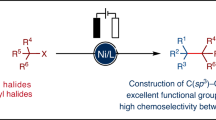
Abstract
Electrochemical catalytic reductive cross couplings are powerful and sustainable methods to construct C−C bonds by using electron as the clean reductant. However, activated substrates are used in most cases. Herein, we report a general and practical electro-reductive Ni-catalytic system, realizing the electrocatalytic carboxylation of unactivated aryl chlorides and alkyl bromides with CO2. A variety of unactivated aryl bromides, iodides and sulfonates can also undergo such a reaction smoothly. Notably, we also realize the catalytic electrochemical carboxylation of aryl (pseudo)halides with CO2 avoiding the use of sacrificial electrodes. Moreover, this sustainable and economic strategy with electron as the clean reductant features mild conditions, inexpensive catalyst, safe and cheap electrodes, good functional group tolerance and broad substrate scope. Mechanistic investigations indicate that the reaction might proceed via oxidative addition of aryl halides to Ni(0) complex, the reduction of aryl-Ni(II) adduct to the Ni(I) species and following carboxylation with CO2.
Similar content being viewed by others
Introduction
Reductive cross couplings represent powerful methods to generate C−C bonds by circumventing extra synthetic steps and handling moisture-sensitive organometallic reagents1. Much progress has been achieved in last decades via transition metal catalysis2,3 and photocatalysis4. Recently, significant advance of electrochemistry5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 has attracted much attention with electron as clean reductant, providing a sustainable solution for chemical synthesis5,6. Compared to direct electrochemistry, electrocatalysis is more attractive and useful due to the lower potential and better functional group tolerance11,12,13,14. Notably, electrochemical transition metal-catalysis15,16,17,18,19,20, especially Ni-catalysis20, is efficient and powerful in reductive cross couplings. Electro-reductive Ni-catalyzed coupling of aryl halides with organo (pseudo)halides21 and carbonyl-containing compounds22 has emerged as a convenient strategy. However, most of such cases are limited to activated aryl halides, which bear electron-withdrawing groups (EWGs) or electron-poor heteroarenes. The electrocatalytic reductive cross couplings of unactivated aryl halides, especially unactivated aryl chlorides, are challenging tasks to resolve23.
Carboxylation of organohalides with CO2 is a direct and effective way to construct valuable carboxylic acids (Fig. 1a) due to the abundant, nontoxic and recyclable properties of CO224,25,26,27,28,29,30. However, the thermodynamic stability and kinetic inertness make it difficult to achieve high efficiency under mild reaction conditions. It is even more challenging when the inexpensive coupling partners are less reactive. Although recent progress has shown some robust catalytic manners in transition-metal catalysis31,32 and photocatalysis33,34,35,36,37,38, the use of stoichiometric amount of extra metallic reductants, expensive Ir-photosensitizer, or reactive organohalides still limites their wide application. Meanwhile, electrocatalytic carboxylation of activated aryl (pseudo) halides22,39,40,41,42,43,44, benzylic and allylic ones45 have been investigated. However, selective electrocatalytic carboxylation of unactivated aryl chlorides and unactivated alkyl halides with CO2 have rarely been realized46,47,48,49,50, which are more challenging due to the low reactivity of both unactivated organohalides and CO2 as well as many competing side reactions, such as protonation, β-H elimination, migration, and homodimerization of the generated organometallic reagents and electrochemical decarboxylation of the products51. Besides the limitations in substrate scope, the reductive electrochemical carboxylations mainly rely on the use of sacrificial electrodes52,53,54,55,56.
Here, we show a general and practical electrochemical Ni-catalytic system, realizing the electrocatalytic carboxylation of challenging aryl chlorides and unactivated alkyl bromides with CO2 (Fig. 1b). A variety of unactivated aryl bromides, iodides, and sulfonates also undergo such a reaction smoothly. Moreover, the catalytic electrochemical carboxylation of aryl (pseudo)halides with CO2 avoiding the use of sacrificial electrodes is also developed.
Results
Optimization of Ni-catalyzed electrochemical carboxylation
We initiated our studies by exploring electro-reductive Ni-catalytic carboxylation of 4-chlorobiphenyl 1a with CO2 (see Supplementary Table 1). Considering that aryl chlorides are less reactive than aryl bromides or iodides, we proposed that a highly reactive Ni-catalytic system should be developed to improve the challenging oxidative addition under mild reaction conditions. We decided to explore proper ligand to keep the Ni-catalyst active and increase the catalytic efficiency. After systematic condition screening, we were delighted to realize the carboxylation of 1a to give 2a in 70% yield in the undivided cell by using Ni-catalyst and mixed nitrogen ligands, which contained ditertbutylbipyridine (L, dtbbpy) and 4-dimethylaminopyridine (DMAP) (Supplementary Table 1, entry 1). Both of these two ligands were important as no or low efficiency was observed in the absence of either one (Supplementary Table 1, entries 2 and 3). Besides, Lewis acidic MgBr2 also promoted the reaction (Supplementary Table 1, entry 4), which might coordinate with CO2 and increase its electrophilicity57,58. Control experiments verified the essential role of nickel catalyst, CO2, and electricity (Supplementary Table 1, entries 5–7). The use of potassium tert-butoxide (KOtBu) had improvement for the carboxylation (Supplementary Table 1, entry 8). Supporting electrolyte also had a significant effect on the reaction (Supplementary Table 1, entry 9). Varying from other parameters, such as solvent and electric current, did not give better results (Supplementary Table 1, entries 10–12).
Substrate scope
With the optimal reaction conditions in hand, we first explored the scope of aryl chlorides (Fig. 2). A variety of unactivated aryl chlorides underwent the carboxylation smoothly to give the desired acids in moderate to good yields (1a-1f). Notably, the substrate 1c bearing free alcohol, which might undergo oxidation via β-H elimination59 or coupling via C−O bond activation60, was not reported in previous carboxylations with CO231,33. Deactivated aryl chlorides (1e and 1f) bearing with electron-donating groups (EDGs) at the para position, which show no or lower reactivity in previous carboxylations31,32,33,34,35,36,37,38, were also amenable to our reaction. Notably, clofibrate (1f), a commercially available drug, underwent the reaction to afford 2f in 53% yield, which might be applied in drug discovery to improve the hydrophilicity and metabolism of drug molecules. As electron-deficient aryl chlorides were more reactive coupling partners, they could take part in the reaction with lower catalyst loading and temperature in the absence of either DMAP or MgBr2. Notably, this electrochemical approach exhibited good tolerance to diverse functional groups, including hydroxyl (1c), fluoro (1d), methoxyl (1e), ester (1f, 1g, and 1i), and ketone (1h and 1j). Besides substituted phenyl chlorides, 2-naphthyl and more sterically hindered 1-naphthyl chlorides were also suitable substrates in our reaction. The substrates with more steric hindrance, such as 2-methylphenyl chloride, were unreactive in our case, which might arise from more difficult oxidative addition of C−Cl bonds.
aReaction conditions, see more details in “Supplementary information”. b5 Å molecular sieve was added. cNiBr2•DME (5 mol%), dtbbpy (5 mol%), DMAP (10 mol%). dNi(acac)2 (5 mol%), dtbbpy (5 mol%), KOtBu (0.5 equiv), I = 8 mA. e50 °C, 8 h. fRoom temperature, 12 h. g3-formylbenzoic acid was obtained as product. hI = 4 mA.
We further examined carboxylation of other unactivated aryl (pseudo)halides (Fig. 2). Besides simple phenyl bromide 3a, a broad range of para- and meta-substituted aryl bromides (3b–3m) reacted well with CO2 to afford the desired carboxylic acids in good yields. Interestingly, the sterically hindered aryl bromide 3n bearing ortho phenyl group could also undergo the carboxylation in moderate efficiency. Moreover, the carboxylation of di-substituted phenyl (3o, 3p) and fused (hetero)aryl bromides (3r–3t) was also smooth. Deactivated aryl bromides (3c, 3e, 3j, 3k, 3p–3t) bearing EDGs were also reactive in this process to give desired carboxylic acids in good yields. A set of functional groups, such as ether (3c, 3d, 3j, 3k, 3p), thioether (3e), hydroxyl (3f), fluoro (3g), ester (3h), alkene (3l), acetal (3m) and heterocycles (3r–3t), were well tolerated. Besides aryl bromides, aryl iodides (3u–3w) also underwent the carboxylation smoothly. Moreover, aryl sulfonates (3x–3ab), such as a mesylate, tosylates, and triflates, which are easily available from phenols, also showed high reactivity and selectivity in this reaction.
Furthermore, we investigated more challenging carboxylation of unactivated alkyl bromides to give important alkyl carboxylic acids. To our delight, a range of primary alkyl bromides (5a–5g) could afford the corresponding carboxylic acids in moderate to good yields with good functional group tolerance under slightly modified conditions (Fig. 3). Moreover, unactivated cyclic alkyl bromides (5h and 5i) could also take part in this reaction to generate carboxylic acids with synthetically useful yields, although these substrates were considered as challenging coupling partners in carboxylation events45,46.
Exploration of the electrochemical carboxylation without sacrificial anode
Although electrocatalytic carboxylation of organo (pseudo) halides shows advantage to those with highly reactive metallic powders by using user-friendly and stable anodes, it would be more appealing and practical to achieve such carboxylation without using sacrificial electrodes52,53,54,55,56. Therefore, we further challenged us with development of an active electrocatalytic system with paired cells. After systematic investigation (see Supplementary Table 2), we were delighted to realize the catalytic electrochemical carboxylation of aryl bromide 3b with CO2 avoiding the use of sacrificial electrodes in 70% isolated yield. Control experiments verified that the pair anodic oxidation was essential, and the electro-oxidative chlorination of toluene61 matched well with the desired Ni-catalyzed reductive carboxylation. Other common anodic oxidations, including the electro-oxidation of triethylamine and triethanolamine (Supplementary Table 2, entries 8–9) gave lower yields. Using this system, we found that a variety of unactivated aryl(hetero) bromides, tosylates, mesylates, and triflates worked well to give the desired carboxylic acids in satisfying yields (Fig. 4, 3a–3ae (selective examples)). Unactivated aryl chlorides (1a–1m, selective examples) also took part in this reaction well under modified conditions with the paired oxidation of triethylamine. More challenging alkyl bromides/sulfonates (5b, 5c, 5j) were also investigated, however, low reactivity was observed.
aReaction conditions, see more details in Table S2 in “Supplementary information”. b0.5 mmol scale. c60 °C. dNiI2 (10 mol%), dtbbpy (10 mol%), DMAP (20 mol%), MgBr2 (1.5 equiv). Anodic chamber: Et3N (0.6 mmol), 3 Å MS (100 mg), NMP/NaI (0.2 M). eDMAP (20 mol%) was added. fNiBr2•DME (10 mol%), dmbby (20 mol%), CsF (1.0 quiv.), NMP/LiClO4 (0.2 M), RT. Anodic chamber: Et3N (0.6 mmol), NMP/LiClO4 (0.2 M). MS = molecule sieve.
Mechanistic studies
To gain more insight into the reaction mechanism, we first applied the synthesized complex LNi(acac)2 as pre-catalyst in the carboxylation of 3 h (Fig. 5a), which provided 4 h in a similar yield to that of standard reaction and higher than that obtained with Ni(COD)2 and dtbbpy (Fig. 5b). Moreover, we carried out the stoichiometric reaction of 3ac with Ni-catalyst to generate an oxidative addition adduct 1-1 (Fig. 5c), which showed similar catalytic activity (Fig. 5d), indicating that 1-1 might be the key intermediate in the catalytic cycle. In addition, the complex 1-2 [ArNi(dtbbpy)(DMAP)Cl] was detected in the catalytic reaction (Fig. 5e).
a Investigation of the effect of nickel complex LNi(acac)2. b Investigation of the effect of Ni(0) catalyst. c Preparation of oxidative addition adduct 1-1. d Investigation of the effect of adduct 1-1. e HRMS detection of oxidation addition adduct. L = dtbbpy. DMAP = N, N-dimethyl-4-aminopyridine. HRMS = High resolution mas spectrometry.
Furthermore, we also tested electrochemical analysis and UV-vis spectroscopy (Fig. 6). We found that both the cyclic voltammogram (CV) and absorption spectra of LNi(acac)2 were almost consistent with the mixture of Ni(acac)2 and dtbbpy in NMP solution (Fig. 6a, b), suggesting that pre-catalyst LNi(acac)2 was formed in situ by mixing Ni(acac)2 and dtbbpy by a ratio of 1:1 in NMP solution. All these results indicated that the coordination occurred between nickel catalyst and ligand. Besides, such nickel catalytic systems had two irreversible reductive peaks in the CV tests (Fig. 6a), which indicated that the nickel catalysts could be reduced into Ni(I) and Ni(0) species on the cathodic surface.
a CV tests of [Ni(acac)2 + L] and LNi(acac)2. Testing conditions: working electrode, glassy carbon electrode; counter electrode, Pt wire; reference electrode, Ag/AgNO3 electrode (10 mM AgNO3 in CH3CN). Scan rate: 100 mV/s. Solvent: NMP/nBu4NPF6 (0.1 M). Samples were tested under N2 atmosphere. Ni(acac)2, 3 mM; L, dtbbpy, 3 mM, LNi(acac)2, 3 mM. b UV-vis spectra of Ni(acac)2, 3 × 10−5 M; L, 3 × 10−5 M; [Ni(acac)2 + L], 3 × 10−5 M + 3 × 10−5 M and LNi(acac)2, 3 × 10−5 M. NMP is chosen as the testing solvent. L = dtbbpy, 4,4′-di-tert-butyl-2,2′-bipyridine; NMP = 1-methyl-2-pyrrolidinone.
We continued to conduct CV tests for Ni(acac)2 and dtbbpy in the presence of 3 h (Fig. 7a) and found a steep reductive peak at −2.73 V (Ep), which indicated a typical catalytic current. Similarly strong catalytic current could also be observed when LNi(acac)2 was used in place of Ni(acac)2 and dtbbpy. Since the pre-formed nickel complex could be reduced into its Ni(0) form according to cyclic voltammetric results, we inferred that the responsive catalytic current was attributed to oxidative addition between 3 h and Ni(0) complex. After the introduction of CO2, the reductive peak current was further increased, indicating the carboxylation reaction occurred. Besides, the deactivated aryl bromide 3b, which is reduced at −2.64 V (Ep), showed the rise of its peak current in the Ni-catalytic system along with DMAP (Fig. 7b), suggesting that DMAP improved the Ni-catalytic efficiency in activating inert aryl bromide to reaction with nickel catalyst. CV test of adduct 1–1 was also investigated in NMP solution to show two irreversible reductive peaks, which might correspond to the reduction of adduct 1–1 at the cathodic surface (Fig. 7c)62. Furthermore, two new reductive peaks at −1.86 V (Ep) and −2.27 V (Ep) were emerged when adduct 1–1 was present in the CO2 atmosphere, which might indicate that oxidative addition complex 1–1 was reduced into its Ni(I) species, which then underwent carboxylation with CO2 and was further reduced into Ni(0) species63. All of the results indicated that adduct 1–1 was generated during the electrolytic process and then reduced to Ni(I) species which underwent carboxylation with CO2.
Testing conditions: working electrode, glassy carbon electrode; counter electrode, Pt wire; reference electrode, Ag/AgNO3 electrode (10 mM AgNO3 in CH3CN). Scan rate: 100 mV/s. Solvent: NMP/nBu4NPF6 (0.1 M). Samples were tested under N2 atmosphere except some cases under CO2 atmosphere. a CV tests of [Ni(acac)2 + dtbbpy] and aryl bromide 3 h. Ni(acac)2, 3 mM; L, dtbbpy, 3 mM; LNi(acac)2, 3 mM; 3 h, ethyl 4-bromobenzoate, 60 mM; CO2 (bubbled in the solution for 10 min). b CV tests for the role of DMAP. [Ni], NiBr2(DME), 3 mM; L, dtbbpy, 3 mM; DMAP, 3 mM, 3b, 4-bromophenyl, 5 mM. c CV tests of adduct 1-1. adduct 1-1, 5 mM; CO2 (bubbled in the solution for 10 min). L, dtbbpy, 4,4′-di-tert-butyl-2,2′-bipyridine; DMAP, 4-dimethylaminopyridine.
Based on these results and the previous work15,16,17,18,19,20,31,32,33,34,35,36,37,38,64, a possible catalytic cycle was proposed (Fig. 8). First, the L″Ni(0) species A is generated from the complex Ni(acac)2 in the presence of bipyridine ligand and DMAP on the cathode. Then the oxidative addition between A and the aryl halide occurred to yield the adduct B, which would be further reduced to Ni(I) species C by cathode. Then, C reacted with CO2 to give the nickel carboxylate intermediate D. Following ligand exchange and reduction of nickel carboxylate complex regenerated the active Ni(0)-catalyst A and afforded carboxylate, which would undergo protonation to give the desired product.
Discussion
In this work, we have developed a general and practical electro-reductive Ni-catalytic system, realizing the electrocatalytic carboxylation of challenging, unactivated aryl chlorides and alkyl bromides with CO2. Unactivated aryl bromides, iodides, and sulfonates can also undergo such an electrocatalytic carboxylation smoothly. The use of DMAP as additional ligand and MgBr2 as Lewis acid improved the efficiency for the electrocarboxylation of unactivated aryl halides. Notably, we also realize the catalytic electrochemical carboxylation of aryl (pseudo)halides with CO2 avoiding the use of sacrificial electrodes. This protocol is operationally simple and robust with mild conditions, undivided cell, safe and cheap electrodes, good functional group tolerance, and broad substrate scope. Mechanistic investigations indicate that the reaction might proceed via oxidative addition of aryl halides to LnNi(0), further reduction to the Ni(I) species, and following carboxylation with CO2. More application of such catalytic system in reductive couplings is underway in our laboratory.
Methods
Synthesis of 1a–1f, 1i–1l, 3a–3c, 3e, 3i–3l, 3p, 3s, and 3aa
In a 50 mL three-neck flask equipped with a carbon felt cathode and a Zn plate, aryl halides (0.3 mmol), NiBr2•DME (0.03 mmol, 10 mol%), 4,4′-di-tert-butyl-2,2′-bipyridine (0.03 mmol, 10 mol%), DMAP (0.06 mmol, 20 mol%), KOtBu (0.15 mmol, 0.5 equiv), anhydrous MgBr2 (0.45 mmol, 1.5 equiv), NaI (1.2 mmol) were loaded in the glove box. Then the mixture was taken out of the box, degassed under vacuum, and back-filled with CO2 gas for five times (each time lasted for 1 min). After that, anhydrous NMP (6 mL) was injected into the flask via a syringe and dissolved the mixture under the strong stirring until the mixture becoming transparent. Then two electrodes were submerged into the solution and conducted under 60 °C. Constant current (10 mA) was passed until the starting material was consumed. After electrolysis, the mixture was acidized by 2 N HCl solution (20 mL) and extracted by EtOAc for four times (30 mL × 4). The combined organic layers were washed with water (20 mL × 2) and brine (20 mL) and concentrated in vacuo. Then the residue was purified by silica gel column chromatography by using the petroleum ether/EtOAc to give out the carboxylic acid.
Synthesis of 3d, 3f–3h, 3m–3o, 3q–3r, 3t–3z and 3ab
In a 50 mL three-neck flask equipped with a carbon felt cathode and a Zn plate, aryl halides (0.3 mmol), Ni(acac)2 (0.015 mmol, 5 mol%), 4,4′-di-tert-butyl-2,2′-bipyridine (0.015 mmol, 5 mol%), KOtBu (0.15 mmol, 0.5 equiv), NaI (1.2 mmol) were loaded in the glove box. Then the mixture was taken out of the box, degassed under vacuum and back-filled with CO2 gas for five times (each time lasted for 1 min). After that, anhydrous NMP (6 mL) was injected into the flask via a syringe and dissolved the mixture under the strong stirring until the mixture becoming transparent. Then two electrodes were submerged into the solution and conducted under room temperature or 50 °C. Constant current (8 mA) was passed until the starting material was consumed. After electrolysis, the mixture was acidized by 2 N HCl solution (20 mL) and extracted by EtOAc for four times (30 mL × 4). The combined organic layers were washed with water (20 mL × 2) and brine (20 mL) and concentrated in vacuo. Then the residue was purified by silica gel column chromatography by using the petroleum ether/EtOAc to give out the carboxylic acid.
Synthesis of 5a–5i
In a 50 mL three-neck flask equipped with a carbon felt cathode and a Zn plate, alkyl bromides (0.5 mmol), NiBr2•DME (0.05 mmol, 10 mol%), 6,6′-di-methyl-2,2′-bipyridine (0.1 mmol, 20 mol%), CsF (0.5 mmol, 1.0 equiv), LiClO4 (1.2 mmol) were loaded in the glove box. Then the mixture was taken out of the box, degassed under vacuum, and back-filled with CO2 gas for five times (each time lasted for 1 min). After that, anhydrous NMP (6 mL) was injected into the flask via a syringe and dissolved the mixture under the strong stirring until the mixture becoming transparent. Then two electrodes were submerged into the solution and conducted under room temperature. Constant current (4 or 8 mA) was passed until the starting material was consumed. After electrolysis, the mixture was acidized by 2 N HCl solution (20 mL) and extracted by EtOAc for four times (30 mL × 4). The combined organic layers were washed with water (20 mL × 2) and brine (20 mL) and concentrated in vacuo. Then the residue was purified by silica gel column chromatography by using the petroleum ether/EtOAc to give out the carboxylic acid.
Synthesis of 3a–3b, 3d–3f, 3j–3k, 3n–3s, 3x–3y, and 3aa–3ae (non-sacrificial anode manner)
In an H-type divided cell equipped with a carbon felt cathode and a carbon felt anode in each chamber, aryl bromides (0.3 mmol), Ni(acac)2 (0.03 mmol, 10 mol%), 4,4′-di-tert-butyl-2,2′-bipyridine (0.03 mmol, 10 mol%), KOtBu (0.15 mmol, 0.5 equiv), 4 Å molecular sieve (100 mg), NaI (1.2 mmol) were loaded in the cathodic chamber. In the anodic chamber, LiCl (1.8 mmol) was added. Then the reaction vial was taken out of the box, degassed under vacuum, and back-filled with CO2 gas for five times (each time lasted for 1 min). After that, anhydrous NMP (6 mL) was injected into each chamber via a syringe and dissolved the mixture under the strong stirring until the solution becoming transparent. Then dry toluene (0.6 mmol) was injected into anodic chamber via syringe. After that, two electrodes were submerged into the solution. Constant cell voltage (3 V) was set to the reaction until the starting material was consumed at room temperature. After electrolysis, the mixture of both chambers was acidized by 2 N HCl solution (20 mL) and extracted by EtOAc for four times (30 mL × 4). The combined organic layers were washed with water (20 mL × 2) and saturated NH4Cl solution (20 mL) and concentrated in vacuo. Then the residue was purified by silica gel column chromatography by using petroleum ether/EtOAc to give out the carboxylic acids. Further details may be found in the Supplemental Information.
Synthesis of 1a, 1c, 1e–1g, 1i, and 1l–1m (non-sacrificial anode manner)
In a H-type divided cell equipped with a carbon felt cathode and a carbon felt anode in each chamber, aryl bromides (0.3 mmol), NiI2 (0.03 mmol, 10 mol%), 4,4′-di-tert-butyl-2,2′-bipyridine (0.03 mmol, 10 mol%), DMAP (0.06 mmol, 20 mol%), KOtBu (0.15 mmol, 0.5 equiv), NaI (1.2 mmol) were loaded in the cathodic chamber. In the anodic chamber, NaI (1.2 mmol) was added. Then the reaction vial was taken out of the box, degassed under vacuum, and back-filled with CO2 gas for five times (each time lasted for 1 min). After that, anhydrous NMP (6 mL) was injected into each chamber via a syringe and dissolved the mixture under the strong stirring until the solution becoming transparent. Then dry Et3N (0.6 mmol) was injected into anodic chamber via syringe. After that, two electrodes were submerged into the solution. Constant cell voltage (3 V) was set to the reaction until the starting material was consumed at room temperature. After electrolysis, the mixture of both chambers was acidized by 2 N HCl solution (20 mL) and extracted by EtOAc for four times (30 mL × 4). The combined organic layers were washed with water (20 mL × 2) and saturated NH4Cl solution (20 mL) and concentrated in vacuo. Then the residue was purified by silica gel column chromatography by using petroleum ether/EtOAc to give out the carboxylic acids. Further details may be found in the Supplemental Information.
Synthesis of 5b, 5c, and 5j (non-sacrificial anode manner)
In a H-type divided cell equipped with a carbon felt cathode and a carbon felt anode in each chamber, alkyl halide (0.5 mmol), NiBr2•DME (0.05 mmol, 10 mol%), 6,6′-di-methyl-2,2′-bipyridine (0.1 mmol, 20 mol%), CsF (0.5 mmol, 1.0 equiv), LiClO4 (1.2 mmol) were loaded in the cathodic chamber. In the anodic chamber, LiClO4 (1.2 mmol) was added. Then the reaction vial was taken out of the box, degassed under vacuum, and back-filled with CO2 gas for five times (each time lasted for 1 min). After that, anhydrous NMP (6 mL) was injected into each chamber via a syringe and dissolved the mixture under the strong stirring until the solution becoming transparent. Then dry Et3N (0.6 mmol) was injected into anodic chamber via syringe. After that, two electrodes were submerged into the solution. Constant cell voltage (3 V) was set to the reaction until the starting material was consumed at room temperature. After electrolysis, the mixture of both chambers was acidized by 2 N HCl solution (20 mL) and extracted by EtOAc for four times (30 mL × 4). The combined organic layers were washed with water (20 mL × 2) and saturated NH4Cl solution (20 mL) and concentrated in vacuo. Then the residue was purified by silica gel column chromatography by using petroleum ether/EtOAc to give out the carboxylic acids. Further details may be found in the Supplemental Information.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplemental Information files. Extra data are available from the author upon request.
References
Krische M. J. Metal catalyzed reductive C—C bond formation (Springer, 2007).
Liu, J., Ye, Y., Sessler, J. L. & Gong, H. Cross-electrophile couplings of activated and sterically hindered halides and alcohol derivatives. Acc. Chem. Res. 53, 1833–1845 (2020).
Weix, D. J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 48, 1767–1775 (2015).
Xia, Q., Dong, J., Song, H. & Wang, Q. Visible-Light photocatalysis of the ketyl radical coupling reaction. Chem. Eur. J. 25, 2949–2961 (2019).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Francke, R., Schille, B. & Roemelt, M. Homogeneously catalyzed electroreduction of carbon dioxide—methods, mechanisms, and catalysts. Chem. Rev. 118, 4631–4701 (2018).
Jiang, Y., Xu, K. & Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 118, 4485–4540 (2018).
Yuan, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 52, 3309–3324 (2019).
Xiong, P. & Xu, H.-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 52, 3339–3350 (2019).
Kingston, C. et al. A survival guide for the “electro-curious”. Acc. Chem. Res. 53, 72–83 (2020).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Jing, Q. & Moeller, K. D. From molecules to molecular surfaces. Exploiting the interplay between organic synthesis and electrochemistry. Acc. Chem. Res. 53, 135–143 (2020).
Wang, F. & Stahl, S. S. Electrochemical oxidation of organic molecules at lower overpotential: accessing broader functional group compatibility with electron−proton transfer mediators. Acc. Chem. Res. 53, 561–574 (2020).
Chang, X., Zhang, Q. & Guo, C. Asymmetric electrochemical transformations. Angew. Chem. Int. Ed. 59, 12612–12622 (2020).
Jutand, A. Contribution of electrochemistry to organometallic catalysis. Chem. Rev. 108, 2300–2347 (2008).
Ackermann, L. Metalla-electrocatalyzed C–H activation by earth-abundant 3d metals and beyond. Acc. Chem. Res. 53, 84–104 (2020).
Jiao, K.-J., Xing, Y.-K., Yang, Q.-L., Qiu, H. & Mei, T.-S. Site-selective C–H functionalization via synergistic use of electrochemistry and transition metal catalysis. Acc. Chem. Res. 53, 300–310 (2020).
Siu, J. C., Fu, N. & Lin, S. Catalyzing electrosynthesis: a homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 53, 547–560 (2020).
Qiu, Y., Zhu, C., Stangier, M., Struwe, J. & Ackermann, L. Rhodaelectro-catalyzed C–H and C–C activation. CCS Chem. 3, 1529–1552 (2021).
Yakhvarov, D. G., Khusnuriyalova, A. F. & Sinyashin, O. G. Electrochemical synthesis and properties of organonickel σ-complexes. Organometallics 33, 4574–4589 (2014).
Kumar, G. S. et al. Nickel-catalyzed chain-walking cross-electrophile coupling of alkyl and aryl halides and olefin hydroarylation enabled by electrochemical reduction. Angew. Chem. Int. Ed. 59, 6513–6519 (2020).
Cao, Y., He, X., Wang, N., Li, H.-R. & He, L.-N. Photochemical and electrochemical carbon dioxide utilization with organic compounds. Chin. J. Chem. 36, 644–659 (2018).
Jiao, K.-J. et al. Nickel-catalyzed electrochemical reductive relay cross-coupling of alkyl halides to aryl halides. Angew. Chem. Int. Ed. 59, 6520–6524 (2020).
Ackermann, L. Transition-metal-catalyzed carboxylation of C–H bonds. Angew. Chem. Int. Ed. 50, 3842–3844 (2011).
Huang, K., Sun, C.-L. & Shi, Z.-J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev. 40, 2435–2452 (2011).
He, M., Sun, Y. & Han, B. Green carbon science: scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 52, 9620–9633 (2013).
Liu, Q., Wu, L., Jackstell, R. & Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 6, 5933 (2015).
Burkart, M. D., Hazari, N., Tway, C. L. & Zeitler, E. L. Opportunities and challenges for catalysis in carbon dioxide utilization. ACS Catal. 9, 7937–7956 (2019).
Zhang, L., Li, Z., Takimoto, M. & Hou, Z. Carboxylation reactions with carbon dioxide using N-heterocyclic carbene-copper catalysts. Chem. Rec. 20, 494–512 (2020).
Song, L. et al. CO2 = CO + [O]: recent advances in carbonylation of C–H bonds with CO2. Chem. Commun. 56, 8355–8367 (2020).
Chen, Y.-G. et al. Transition-metal-catalyzed carboxylation of organic halides and their surrogates with carbon dioxide. Synthesis 50, 35–48 (2018).
Tortajada, A., Juliá-Hernández, F., Börjesson, M., Moragas, T. & Martin, R. Transition-metal-catalyzed carboxylation reactions with carbon dioxide. Angew. Chem. Int. Ed. 57, 15948–15982 (2018).
Yeung, C. S. Photoredox catalysis as a strategy for CO2 incorporation: direct access to carboxylic acids from a renewable feedstock. Angew. Chem. Int. Ed. 58, 5492–5502 (2019).
Ye, J.-H., Ju, T., Huang, H., Liao, L.-L. & Yu, D.-G. Radical carboxylative cyclizations and carboxylations with CO2. Acc. Chem. Res. 54, 2518–2531 (2021).
Cai, B., Cheo, H. W., Liu, T. & Wu, J. Light-promoted organic transformations utilizing carbon-based gas molecules as feedstocks. Angew. Chem. Int. Ed. 60, 18950–18980 (2021).
Zhang, Z. et al. Visible-light-driven catalytic reductive carboxylation with CO2. ACS Catal. 10, 10871–10885 (2020).
He, X., Qiu, L.-Q., Wang, W.-J., Chen, K.-H. & He, L.-N. Photocarboxylation with CO2: an appealing and sustainable strategy for CO2 fixation. Green. Chem. 22, 7301–7320 (2020).
Fan, Z., Zhang, Z. & Xi, C. Light-Mediated carboxylation using carbon dioxide. ChemSusChem 13, 6201–6218 (2020).
Matthessen, R., Fransaer, J., Binnemans, K. & De Vos, D. E. Electrocarboxylation: towards sustainable and efficient synthesis of valuable carboxylic acids. Beilstein J. Org. Chem. 10, 2484–2500 (2014).
Senboku, H. & Katayama, A. Electrochemical carboxylation with carbon dioxide. Curr. Opin. Green. Sustain. Chem. 3, 50–54 (2017).
Torii, S. et al. Pd(0)-catalyzed electroreductive carboxylation of aryl halides, β-bromostyrene, and allyl acetates with CO2. Chem. Lett. 15, 169–172 (1986).
Jutand, A., Négri, S. & Mosleh, A. Palladium catalysed electrosynthesis using aryl trifluoromethanesulfonates (triflates). Synthesis of biaryls and aromatic carboxylic acids. J. Chem. Soc. Chem. Commun. 23, 1729–1730 (1992).
Jutand, A. & Négri, S. Activation of aryl and vinyl triflates by palladium and electron transfer – electrosynthesis of aromatic and α,β-unsaturated carboxylic acids from carbon dioxide. Eur. J. Org. Chem. 1998, 1811–1821 (1998).
Medeiros, M. J., Pintaric, C., Olivero, S. & Dunach, E. Nickel-catalysed electrochemical carboxylation of allylic acetates and carbonates. Electrochim. Acta 56, 4384–4389 (2011).
Jiao, K.-J. et al. Palladium-catalyzed reductive electrocarboxylation of allyl esters with carbon dioxide. Org. Chem. Front. 5, 2244–2248 (2018).
Sock, O., Troupel, M. & Perichon, J. Electrosynthesis of carboxylic acids from organic halides and carbon dioxide. Tetrahedron Lett. 26, 1509–1512 (1985).
Niu, D.-F. et al. Electrocatalytic carboxylation of aliphatic halides at silver cathode in acetonitrile. Tetrahedron 64, 10517–10520 (2008).
Yang, H. P. et al. Platinum/nitrogen-doped carbon/carbon cloth: a bifunctional catalyst for the electrochemical reduction and carboxylation of CO2 with excellent efficiency. Chem. Commun. 54, 4108–4111 (2018).
Yang, H. P. et al. A core-shell-structured silver nanowire/nitrogen-doped carbon catalyst for enhanced and multifunctional electrofixation of CO2. ChemSusChem 11, 3905–3910 (2018).
Bazzi, S., Le Duc, G., Schulz, E., Gosmini, C. & Mellah, M. CO2 activation by electrogenerated divalent samarium for aryl halide carboxylation. Org. Biomol. Chem. 17, 8546–8550 (2019).
Shi, S.-H., Liang, Y. & Jiao, N. Electrochemical oxidation induced selective C–C bond cleavage. Chem. Rev. 121, 485–505 (2021).
Matthessen, R., Fransaer, J., Binnemans, K. & De Vos, D. E. Paired electrosynthesis of diacid and diol precursors using dienes and CO2 as the carbon source. ChemElectroChem 2, 73–76 (2015).
Yang, D.-T. et al. Direct electrochemical carboxylation of benzylic C–N bonds with carbon dioxide. ACS Catal. 9, 4699–4705 (2019).
Alkayal, A. et al. Harnessing applied potential: selective β-hydrocarboxylation of substituted olefins. J. Am. Chem. Soc. 142, 1780–1785 (2020).
Sheta, A. M. et al. Selective α,δ-hydrocarboxylation of conjugated dienes utilizing CO2 and electrosynthesis. Chem. Sci. 11, 9109–9114 (2020).
Gao, X.-T. et al. Direct electrochemical defluorinative carboxylation of α-CF3 alkenes with carbon dioxide. Chem. Sci. 11, 10414–10420 (2020).
Metzger, A., Bernhardt, S., Manolikakes, G. & Knochel, P. MgCl2-accelerated addition of functionalized organozinc reagents to aldehydes, ketones, and carbon dioxide. Angew. Chem. Int. Ed. 49, 4665–4668 (2010).
Sayyed, F. B. & Sakaki, S. The crucial roles of MgCl2 as a non-innocent additive in the Ni-catalyzed carboxylation of benzyl halide with CO2. Chem. Commun. 50, 13026–13029 (2014).
Fuse, H., Mitsunuma, H. & Kanai, M. Catalytic acceptorless dehydrogenation of aliphatic alcohols. J. Am. Chem. Soc. 142, 4493–4499 (2020).
Yu, D.-G. et al. Direct arylation/alkylation/magnesiation of benzyl alcohols in the presence of Grignard reagents via Ni-, Fe-, or Co-catalyzed sp3 C–O bond activation. J. Am. Chem. Soc. 134, 14638–14641 (2012).
So, Y. H. Selective chlorination of toluene by anodic oxidation. J. Org. Chem. 50, 5895–5896 (1985).
Shields, B. J. & Doyle, A. G. Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc. 138, 12719–12722 (2016).
Sayyed, F. B., Tsuji, Y. & Sakaki, S. The crucial role of a Ni(I) intermediate in Ni-catalyzed carboxylation of aryl chloride with CO2: a theoretical study. Chem. Commun. 49, 10715–10717 (2013).
Diccianni, J. B., Hu, C. T. & Diao, T. Insertion of CO2 mediated by a (Xantphos)NiI–alkyl species. Angew. Chem. Int. Ed. 58, 13865–13868 (2019).
Acknowledgements
Financial support is provided by the National Natural Science Foundation of China (21822108), Sichuan Science and Technology Program (20CXTD0112), Fundamental Research Funds from Sichuan University (2020SCUNL102), and the Fundamental Research Funds for the Central Universities. We thank Xiaoyan Wang from the Analysis and Testing Center of Sichuan University for helping us perform NMR studies and the comprehensive training platform of the Specialized Laboratory in the College of Chemistry at Sichuan University for compound testing.
Author information
Authors and Affiliations
Contributions
D.G.Y. and G.Q.S. conceived and designed the study, and wrote the manuscript. G.Q.S., W.Z., L.L.L., L.L., Z.H.N., J.G.W., and Z.Z. performed the experiments, mechanistic studies, and revised the manuscript. All authors contributed to the analysis and interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing financial interest(s): A Chinese Patent on this work has been authorized with the number (202010244592.7). The authors declare no other competing interests.
Peer review information
Nature Communications thanks Marta Feroci and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, GQ., Zhang, W., Liao, LL. et al. Nickel-catalyzed electrochemical carboxylation of unactivated aryl and alkyl halides with CO2. Nat Commun 12, 7086 (2021). https://doi.org/10.1038/s41467-021-27437-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-27437-8
This article is cited by
-
Efficient palladium-catalyzed electrocarboxylation enables late-stage carbon isotope labelling
Nature Communications (2024)
-
Electrocarboxylation of CO2 with Organic Substrates: Toward Cathodic Reaction
Transactions of Tianjin University (2023)
-
Facile and general electrochemical deuteration of unactivated alkyl halides
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.