Abstract
Acyclic quaternary stereocenters are widely present in a series of biologically active natural products and pharmaceuticals. However, development of highly efficient asymmetric catalytic methods for the construction of these privileged motifs represents a longstanding challenge in organic synthesis. Herein, an efficient chiral phosphoric acid catalyzed direct asymmetric addition of α-alkynyl acyclic ketones with allenamides has been developed, furnishing the acyclic all-carbon quaternary stereocenters with excellent regioselectivities and high enantioselectivities. Extensive and detailed experimental mechanistic studies were performed to investigate the mechanism of this reaction. Despite a novel covalent allyl phosphate intermediate was found in these reactions, further studies indicated that a SN2-type mechanism with the ketone nucleophiles is not very possible. Instead, a more plausible mechanism involving the elimination of the allyl phosphate to give the α,β-unsaturated iminium intermediate, which underwent the asymmetric conjugate addition with the enol form of ketone nucleophiles under chiral anion catalysis, was proposed. In virtue of the fruitful functional groups bearing in the chiral products, the diverse derivatizations of the chiral products provided access to a wide array of chiral scaffolds with quaternary stereocenters.
Similar content being viewed by others
Introduction
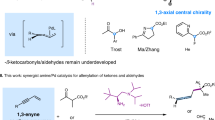
All-carbon quaternary stereocenters, carbon centers with four distinct carbon substituents, are abundant in many biologically active small molecules and natural products1. However, the catalytic enantioselective construction of these important structural motifs remains synthetically challenging, due to their congested nature and required stereoselectivities in forming the new C–C bond. Despite these challenges, the last two decades have witnessed tremendous progress in the field of enantioselective construction of all-carbon quaternary stereocenters, and a number of elegant strategies have been developed2,3,4. To this end, the asymmetric functionalizations of α,α-disubstituted carbonyl compounds represent one of the most productive strategies. However, in contrast to the well-developed methods for asymmetric construction of quaternary stereocenters of α-branched aldehydes via enamine catalysis5,6,7,8,9 and others10,11,12,13,14, the analogous methods for α-branched ketones are more challenging, probably due to their low reactivity and poor regioselectivity issues. To date, existing methods are mostly limited to activated ketone substrates (i.e., ketones with strong α-electron-withdrawing groups), such as β-ketoesters15,16,17,18,19,20,21,22,23 and others24,25,26 (Fig. 1a). For α-branched ketones without these strong activation groups, their relatively low reactivity is a major obstacle in these reactions. Recently, several ingenious methods have been developed through direct asymmetric functionalizations of α-branched cyclic ketones, such as Michael additions27,28,29,30,31, allylic alkylation32,33,34, arylation35,36,37,38, and others39,40 (Fig. 1b). Notably, the chiral phosphoric acids (CPA) catalyzed regio- and enantioselective functionalizations of α-branched cyclic ketones have become a powerful strategy for the construction of α-quaternary stereocenters for cyclic ketones28,32,41,42,43,44,45,46. In these reactions, the formation of thermodynamic favored more-substituted enol intermediate under acid-catalysis well addressed the issue of regioselectivity.
a Asymmetric construction of quaternary stereocenters of activated ketones. b Asymmetric construction of quaternary stereocenters of α-branched cyclic ketones. c Unsaturation-enabled asymmetric Mannich reaction of α-branched acyclic ketones. d Asymmetric construction of acyclic quaternary stereocenters of α-alkynyl acyclic ketones.
The direct asymmetric functionalizations of α-branched acyclic ketones with less-stabilized enol/enolate intermediates is still an unconquered challenge47,48,49,50, since the extra requirement for the formation of geometrically defined enol/enolate intermediate in the ketone activation step. To overcome this hurdle, some elegant indirect methods employing stereodefined acyclic enolate as nucleophiles have been developed via asymmetric alkylation51 and allylic alkylations52,53,54. To the best of our knowledge, only one example of direct asymmetric functionalization of acyclic α-branched ketones for the construction of quaternary stereocenter has been disclosed by Trost and co-workers, who developed the unsaturation-enabled asymmetric Mannich reactions using Zn-Prophenol catalysis55 (Fig. 1c). The unsaturation introduced in the ketone substrates was believed to play two roles: (1) serving as a blocking group to overcome the issue of regioselectivity; (2) modifying the activity of substrates by adjusting the pKa of the α-protons. With our continuous interest in CPA-catalyzed asymmetric addition reactions56,57,58,59, we explored the possibility of using acyclic α-branched ketones as the nucleophiles in these reactions.
Herein, we disclose an efficient method for the construction of acyclic all-carbon quaternary stereocenters via enantioselective additions of α-alkynyl branched ketones with allenamides60,61,62,63,64, which gives access to ketones bearing α-quaternary stereocenters with broad substrate scope and high enantioselectivities (Fig. 1d).
Results
Reaction condition optimizations
Since the diverse transformations of the alkynyl groups and their weak ability to activate the α-position of carbonyl compounds due to the high s character of alkynyl groups65,66, we started our study by employing racemic α-alkynyl ketone 1a as the model substrate. Encouragingly, when the reaction between 1a and allenamide 2a was investigated under the catalysis of CPA A1 (10 mol%) in CCl4 at ambient temperature, the desired product 3a bearing an all-carbon quaternary stereocenter was exclusively generated in 61% yield with 71:29 enantiomeric ratio (er), without detecting the potential α’- or γ-regioisomers (Table 1, entry 1). Next, a series of BINOL-derived CPA catalysts were screened in this reaction (entries 2–8), which indicated that the sterically bulky CPA catalyst A7 (TRIP) to be the optimal one (70%, 95:5 er, entry 7). Furthermore, switching the chiral scaffold of TRIP from BINOL-type to H8-BINOL-type led to further improved enantioselectivity (75%, 97.5:2.5 er, entry 9). In addition, 4 Å molecular sieves (MS) was found to be effective in promoting the reaction with higher yield (95%, entry 10), probably because of the inhibition of hydrolysis of allenamide under these conditions. A range of solvents was also examined in this reaction (entries 11–14). Interestingly, generally high enantioselectivities were observed with these solvents; however, none of them could provide superior results than CCl4.
Substrates scope investigation
With the optimal conditions in hand, we set out to explore the scope of α-alkynyl ketones for the asymmetric construction of acyclic quaternary stereocenters (Fig. 2). A range of substituted phenyl groups (with various substituents at the para- and meta-positions) at the α-position of ketones were examined, which were well tolerated with the optimal reaction conditions (3b–3h), as well as a 2-naphthyl group (3i). Moreover, switching the α-substitution from an aryl group to an alkyl group was also amenable, providing the addition product in high yield and enantioselectivity (3j). Subsequently, the scope of the alkynyl groups was also studied, which suggested that both the arylacetylene- and alkylacetylene-substituted acyclic ketones were compatible with the optimal conditions, albeit generating products with slightly diminished enantioselectivities (3k–3m). Additionally, using an α-acetylene-substituted ketone as substrate under the standard conditions afforded the desired product with 92.5:7.5 er and moderate yield (3n). The absolute configuration of these addition products was assigned as (S) by analogy to the structure of 3n, which was unambiguously determined by X-ray crystallography. Finally, the variants on the other side of the ketone substrates were also investigated. Replacing the Me group with other alkyl groups (e.g., Et, n-Bu, and iPr group) was feasible, which afforded the addition products with excellent stereoselectivities, albeit in moderate yields due to their relatively low reactivities (3o–3q).
With the excellent compatibility of α-alkynyl ketone substrates, we turned our attention to the scope of allenamides (Fig. 3). A series of substituted N-aryl groups were examined in this reaction, which were feasible variants under the standard conditions, regardless of the electronic nature and substitution sites of these substituents (3r–3x). Next, the scope of the amide moieties was also studied. Switching the alkylacyl group from Piv group to the less sterically demanding groups were amenable in these reactions, and the desired products could be uniformly obtained with high enantioselectivities (3y and 3z).
Mechanistic studies
In order to probe the effect of the α-alkynyl groups in these reactions, a number of α-substituted acyclic ketones were prepared and investigated in this reaction (Fig. 4). Although relatively low reactivity of the α-styrenyl-substituted ketone 4a was observed under the standard conditions (with CPA (S)-A9), raising the reaction temperature to 45 °C provided the acyclic quaternary stereocenter containing product 5a in 73% yield with 80.5:19.5 er. Brief optimizations of the CPA catalyst indicated that high enantioselectivity could be afforded in the presence of CPA (R)-A6 at 0 °C, which generated 5a in 57% yield with 7:93 er. These results indicated that this reaction is not only feasible for α-alkynyl-substituted ketones, but could also be extended to broader ketone scope. However, switching the alkynyl group to alkyl group (4b) or using Trost’s α-branched ynone 4c55 as substrates could not provide the desired adducts, even at elevated reaction temperature (55 °C, Fig. 4b). Surprisingly, the activated ketone substrate (e.g., β-ketoester 4d) also exhibited low reactivity under these conditions, which only gave the desired addition product 5d in 18% yield, with poor enantioselectivity (59.5:40.5 er) as well. These results together with the predicted pKa values67 (obtained from http://pka.luoszgroup.com/) of these acyclic ketone substrates indicated that although the α-alkynyl substitutions could increase the acidity of the α-protons, the reactivities of these ketones in this reaction were not positively correlated with the pKa values of the α-protons (Fig. 4c). We believe that both the electronic effect and the steric effect of the α-substituents play important roles in modulating the reactivities of these α-branched ketones in this reaction.
The kinetic profile experiments were conducted on the model reaction to study the mechanism of these reactions. A linear relationship between the initial reaction rate and the concentration of CPA A7 and ketone 1a was observed, respectively, which suggested a first-order dependence on CPA catalyst and ketone substrate (Fig. 5a, b). In addition, a negative correlation between the initial reaction rate and concentration of allenamide 2a and an amide additive 2a’ was found respectively, which suggested that the allenamide is probably not involved in the rate-determining step and the basicity of the amide group may inhibit this acid-catalyzed reaction (Fig. 5c, d). Moreover, a parallel kinetic isotopic effect (KIE) experiment of the α-H of the ketone substrate 1a was performed and gave a KIE value of 4.1, which suggested that the cleavage of this α-C–H bond is probably involved in the rate-determining step (Fig. 5e). Interestingly, treatment of 1a with Et3N (0.2 equiv.) led to the formation of an inseparable mixture of 1a and allenyl ketones 1a’ (1a:1a’ = 0.46:0.54), in which 1a and 1a’ were in equilibrium (Fig. 5f). Monitoring the reaction of this 1a/1a’ mixture with allenamide 2a under the standard conditions using 1H NMR suggested that the allenyl ketone 1a’ was actually more reactive in this reaction, as indicated by the conversion results at 0.5 h and 8 h. Moreover, the enantioselectivities at the early stage (0.5 h) and late stage (8 h) of this reaction retained the same value, which indicated that both reactions of 1a and 1a’ could go through the same reaction intermediate, namely the enol form of the ketone. The different reaction rates of 1a and 1a’ also indicated that the ability to form the active enol intermediate may determine the reaction rate of this reaction.
a Relationship between the initial reaction rate and concentration of the CPA catalyst A7. b Relationship between the initial reaction rate and concentration of ketone 1a. c Relationship between the initial reaction rate and concentration of the allenamide 2a. d Relationship between the initial reaction rate and concentration of the amide additive 2a’. e KIE experiment of the α-H of ketone 1a. f Isomerization of 1a into allenyl ketone 1a’ and its reaction with allenamide 2a under the standard conditions.
In order to gain more details of the asymmetric induction model68, the nonlinear effect study was first performed. A highly linear relationship between the ee of product 3a and the ee of CPA A9 was observed, which indicated the presence of only one chiral catalyst (intermediate) in the enantio-determining step (Fig. 6a). We also studied the potential reaction intermediate by mixing allenamide 2a with stoichiometric amount of CPA A7 in D6-benzene, which readily afforded a mixture of acrolein (the hydrolysis product of 2a) and an unknown product, as detected by 1H NMR (Fig. 6b). However, an attempt to isolate this compound by silica gel chromatography resulted in no success. Nevertheless, HRMS analysis of the mixture implied the formation of an adduct between allenamide and CPA. A set of NMR spectroscopies (including 1H NMR, 13C NMR, 2D NMR, 31P NMR) were directly performed on this mixture, which suggested the formation of allyl phosphate adduct INT-A (see Supplementary Information for details). The characteristic NMR signals include the doublet 13C NMR peaks of Cγ and Cβ through the coupling with the phosphorus atom of phosphate moiety and the triplet 31P NMR peak via the coupling with the two H atoms of the Cγ-methylene moiety. Furthermore, the addition of α-alkynyl ketone 1a into this mixture provided the same addition product 3a in 80% yield with 95:5 er after 20 h. In addition, another allyl phosphate intermediate (INT-A2w) formed between CPA A7 and the N-fluorobenzene-substituted allenamide 2w could be clearly observed by 19F NMR at −110.9 ppm, which facilitated the monitoring of this reaction. Interestingly, the INT-A2w could be detected at the beginning of this reaction, however, which disappeared after 2 h while the reaction was still proceeding (see Supplementary Information for details).
a Nonlinear effect study. b Detection and characterization of the reaction intermediate. c Preparation of allyl phosphate of CPA A7 and its reaction with ketone 1a under the standard conditions. d Cross experiment of INT-A2w. e Asymmetric reaction of allyl benzyl ether 6a with ketone 1a under the standard conditions.
Although the covalent phosphate INT-A was unambiguously assigned and it could undergo the addition reaction with α-alkynyl ketone 1a to give the enantioenriched addition product 3a, there are still two plausible reaction pathways of INT-A. The first one is that the enol form of the ketone substrate would directly attack the INT-A through a SN2 substitution reaction. While the second one is that the INT-A would go through the elimination reaction to give the α,β-unsaturated iminium intermediate pairing with the chiral phosphate anion, which would receive the conjugate addition of the ketone to give the corresponding addition product. To figure out which reaction pathway INT-A is taking part in, some control experiments were performed. First, an allyl phosphate of CPA A7 (A7-allyl) was prepared, whose characteristic NMR signals are well matched with that of INT-A, again confirming the structure of INT-A (Fig. 6c). However, A7-allyl is rather stable and could be readily purified by silica gel column chromatography. Moreover, the subjection of A7-allyl into the standard conditions with ketone 1a did not provide the expected ketone α-allylation product. Second, a cross experiment was performed (Fig. 6d). Mixing allenamide 2w with (R)-CPA A7 gave the mixture containing (R)-INT-A2w, which was followed by adding a mixture of ketone 1a and CPA (S)-A7. On the other hand, another experiment was conducted by mixing 2w with (S)-A7 first to generate (S)-INT-A2w, which was followed by adding the mixture of ketone 1a and CPA (R)-A7. We anticipated that if the covalent INT-A2w would directly participate in the SN2 reaction with the enol form of the ketone, these two reactions would provide products with distinct er values at the early stage of the reactions, since the enantioselectivities would be mainly controlled by the corresponding chiral covalent INT-A2w. However, both reactions afforded products 3w in almost racemic version at the early stage of the reactions (at 5 min). Furthermore, allyl benzyl ether 6a was prepared, which was anticipated to undergo the facile E1cb-type elimination to give the α,β-unsaturated iminium under acidic conditions. The subjection of the allyl benzyl ether 6a into the standard conditions with ketone 1a afforded the same addition product 3w in 46% yield with 97:3 er, which suggested that 6a probably underwent the same reaction intermediate as allenamide 2w (Fig. 6e). However, it is worth mentioning that no covalent intermediate INT-A2w was observed in 31P NMR and 19F NMR by mixing 6a with CPA (S)-A7 in D6-benzene.
Based on these experimental results, a plausible reaction mechanism for this reaction was proposed, in which the CPA catalyst played two roles (Fig. 7). First, CPA catalyzed the isomerization of ketone 1a to generate the E-configurated enol intermediate INT-B (the less sterically hindered enol intermediate), which is the rate-determining step (RDS) of this reaction. The isomerization of allenyl ketone 1a’ to INT-B was also feasible and more efficient, which may be available to develop new asymmetric reactions65. The second role of CPA is the facile addition with the allenamide 2a to form the allyl phosphate adduct INT-A, however, which is not the real active intermediate and not involved in the key asymmetric transition state. We anticipated that the covalent phosphate INT-A would undergo the facile E1cb-type elimination to give the α,β-unsaturated iminium INT-C69, paired with the corresponding chiral phosphate anion, which was believed to be the real active intermediate. The asymmetric conjugate addition of the enol INT-B with this α,β-unsaturated iminium INT-C under the guidance of chiral phosphate anion was proposed to be the enantio-determining step (EDS) of this reaction, and the hydrogen-bonding between the enol −OH group and the P = O moiety of phosphate INT-C was believed to be crucial. Consequently, the Re-face attack of the enol intermediate INT-B to the α,β-unsaturated iminium under the dual-activation of the phosphate anion of CPA (S)-A9 afforded the enantioenriched addition product 3a with S-configuration.
Derivatizations of chiral products
A large-scale asymmetric reaction of 1a (2.0 mmol) with allenamide 2a was performed under the optimal conditions to showcase the practicability of this method, which readily provided the desired addition product 3a in 78% yield with 97:3 er (Fig. 8a). To demonstrate the synthetic utilities of these chiral products bearing fruitful functional groups, a range of transformations were studied. Hydrogenation of 3a using Pd/C as catalyst under 1 atm of H2 at 0 °C provided the enamide reduced product 7a; while performing the hydrogenation under 50 atm of H2 provided both enamide and alkyne hydrogenated product 8a (Fig. 8b). Cu-catalyzed cycloaddition between the acetylene motif of 3 m and benzyl azide proceeded smoothly to give the triazole product 9 m in 68% yield with 97:3 er (Fig. 8c). Treatment of 3a with HCl/EA solution facilely hydrolyzed the enamide moiety, generating the 5-keto aldehyde 10a in high yield, which upon treatment with TsOH at 80 °C provided the aldol reaction product cyclohexenone 11a in 88% yield with retained optical purity (Fig. 8d). The ketone moiety in product 3a could be stereoselectively reduced by l-selectride at −78 °C to give the alcohol 12a in 97% yield with >20:1 dr (Fig. 8e). Further cyclization of 12a under the catalysis of PdCl2(PhCN)2 provided access to dihydropyran derivative 13a in 43% yield and cyclic aminal 13b in 21% yield, without erosion of enantiomeric purity. The relative configurations of the products were assigned by NOE analysis of 13a and 13b.
Discussion
In conclusion, we have disclosed a practical method for asymmetric construction of acyclic all-carbon quaternary stereocenters via direct enantioselective additions of α-alkynyl ketones to allenamides enabled by chiral phosphoric acid catalysis. A broad range of α-substitutions, alkynyl groups, and substituents on allenamide were well compatible within these reactions, generating acyclic ketones bearing α-quaternary stereocenters with high enantioselectivities. Detailed mechanistic studies were performed to elucidate the mechanism of these reactions, and a CPA catalyzed enol formation step was proposed as the rate-determining step. Although a novel covalent allyl phosphate INT-A was well identified by various spectroscopic methods in these reactions, more mechanistic studies suggested that it was probably not involved in the crucial asymmetric transition state. A more plausible mechanism involving the enantioselective addition of the enol form of ketones with the α,β-unsaturated iminium under the guidance of chiral phosphate anion as the enantio-determining step was proposed. The abundance of various functional groups within the chiral products facilitated the diverse derivatizations of the chiral products, demonstrating the synthetic utilities of these methods.
Methods
To a dried sealed tube was added 4 Å molecular sieves (200 mg), allenamide 2 (0.22 mmol) and (S)-A9 (15.2 mg 0.02 mmol) sequentially, which was followed by adding a solution of ketone 1 (0.2 mmol) in CCl4 (2 mL). After stirring at room temperature for 20 h, the mixture was filtered through Celite and the filtrate was concentrated under vacuum to give a residue, which was purified by column chromatography on silica gel (petroleum ether:EtOAc, 30:1 ~10:1) to give the products 3.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2047788 (3n). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Vries, J. G. D. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis (Wiley-VCH, 2006).
Corey, E. J. & Guzman-Perez, A. The catalytic enantioselective construction of molecules with quaternary carbon stereocenters. Angew. Chem. Int. Ed. 37, 388–401 (1998).
Trost, B. M. & Jiang, C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis 2006, 369–396 (2006).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181 (2014).
Mase, N., Tanaka, F. & Barbas, C. F. III Synthesis of β-hydroxyaldehydes with stereogenic quaternary carbon centers by direct organocatalytic asymmetric aldol reactions. Angew. Chem. Int. Ed. 43, 2420–2423 (2004).
Lalonde, M. P., Chen, Y. & Jacobsen, E. N. A chiral primary amine thiourea catalyst for the highly enantioselective direct conjugate addition of α,α-disubstituted aldehydes to nitroalkenes. Angew. Chem. Int. Ed. 45, 6366–6370 (2006).
Brown, A. R., Kuo, W.-H. & Jacobsen, E. N. Enantioselective catalytic α-alkylation of aldehydes via an SN1 pathway. J. Am. Chem. Soc. 132, 9286–9288 (2010).
Krautwald, S., Schafroth, M. A., Sarlah, D. & Carreira, E. M. Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis. J. Am. Chem. Soc. 136, 3020–3023 (2014).
Mo, X. & Hall, D. G. Dual catalysis using boronic acid and chiral amine: acyclic quaternary carbons via enantioselective alkylation of branched aldehydes with allylic alcohols. J. Am. Chem. Soc. 138, 10762–10765 (2016).
Mukherjee, S. & List, B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 129, 11336–11337 (2007).
Wang, P.-S., Lin, H.-C., Zhai, Y.-J., Han, Z.-Y. & Gong, L.-Z. Chiral counteranion strategy for asymmetric oxidative C(sp3)-H/C(sp3)-H coupling: enantioselective α-allylation of aldehydes with terminal alkenes. Angew. Chem. Int. Ed. 53, 12218–12221 (2014).
Wright, T. B. & Evans, P. A. Enantioselective rhodium-catalyzed allylic alkylation of prochiral α,α-disubstituted aldehyde enolates for the construction of acyclic quaternary stereogenic centers. J. Am. Chem. Soc. 138, 15303–15306 (2016).
Meng, J., Fan, L.-F., Han, Z.-Y. & Gong, L.-Z. α-quaternary chiral aldehydes from styrenes, allylic alcohols, and syngas via multi-catalyst relay catalysis. Chem. 4, 1047–1058 (2018).
Trost, B. M., Hung, C.-I., Saget, T. & Gnanamani, E. Branched aldehydes as linchpins for the enantioselective and stereodivergent synthesis of 1,3-aminoalcohols featuring a quaternary stereocentre. Nat. Catal. 1, 523–530 (2018).
Hamashima, Y., Hotta, D. & Sodeoka, M. Direct generation of nucleophilic chiral palladium enolate from 1,3-dicarbonyl compounds: catalytic enantioselective Michael reaction with enones. J. Am. Chem. Soc. 124, 11240–11241 (2002).
Nemoto, T. et al. P-chirogenic diaminophosphine oxide: a new class of chiral phosphorus ligands for asymmetric catalysis. J. Am. Chem. Soc. 126, 3690–3691 (2004).
Corkey, B. K. & Toste, F. D. Catalytic enantioselective Conia-Ene reaction. J. Am. Chem. Soc. 127, 17168–17169 (2005).
Wu, F., Hong, R., Khan, J., Liu, X. & Deng, L. Asymmetric synthesis of chiral aldehydes by conjugate additions with bifunctional organocatalysis by cinchona alkaloids. Angew. Chem. Int. Ed. 45, 4301–4305 (2006).
Xie, X., Chen, Y. & Ma, D. Enantioselective arylation of 2-methylacetoacetates catalyzed by CuI/trans-4-hydroxy-l-proline at low reaction temperatures. J. Am. Chem. Soc. 128, 16050–16051 (2006).
Bernardi, L., López-Cantarero, J., Niess, B. & Jørgensen, K. A. Organocatalytic asymmetric 1,6-additions of β-ketoesters and glycine imine. J. Am. Chem. Soc. 129, 5772–5778 (2007).
Liu, W.-B., Reeves, C. M. & Stoltz, B. M. Enantio-, diastereo-, and regioselective iridium-catalyzed asymmetric allylic alkylation of acyclic β-ketoesters. J. Am. Chem. Soc. 135, 17298–17301 (2013).
You, Y. E., Zhang, L., Cui, L., Mi, X. & Luo, S. Catalytic asymmetric Mannich reaction with N-carbamoyl imine surrogates of formaldehyde and glyoxylate. Angew. Chem. Int. Ed. 56, 13814–13818 (2017).
Zhou, H., Wang, Y., Zhang, L., Cai, M. & Luo, S. Enantioselective terminal addition to allenes by dual chiral primary amine/palladium catalysis. J. Am. Chem. Soc. 139, 3631–3634 (2017).
Wang, Y., Liu, X. & Deng, L. Dual-function cinchona alkaloid catalysis: catalytic asymmetric tandem conjugate addition−protonation for the direct creation of nonadjacent stereocenters. J. Am. Chem. Soc. 128, 3928–3930 (2006).
Wang, B., Wu, F., Wang, Y., Liu, X. & Deng, L. Control of diastereoselectivity in tandem asymmetric reactions generating nonadjacent stereocenters with bifunctional catalysis by cinchona alkaloids. J. Am. Chem. Soc. 129, 768–769 (2007).
Bella, M. & Jørgensen, K. A. Organocatalytic enantioselective conjugate addition to alkynones. J. Am. Chem. Soc. 126, 5672–5673 (2004).
Kang, J. Y. & Carter, R. G. Primary amine, thiourea-based dual catalysis motif for synthesis of stereogenic, all-carbon quaternary center-containing cycloalkanones. Org. Lett. 14, 3178–3181 (2012).
Felker, I., Pupo, G., Kraft, P. & List, B. Design and enantioselective synthesis of cashmeran odorants by using “enol catalysis”. Angew. Chem. Int. Ed. 54, 1960–1964 (2015).
Yang, X. & Toste, F. D. Asymmetric addition of α-branched cyclic ketones to allenamides catalyzed by a chiral phosphoric acid. Chem. Sci. 7, 2653–2656 (2016).
Urruzuno, I., Mugica, O., Oiarbide, M. & Palomo, C. Bifunctional Brønsted Base catalyst enables regio-, diastereo-, and enantioselective Cα-alkylation of β-tetralones and related aromatic-ring-fused cycloalkanones. Angew. Chem. Int. Ed. 56, 2059–2063 (2017).
Urruzuno, I. et al. α-branched ketone dienolates: base-catalysed generation and regio- and enantioselective addition reactions. Chem. Eur. J. 25, 9701–9709 (2019).
Pupo, G., Properzi, R. & List, B. Asymmetric catalysis with CO2: the direct α-allylation of ketones. Angew. Chem. Int. Ed. 55, 6099–6102 (2016).
Trost, B. M. & Schroeder, G. M. Palladium-catalyzed asymmetric alkylation of ketone enolates. J. Am. Chem. Soc. 121, 6759–6760 (1999).
Trost, B. M., Schroeder, G. M. & Kristensen, J. Palladium-catalyzed asymmetric allylic alkylation of α-aryl ketones. Angew. Chem. Int. Ed. 41, 3492–3495 (2002).
Hamada, T., Chieffi, A., Åhman, J. & Buchwald, S. L. An improved catalyst for the asymmetric arylation of ketone enolates. J. Am. Chem. Soc. 124, 1261–1268 (2002).
Rao, X. et al. Efficient synthesis of (−)-corynoline by enantioselective palladium-catalyzed α-arylation with sterically hindered substrates. Angew. Chem. Int. Ed. 57, 12328–12332 (2018).
Liao, X., Weng, Z. & Hartwig, J. F. Enantioselective α-arylation of ketones with aryl triflates catalyzed by difluorphos complexes of palladium and nickel. J. Am. Chem. Soc. 130, 195–200 (2008).
Ge, S. & Hartwig, J. F. Nickel-catalyzed asymmetric α-arylation and heteroarylation of ketones with chloroarenes: effect of halide on selectivity, oxidation state, and room-temperature reactions. J. Am. Chem. Soc. 133, 16330–16333 (2011).
Teng, B. et al. Pentanidium- and bisguanidinium-catalyzed enantioselective alkylations using silylamide as Brønsted probase. J. Am. Chem. Soc. 138, 9935–9940 (2016).
Trost, B. M., Saget, T. & Hung, C.-I. Direct catalytic asymmetric Mannich reactions for the construction of quaternary carbon stereocenters. J. Am. Chem. Soc. 138, 3659–3662 (2016).
Zheng, Y. & Deng, L. Catalytic asymmetric direct aldol reaction of [small alpha]-alkyl azlactones and aliphatic aldehydes. Chem. Sci. 6, 6510–6514 (2015).
Yang, X. & Toste, F. D. Direct asymmetric amination of α-branched cyclic ketones catalyzed by a chiral phosphoric acid. J. Am. Chem. Soc. 137, 3205–3208 (2015).
Shevchenko, G. A., Oppelaar, B. & List, B. An unexpected α-oxidation of cyclic ketones with 1,4-benzoquinone by enol catalysis. Angew. Chem. Int. Ed. 57, 10756–10759 (2018).
Shevchenko, G. A., Pupo, G. & List, B. Direct asymmetric α-hydroxylation of cyclic α-branched ketones through enol catalysis. Synlett 30, 49–53 (2019).
Pousse, G., Cavelier, F. L., Humphreys, L., Rouden, J. & Blanchet, J. Brønsted acid catalyzed asymmetric aldol reaction: a complementary approach to enamine catalysis. Org. Lett. 12, 3582–3585 (2010).
Shevchenko, G. A., Pupo, G. & List, B. Catalytic asymmetric α-amination of α-branched ketones via enol catalysis. Synlett 26, 1413–1416 (2015).
Das, J. P. & Marek, I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 47, 4593–4623 (2011).
Marek, I. et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical step. J. Am. Chem. Soc. 136, 2682–2694 (2014).
Eppe, G., Didier, D. & Marek, I. Stereocontrolled formation of several carbon–carbon bonds in acyclic systems. Chem. Rev. 115, 9175–9206 (2015).
Marek, I., Masarwa, A., Delaye, P.-O. & Leibeling, M. Selective carbon–carbon bond cleavage for the stereoselective synthesis of acyclic systems. Angew. Chem. Int. Ed. 54, 414–429 (2015).
Doyle, A. G. & Jacobsen, E. N. Enantioselective alkylation of acyclic α,α-disubstituted tributyltin enolates catalyzed by a 51 complex. Angew. Chem. Int. Ed. 46, 3701–3705 (2007).
Starkov, P., Moore, J. T., Duquette, D. C., Stoltz, B. M. & Marek, I. Enantioselective construction of acyclic quaternary carbon stereocenters: palladium-catalyzed decarboxylative allylic alkylation of fully substituted amide enolates. J. Am. Chem. Soc. 139, 9615–9620 (2017).
Alexy, E. J., Zhang, H. & Stoltz, B. M. Catalytic enantioselective synthesis of acyclic quaternary centers: palladium-catalyzed decarboxylative allylic alkylation of fully substituted acyclic enol carbonates. J. Am. Chem. Soc. 140, 10109–10112 (2018).
Alexy, E. J., Fulton, T. J., Zhang, H., Stoltz & Brian, M. Palladium-catalyzed enantioselective decarboxylative allylic alkylation of fully substituted N-acyl indole-derived enol carbonates. Chem. Sci. 10, 5996–6000 (2019).
Trost, B. M., Hung, C.-I. J. & Gnanamani, E. Tuning the reactivity of ketones through unsaturation: construction of cyclic and acyclic quaternary stereocenters via Zn-prophenol catalyzed Mannich reactions. ACS Catal. 9, 1549–1557 (2019).
Ye, X., Pan, Y. & Yang, X. Direct enantioselective Mannich reactions of α-azido cyclic ketones: asymmetric construction of chiral azides possessing an α-quaternary stereocenter. Chem. Commun. 56, 98–101 (2020).
Ye, X., Pan, Y., Chen, Y. & Yang, X. Enantioselective construction of sulfur-containing tetrasubstituted stereocenters via asymmetric functionalizations of α-sulfanyl cyclic ketones. Adv. Synth. Catal. 362, 3374–3379 (2020).
Rajkumar, S. et al. Regioselective and enantioselective synthesis of β-indolyl cyclopentenamides by chiral anion catalysis. Angew. Chem. Int. Ed. 57, 13489–13494 (2018).
Wang, J. et al. Chiral phosphoric acid-catalyzed stereodivergent synthesis of trisubstituted allenes and computational mechanistic studies. Nat. Commun. 11, 5527 (2020).
Romano, C., Jia, M., Monari, M., Manoni, E. & Bandini, M. Metal-free enantioselective electrophilic activation of allenamides: stereoselective dearomatization of indoles. Angew. Chem. Int. Ed. 53, 13854–13857 (2014).
Zhou, H. et al. From palladium to Brønsted acid catalysis: highly enantioselective regiodivergent addition of alkoxyallenes to pyrazolones. Angew. Chem. Int. Ed. 56, 1077–1081 (2017).
Yang, K. et al. Asymmetric addition of pyrazolones to allenamides catalyzed by a chiral phosphoric acid. Eur. J. Org. Chem. 2018, 6469–6473 (2018).
Yang, B. et al. Organocatalyzed intermolecular asymmetric allylic dearomatization of both α- and β-naphthols. Org. Lett. 21, 330–334 (2019).
Biswas, S., Kim, H., Le Anh Cao, K. & Shin, S. Enantioselective dearomative cyclization of homotryptamines with allenamides into indolo[2,3-b]quinolines. Adv. Synth. Catal. 362, 1841–1845 (2020).
Hashimoto, T., Sakata, K. & Maruoka, K. α-chiral acetylenes having an all-carbon quaternary center: phase transfer catalyzed enantioselective α alkylation of α-alkyl-α-alkynyl esters. Angew. Chem. Int. Ed. 48, 5014–5017 (2009).
Zhong, F., Xue, Q.-Y. & Yin, L. Construction of chiral 2,3-allenols through a copper(I)-catalyzed asymmetric direct alkynylogous aldol reaction. Angew. Chem. Int. Ed. 59, 1562–1566 (2020).
Yang, Q. et al. Holistic prediction of the pKa in diverse solvents based on a machine-learning approach. Angew. Chem. Int. Ed. 59, 19282–19291 (2020).
Giacinto, P., Bottoni, A., Garelli, A., Miscione, G. P. & Bandini, M. Covalent or non-covalent? A mechanistic insight into the enantioselective brønsted acid catalyzed dearomatization of indoles with allenamides. ChemCatChem 10, 2442–2449 (2018).
Lv, J., Zhang, Q., Zhong, X. & Luo, S. Asymmetric latent carbocation catalysis with chiral trityl phosphate. J. Am. Chem. Soc. 137, 15576–15583 (2015).
Acknowledgements
We gratefully acknowledge NSFC (Grant No. 22171186) and ShanghaiTech University start-up funding for financial support. The authors thank the support from Analytical Instrumentation Center (contract no. SPST-AIC10112914), SPST, ShanghaiTech University, and Dr. Na Yu for the X-ray crystallographic analysis. We thank Prof. De-Wei Gao (ShanghaiTech University) for proofreading.
Author information
Authors and Affiliations
Contributions
J.W. and F.H. performed the experiments. X.Y. directed the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., He, F. & Yang, X. Asymmetric construction of acyclic quaternary stereocenters via direct enantioselective additions of α-alkynyl ketones to allenamides. Nat Commun 12, 6700 (2021). https://doi.org/10.1038/s41467-021-27028-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-27028-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.