that were processed using the TaqPath Kit.
Statistical analysis
Study samples were described using frequency distributions and measures of central tendency. Groups were compared using standardized mean differences (SMDs), defined as the difference in the mean of a covariate between groups, divided by the pooled standard deviation. SMD < 0.1 indicated adequate matching54. The odds ratio (and 95% confidence interval (CI)), comparing odds of vaccination among cases to that among controls, was estimated using conditional logistic regression factoring the matching in the study design. This analytical approach was implemented to reduce potential bias due to variation in epidemic phase11,55, gradual vaccination roll-out11,55, and other confounders15,17,18,19,56,57. CIs did not factor multiplicity and should not be used to infer definitive differences between study groups. Interactions were not examined. Vaccine effectiveness at different time frames and its associated 95% CI were then estimated using11,12:
Since we used a test-negative study design, some persons were tested, PCR-positive or PCR-negative, after one vaccine dose, but before the next vaccine dose. This allowed us to estimate effectiveness after each dose. In each time-since-vaccination stratum, for first, second, and third doses, we analyzed only those vaccinated in this specific time-since-vaccination stratum and those unvaccinated (our reference group). Accordingly, the sample size for cases (and controls) varied in the different time-since-vaccination analyses. To assess booster effectiveness over longer time interval, the analysis for effectiveness against any symptomatic Omicron infection was subsequently extended until April 11, 2022. This extended analysis was done for only effectiveness against any symptomatic Omicron infection to optimize statistical precision with the larger case numbers. Effectiveness was estimated by one or more months in which 1 month was defined as 30 days, or by one or more weeks where 1 week was defined as 7 days.
Sensitivity analyses were conducted to assess the impact on effectiveness estimates of adjusting for documented prior infection and healthcare worker status in conditional logistic regression. With the majority of those unvaccinated being children or young persons, and therefore not necessarily representative of total population demographics, additional analyses were conducted to assess the impact of excluding children <12 years of age and individuals <20 years of age on effectiveness estimates. A case-only logistic regression analysis was conducted to examine differential waning for BA.1 versus BA.2, by comparing odds of BA.2 infection to odds of BA.1 infection among those vaccinated, with exposure being time-since vaccination, and with adjustment for sex, 10-year age groups, and 10 nationality groups. Statistical analyses were conducted in STATA/SE version 17.058.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The dataset of this study is a property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Her Excellency the Minister of Public Health (https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-Health-Communication-Center.aspx). The raw data are protected and are not available due to data privacy laws. Data were available to authors through.csv files where information has been downloaded from the CERNER database system (no links/accession codes were available to authors). Aggregate data are available within the paper and its Supplementary Information.
Code availability
Standard epidemiological analyses were conducted using standard commands in STATA/SE 17.058. The commands/code are accessible using URL: https://github.com/IDEGWCMQ/VE-Code/blob/main/VE%20Code.do59.
References
World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 25 Jan 2022. (2022).
National Project of Surveillance for Variants of Concern and Viral Genome Sequencing. Qatar viral genome sequencing data. Data on randomly collected samples. https://www.gisaid.org/phylodynamics/global/nextstrain/. Accessed 6 Feb 2022. (2022).
Altarawneh, H. N. et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 386, 1288–1290 (2022).
Chemaitelly, H. et al. Protection of Omicron sub-lineage infection against reinfection with another Omicron sub-lineage. medRxiv https://doi.org/10.1101/2022.02.24.22271440 (2022).
Abu-Raddad, L. J. et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N. Engl J. Med. 386, 1804–1816 (2022).
Kumar, S., Karuppanan, K. & Subramaniam, G. Omicron (BA.1) and sub-variants (BA.1, BA.2 and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: a comparative sequence and structural-based computational assessment. bioRxiv https://doi.org/10.1101/2022.02.11.480029 (2022).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
World Health Organization. COVID-19 clinical management: living guidance. Available from: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-2021-2. Accessed 3 Mar 2022. (2022).
World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. https://www.paho.org/en/documents/international-guidelines-certification-and-classification-coding-covid-19-cause-death. Document Number: WHO/HQ/DDI/DNA/CAT. Accessed 3 Mar 2022. (2022).
Jackson, M. L. & Nelson, J. C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 31, 2165–2168 (2013).
Verani, J. R. et al. Case-control vaccine effectiveness studies: preparation, design, and enrollment of cases and controls. Vaccine 35, 3295–3302 (2017).
Chemaitelly, H. et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 385, e83 (2021).
Abu-Raddad, L. J., Chemaitelly, H. & Bertollini, R. National Study Group for C-V. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 386, 1091–1093 (2022).
Abu-Raddad, L. J. et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci. Rep. 11, 6233 (2021).
Ayoub, H. H. et al. Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J. Glob. Health 11, 05005 (2021).
Coyle, P. V. et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: An analysis of antibody testing on a sample of 112,941 individuals. iScience 24, 102646 (2021).
Al-Thani, M. H. et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect. Dis. 8, ofab221 (2021).
Jeremijenko, A. et al. Herd immunity against Severe Acute Respiratory Syndrome Coronavirus 2 Infection in 10 communities, Qatar. Emerg. Infect. Dis. 27, 1343–1352 (2021).
Ayoub, H. H. et al. Estimating protection afforded by prior infection in preventing reinfection: Applying the test-negative study design. medRxiv https://doi.org/10.1101/2022.01.02.22268622 (2022).
Chemaitelly, H. et al. Protection of prior natural infection compared to mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar. medRxiv https://doi.org/10.1101/2022.03.17.22272529 (2022).
Altarawneh, H. N. et al. Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar. medRxiv https://doi.org/10.1101/2022.03.22.22272745 (2022).
Tenforde, M. W. et al. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb. Mortal. Wkly Rep. 71, 459–465 (2022).
Wolter, N. et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 399, 437–446 (2022).
Butt, A. A. et al. COVID-19 Disease severity in children infected with the Omicron variant. Clin. Infect. Dis. ciac275. https://doi.org/10.1093/cid/ciac275 (2022).
Seedat, S. et al. SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates in Qatar. Sci. Rep. 11, 18182 (2021).
UK Health Security Agency (UKHSA). SARS-CoV-2 variants of concern and variants under investigation in England: Technical briefing 34. Ref: UKHSA publications gateway number GOV-12276 (2022). Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1077180/Technical-Briefing-42-20May2022.pdf, Accessed on May 27, 2022. UKHSA: London.
Abu-Raddad, L. J. et al. Introduction and expansion of the SARS-CoV-2 B.1.1.7 variant and reinfections in Qatar: A nationally representative cohort study. Plos Med. 18, e1003879 (2021).
Challen, R. et al. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 372, n579 (2021).
Chemaitelly, H. et al. SARS-CoV-2 vaccine effectiveness in immunosuppressed kidney transplant recipients. medRxiv https://doi.org/10.1101/2021.08.07.21261578 (2021).
Makhoul, M. et al. Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. Vaccines 8, 668 (2020).
Usherwood, T., LaJoie, Z. & Srivastava, V. A model and predictions for COVID-19 considering population behavior and vaccination. Sci. Rep. 11, 12051 (2021).
Andersson, O., Campos-Mercade, P., Meier, A. N. & Wengström, E. Anticipation of COVID-19 vaccines reduces social distancing. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3765329. (SSRN, 2021).
Ray, G. T. et al. Depletion-of-susceptibles bias in analyses of intra-season waning of influenza vaccine effectiveness. Clin. Infect. Dis. 70, 1484–1486 (2020).
Abu-Raddad, L. J. et al. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J. Travel. Med. 28, taab083. https://doi.org/10.1093/jtm/taab083 (2021).
Chemaitelly, H. et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 27, 1614–1621 (2021).
Abu-Raddad, L. J., Chemaitelly, H. & Bertollini, R. National Study Group for C-V. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N. Engl. J. Med. 386, 799–800 (2022).
Abu-Raddad, L. J., Chemaitelly, H. & Butt, A. A. National Study Group for Covid Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 385, 187–189 (2021).
Butt, A. A. et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J. Clin. Investig. 131, e153662. https://doi.org/10.1172/JCI153662 (2021).
Lopez Bernal, J. et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 385, 585–594 (2021).
Planning and Statistics Authority-State of Qatar. Qatar Monthly Statistics. https://www.psa.gov.qa/en/pages/default.aspx. Accessed 26 May 2020. (2020).
Andrews, N. et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 386, 1532–1546 (2022).
Buchan, S. A. et al. Effectiveness of COVID-19 vaccines against Omicron or Delta infection. medRxiv https://doi.org/10.1101/2021.12.30.21268565 (2022).
Hansen, C. H. et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv https://doi.org/10.1101/2021.12.20.21267966 (2021).
Patalon, T. et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. medRxiv https://doi.org/10.1101/2022.02.25.22271494 (2022).
Šmíd, M. et al. Protection by vaccines and previous infection against the Omicron variant of SARS-CoV-2. medRxiv https://doi.org/10.1101/2022.02.24.22271396 (2022).
Tseng, H. F. et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 28, 1063–1071 (2022).
Powell, A. A. et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect. Dis. 22, 581–583 (2022).
Vogels, C. F. J. & Grubaugh, N. Multiplexed RT-qPCR to screen for SARS-COV-2 B.1.1.7, B.1.351, and P.1 variants of concern V.3. dx. https://doi.org/10.17504/protocols.io.br9vm966. (2021).
Benslimane, F. M. et al. One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. Front. Cell Infect. Microbiol. 11, 768883 (2021).
Hasan, M. R. et al. Real-time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int. J. Infect. Dis. 112, 52–54 (2021).
Saththasivam, J. et al. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 774, 145608 (2021).
Thermo Fisher Scientific. TaqPath COVID‑19 CE‑IVD RT‑PCR Kit instructions for use. Available from: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019215_TaqPathCOVID-19_CE-IVD_RT-PCR%20Kit_IFU.pdf. Accessed 02 Dec 2020. (2020).
Austin, P. C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 38, 1228–1234 (2009).
Jacoby, P. & Kelly, H. Is it necessary to adjust for calendar time in a test negative design?: Responding to: Jackson ML, Nelson JC. The test negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31(April (17)):2165-8. Vaccine 32, 2942 (2014).
Pearce, N. Analysis of matched case-control studies. BMJ 352, i969 (2016).
Rothman, K. J., Greenland, S. & Lash, T. L. Modern epidemiology, 3rd edn. (Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008).
StataCorp. Stata Statistical Software: Release 17. (StataCorp LLC, 2021).
Chemaitelly, H. VE code. Zenodo repository. https://doi.org/10.5281/zenodo.6528694 (2022).
Acknowledgements
We acknowledge the many dedicated individuals at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, the Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine—Qatar for their diligent efforts and contributions to make this study possible. The authors are grateful for support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine—Qatar, as well as for support provided by the Ministry of Public Health, Hamad Medical Corporation, and Sidra Medicine. The authors are also grateful for the Qatar Genome Program and Qatar University Biomedical Research Center for institutional support for the reagents needed for the viral genome sequencing. Statements made herein are solely the responsibility of the authors. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
Author information
Authors and Affiliations
Contributions
H.C. co-designed the study, performed the statistical analyses, and co-wrote the first draft of the article. L.J.A. conceived and co-designed the study, led the statistical analyses, and co-wrote the first draft of the article. P.T. and M.R.H. conducted the multiplex, RT-qPCR variant screening and viral genome sequencing. H.Y., H.A.K., and M.K.S. conducted viral genome sequencing. H.C., H.H.A., S.A., P.C., P.T., H.M.Y., H.A.A., M.K.S., M.R.H., Z.A., E.A., A.J., A.H.K., A.N.L., R.M.S., H.F.A., G.K.N., M.G.A., A.A.B., H.E.A., M.H.A., A.A., R.B., and L.J.A. contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the paper. All authors have read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
A.A.B. has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. Otherwise, we declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jennifer Nguyen, Jonathan Sugimoto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chemaitelly, H., Ayoub, H.H., AlMukdad, S. et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun 13, 3082 (2022). https://doi.org/10.1038/s41467-022-30895-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30895-3
This article is cited by
-
Modelling the impact of hybrid immunity on future COVID-19 epidemic waves
BMC Infectious Diseases (2024)
-
Confronting Health Misinformation Surrounding COVID-19 Vaccines in the State of Florida
Journal of General Internal Medicine (2024)
-
Cost-Effectiveness of the Second COVID-19 Booster Vaccination in the USA
Applied Health Economics and Health Policy (2024)
-
Impact of vaccination against severe COVID-19 in the French population aged 50 years and above: a retrospective population-based study
BMC Medicine (2023)
-
Dementia and psychotropic medications are associated with significantly higher mortality in geriatric patients hospitalized with COVID-19: data from the StockholmGeroCovid project
Alzheimer's Research & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.