Abstract
A highly efficient, low-cost and environmentally friendly photocathode with long-term stability is the goal of practical solar hydrogen evolution applications. Here, we found that the Cu3BiS3 film-based photocathode meets the abovementioned requirements. The Cu3BiS3-based photocathode presents a remarkable onset potential over 0.9 VRHE with excellent photoelectrochemical current densities (~7 mA/cm2 under 0 VRHE) and appreciable 10-hour long-term stability in neutral water solutions. This high onset potential of the Cu3BiS3-based photocathode directly results in a good unbiased operating photocurrent of ~1.6 mA/cm2 assisted by the BiVO4 photoanode. A tandem device of Cu3BiS3-BiVO4 with an unbiased solar-to-hydrogen conversion efficiency of 2.04% is presented. This tandem device also presents high stability over 20 hours. Ultimately, a 5 × 5 cm2 large Cu3BiS3-BiVO4 tandem device module is fabricated for standalone overall solar water splitting with a long-term stability of 60 hours.
Similar content being viewed by others
Introduction
Solar water splitting using sunlight irradiation has been widely believed to be a clean way to produce hydrogen energy without CO2 emission1,2,3,4. As an appreciable solar energy utilization/conversion mode, photoelectrochemical (PEC) solar water splitting has been extensively studied based on various semiconductors5,6,7. Since Honda and Fujishima found that TiO2 photoelectrodes showed promising PEC water splitting properties8, various semiconductor materials have been investigated to improve the solar-to-hydrogen (STH) conversion efficiency and device working stability9. The core of the PEC water splitting device is at the semiconductor/liquid interface junction, where minority carriers excited in the semiconductor are driven into the liquid by the electric field in the depletion region at the semiconductor/liquid interface6. For example, redox reactions, such as the reduction of H+ to H2, are driven at the interface between the P-type semiconductor and liquid. To date, many p-type semiconductors have been studied as photocathodes for PEC water splitting.
P-type semiconductors that are used on photovoltaic devices such as c-Si10, a-Si11, Cu(In,Ga)Se12,13, CuInS214, Cu2ZnSnS4 (CZTS)15,16, CuSbS217, Sb2Se318 and CdTe19 are always suitable as the photocathode for PEC water splitting due to their high light absorption coefficient and suitable optical band gap. In recent years, Cu-chalcogenide compound semiconductors have attracted wide attention for PEC water splitting. Although Cu-chalcogenide photocathodes such as CuInS214, CuGaSe220, CuGa3Se521, (Ag,Cu)GaSe222, and Cu(In,Ga)Se212,13, show excellent STH conversion efficiency, there are many difficulties in their large-scale production with regards to low throughput and material utilization because of the limited availability of elements (i.e., In, Ga and Se). Therefore, cost-effective and environmentally protective CZTS semiconductors are believed to be promising photocathode materials that have been investigated for ~10 years since the first report from Prof. Domen’s group in 201023. During the last few years, many researchers, including us, have made efforts to improve the STH conversion efficiency and working stability15,16,24,25,26,27,28. Recently, we achieved a record applied bias photon-to-current efficiency (ABPE) over 2.7% and 3.5% with the highest onset potential of 0.7 VRHE and over 10 h of PEC stability for the CZTS-based photocathode by various surface modifications25,28. However, the complex element consistency and numerous antisite/vacancy defects of CZTS compounds restrict their photovoltage. The record onset potential we achieved at ~0.7 VRHE is still not appreciable for the further fabrication of efficient tandem cells with suitable photoanodes such as BiVO4 and Fe2O329,30. Meanwhile, the required postsulfurization process of the CZTS film also restricted its large-scale industrial integration for solar hydrogen evolution16,25,28. Cu2O-based photocathodes have been widely investigated due to their high PEC photovoltage, which has reached as high as 1.2 VRHE, but their low stability is still a major problem31,32. Fortunately, ternary I − V − VI compounds of Cu3BiS3 are believed to be promising chalcogenide compounds for photovoltaic devices due to their perfect optical band gap (Eg ≈ 1.4–1.7 eV), high absorption coefficient (>105 cm−1) compared to those of CuInSe2 and Cu2ZnSnS4, and p-type conductivity with a carrier concentration of ~2 × 1016 cm−3 33,34,35. Cu3BiS3, naturally occurring in the “Wittichenite” mineral form, is stable for a broad range of processing temperatures36. Moreover, it is made of inexpensive, nontoxic, and earth-abundant elements, and we found that the Cu3BiS3 film can be easily produced on a large scale by any suitable low-cost approach not under vacuum, such as printing and spraying34,37. Recently, Cu3BiS3 has attracted much attention, and remarkable progress has been made by many groups using different fabrication approaches and theoretical studies38,39,40.
However, the previously reported photovoltaic performances and PEC water splitting efficiencies of Cu3BiS3-based solar cells or photocathode devices are still not significant35,37,38,39. The best conversion efficiency achieved by a Cu3BiS3-based solar cell is 0.17% (0.11 cm2 active area)37, and the best reported Cu3BiS3-based photocathode only presented a 0.1 mA/cm2 photocurrent density at 0 VRHE (J0), with a 0.65 VRHE onset potential value (Voc)38. Moreover, the reported devices are all on the lab scale, and their stabilities are all not appreciable (<60 min)37,38,39. The reported low efficiency and poor PEC performance of Cu3BiS3-based photoelectrodes are far from their theoretical limit considering their perfect optical band gap value of ~1.7 eV for solar water splitting, and the low working stability itself restricts their further industrial utilization38,39. The low solar-to -hydrogen conversion efficiency should be caused by the low crystallinity of the material, poor surface/interface conditions and obvious internal defects33,38. In this work, we presented a world record ABPE of 1.7% for our Cu3BiS3-based photocathode for solar water splitting, and the photocurrent density (J0) of ~7 mA/cm2, onset potential (Voc) of 0.9 VRHE and ABPE of 1.7%. The obtained photocurrent density (J0) of ~7 mA/cm2 are almost 70 times higher than their previously reported highest values of J0 (0.1 mA/cm2)38. In addition, due to the high onset potential of the Cu3BiS3-based photocathode, we first fabricated a tandem cell of Cu3BiS3-BiVO4 for unbiased solar water splitting, and an unassisted bright STH conversion efficiency over 2% was also achieved. More importantly, a large-scale Cu3BiS3-based single photocathode over 5 × 5 cm2 was also fabricated. Finally, with the help of the BiVO4 photoanode, a large 5 × 5 cm2 Cu3BiS3-BiVO4 tandem cell was fabricated. We were excited to find that the large-scale Cu3BiS3-BiVO4 tandem cell showed long-term working stability, and the device working stability was found to be very high; the solar water splitting photocurrents were not degraded over 60 h (70% of their initial photocurrent density was maintained).
This is the first report of an efficient and large-sized Cu3BiS3-based photocathode and Cu3BiS3-BiVO4 tandem cell for unbiased solar water splitting. The large device size, appreciable STH conversion efficiency and high device working stability that were presented in this work indicated the promising practical application potentials of the Cu3BiS3-based photoelectrode.
Results
Fabrication and optimization of the Cu3BiS3 thin films
In this work, Cu3BiS3 thin films were prepared by spray pyrolysis in one step. The detailed raw materials and process for the preparation of Cu3BiS3 films are given in the Supporting Information. We found that the quality of the Cu3BiS3 films was significantly influenced by the substrate temperature and Cu: Bi molar ratio of the precursor solution.
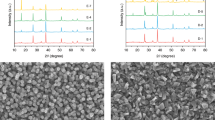
Figure 1a shows the XRD patterns of the Cu3BiS3 films sprayed at various substrate temperatures (360 °C, 380 °C, 400 °C and 420 °C). It was found that the intensity of major diffraction peaks increased with increasing substrate temperature, indicating that the crystallinity of Cu3BiS3 films was improved with the substrate temperature. Nevertheless, some impurity phases, such as Mo2-xS and Cu3Bi3S7, were also easily formed at high substrate temperatures (marked with a yellow background in Fig. 1a). Furthermore, the substrate temperature-dependent Raman spectrum of the Cu3BiS3 films is shown in Fig. 1b. We observed two major vibrational peaks at 279 cm−1 and 467 cm−1 from the Raman spectrum, which matched well with the reported Raman shifts of Cu3BiS3 films33,38. The half-widths of the vibrational peaks at ~279 cm−1 and 467 cm−1 decreased with increasing substrate temperature, indicating that the crystallinity increased with increasing substrate temperature. In addition, an extra Raman shift peak at ~410 cm−1 (corresponding to the MoS2 Raman peak) appeared when the substrate temperature increased to above 400 °C. Notably, post-high-temperature sulfurization is generally necessary for the further crystal growth of multimetal sulfide materials13,14,15,16,17. Nevertheless, the one-step sprayed Cu3BiS3 films in this work possessed good crystalline quality and pure phases with no obvious secondary phase or impurity phase, indicating that no postannealing process was required, which was instrumental in scalability and practical applications for Cu3BiS3-based photocathodes.
(a) XRD patterns and (b) Raman spectra of the Cu3BiS3 films sprayed at various substrate temperatures. surface SEM images of the Cu3BiS3 films sprayed at various substrate temperatures of (c) 360 °C, (d) 380 °C, (e) 400 °C and (f) 420 °C. Source data are provided as a Source Data file.
The microstructural morphologies of the Cu3BiS3 films sprayed at various substrate temperatures (360 °C, 380 °C, 400 °C and 420 °C) are shown in Fig. 1c–f. The films sprayed at a relatively low substrate temperature of 360 °C were not compact, various small crystals composed the Cu3BiS3 films, and obvious fluctuations with large gaps were observed from their surface morphology (Fig. 1c). When we increased the substrate temperature to 380 °C, the Cu3BiS3 crystal size significantly increased to ~1000 nm, and the films were observed to be compact and flat (Fig. 1d). Although the Cu3BiS3 films sprayed at 400 °C were dense and smooth, the crystal size of Cu3BiS3 was slightly decreased (Fig. 1e). Moreover, when the substrate temperature increased to 420 °C, the size of the Cu3BiS3 crystals obviously decreased to ~300–500 nm (Fig. 1f).
Figure 2a shows the PEC performance of the Cu3BiS3-based photocathodes sprayed at various substrate temperatures determined by LSV under chopped sunlight irradiation. The photocathode based on the Cu3BiS3 film sprayed at 380 °C exhibited the highest photocurrent density (~7 mA/cm2), onset potential (~0.9 VRHE) and fill factor. Meanwhile, the statistical plots shown in Fig. S1 also persuasively suggested this trend. The flat band potential (Vfb) of the Cu3BiS3-based photocathodes sprayed at various substrate temperatures can be determined from the intersecting points of the Mott–Schottky curves and X axis (shown in Fig. 2b)41. The photocathodes based on the Cu3BiS3 films sprayed at 360 °C, 380 °C, 400 °C and 420 °C exhibited Vfb values of −0.55 VRHE, −0.57 VRHE, −0.53 VRHE and −0.48 VRHE, respectively. The most negative Vfb of −0.57 VRHE (corresponding to the photocathode based on the Cu3BiS3 film sprayed at 380 °C) implied the highest energy level of photogenerated electrons, indicating that it was easier for photogenerated electrons to transfer from the electrode into the electrolyte and participate in the hydrogen evolution reaction41.
(a) Chopped photocurrent density-potential curves, (b) corresponding Mott–Schottky curves and (c) corresponding transit photocurrent spectra of the Cu3BiS3-based photocathodes sprayed at various substrate temperatures (360 °C, 380 °C, 400 °C and 420 °C). Source data are provided as a Source Data file.
Figure 2c shows the transient photocurrent decay of the photocathodes based on the Cu3BiS3 films sprayed at various substrate temperatures (360 °C, 380 °C, 400 °C and 420 °C). We found that the photocathode based on the Cu3BiS3 film sprayed at 380 °C exhibited a smaller photocurrent spike and wider saturated photocurrent region, illustrating less charge carrier recombination and efficient photogenerated electron transfer within the electrode28. In summary, the photocathode based on the Cu3BiS3 film sprayed at 380 °C invariably presented excellent charge-transfer kinetics, which may be attributed to the fact that the Cu3BiS3 film sprayed at the 380 °C substrate temperature possessed fewer spurious phases, fewer bulk defects and higher crystallinity. As shown in Fig. S2, electrochemical impedance spectroscopy (EIS) measurements were undertaken to further identify the charge-transfer kinetics mechanism of the Cu3BiS3-based photocathodes sprayed at various substrate temperature. The EIS measurements were carried out in phosphate buffer (pH = 6.5) under AM 1.5 G illumination biased at 0.3 VRHE with a frequency sweep from 100 kHz to 0.1 Hz. An equivalent circuit model (shown in the inset of Fig. S2) consisting of two serially connected resistor–capacitor blocks with a resistance (R) and a constant phase element was employed to fit the EIS results42. The fitted resistance parameters are shown in Table S1, in which Rs is mainly composed of the sheet resistance of the contact and external wire. R1 is generally influenced by the transport of charge carriers inside the electrode, and R2 represents the impedance at the electrode/electrolyte interface33,34,35,36,37,38,39,40. We found that the samples sprayed at 380 °C showed a significantly lower bulk resistance and lower interfacial/bulk recombination ratio of photoexcited carriers due to the compact film structure and appreciate crystalline quality of the Cu3BiS3 film (380 °C), as shown in Fig. 1d. The Cu3BiS3 films we studied below were all sprayed at 380 °C substrate temperature.
The effects of the Cu:Bi molar ratio of the precursor solution on the crystallinity and purity of the sprayed Cu3BiS3 films were also systematically investigated in this work. Figure S3 shows a comparison of the X-ray diffraction (XRD) patterns of Cu3BiS3 films sprayed from precursor solutions with various Cu: Bi molar ratios. Clearly, all of the observed diffraction peaks of the Cu3BiS3 film sprayed from the precursor solution with a Cu:Bi = 3:1 ratio corresponded well to the reference (JCPDS NO. 43-1479)33,34,35,36,37,38,39,40. Nevertheless, we observed obvious secondary phases and impurity phases, such as Cu2-xS and Cu3Bi3S7 (marked with a yellow background in Fig. S3), from the diffraction peaks of the Cu3BiS3 films sprayed from the precursor solution with Cu:Bi ratios of 1:1, 2:1, 4:1 and 5:1. Meanwhile, the high intensity of sharp diffraction characteristic peaks of the Cu3BiS3 film (with a Cu:Bi = 3:1 ratio) indicated its good crystallinity, which was preferred for high-performance water splitting device fabrication.
Furthermore, the PEC performance of the Cu3BiS3-based photocathode as a function of various Cu:Bi molar ratios was also discussed. As we expected, the Cu3BiS3-based photocathode with a Cu:Bi = 3:1 ratio exhibited the highest photocurrent density and onset potential, as shown in Fig. S4a. We repeated the experiments many times and found the real trend, as shown in Fig. S4b. Relatively, the samples with an excess of Cu tended to show a higher PEC performance than Cu-deficient samples, but this trend was reversed when the molar ratio of Cu:Bi exceeded 3:1. Therefore, the stoichiometric samples possessed the highest PEC performance (including photocurrent density, onset potential and ABPE). The EIS spectra (Fig. S5) and the fitted resistance parameters results (Table S2) of these Cu3BiS3 photocathodes also indicated that the stoichiometric samples exhibited the smallest values of R1´ and R2´, which suggested less charge recombination inside of the electrode and better charge transfer at the electrode/electrolyte interface42. The EIS analyses were consistent with the results of linear sweep voltammetry (LSV) measurements (Fig. S4a). As a result, the optimal Cu:Bi ratio of the precursor solution for the sprayed Cu3BiS3 films was 3:1.
On the basis of these studies, the Cu3BiS3 film sprayed at a 380 °C substrate temperature and with a Cu:Bi = 3:1 molar ratio precursor solution was selected as the absorption layer for the Cu3BiS3 photocathode. In this context, transmission electron microscopy (TEM) of the optimum Cu3BiS3 film (Fig. S6a) further confirmed the large Cu3BiS3 crystalline size of ~1000 nm. The STEM-EDX elemental mappings of Cu, Bi and S in Fig. S6 b–d clearly showed that the elements were homogeneously distributed in the grains. In addition, the high-resolution transmission electron microscopy image of the as-prepared Cu3BiS3 crystals (Fig. S6e) indicated that the measured interplanar distance of 2.8 Å and 3.0 Å corresponds to the (131) and (102) plane of the Wittichenite Cu3BiS3 (PDF No. 43-1479). The typical selected area electron diffraction pattern (Fig. S6f) indicated that our Cu3BiS3 film possessed a good crystalline quality.
PEC performance of the Cu3BiS3-based photocathode
As we have found in the CZTS-based photocathode, surface coverage of the CdS buffer layer is an effective way to enhance the PEC performance due to the p–n junction formed at the interface of CZTS/CdS16,25,26,28, and further deposition of the TiO2 protective layer under CdS would enhance the PEC stability43. In this work, a TiO2/CdS overlayer and a Pt particle catalyst were also modified under the Cu3BiS3 absorption layer, and the detailed deposition processes of these modification layers are given in the Supporting Information. The microscopic cross-sectional structure of the finished Pt-TiO2/CdS/Cu3BiS3 electrode is shown in Fig. S7a, indicating that a very thin TiO2 (~50 nm)/CdS (~80 nm) double layer was modified onto the surface of the Cu3BiS3 layer (~1000 nm). The EDS mapping results (Fig. S7b–d) clearly show the interfaces between the multilayer structure of TiO2/CdS/Cu3BiS3, demonstrating the compact coverage or passivation of the Cu3BiS3 films by CdS and TiO2 over layers.
The PEC performances of the Pt-Cu3BiS3, Pt-CdS/Cu3BiS3 and Pt-TiO2/CdS/Cu3BiS3 photocathodes were investigated by LSV, as shown in Fig. 3a. Pt catalytic particles are necessary to provide active sites for surface reactions43. However, the Pt-Cu3BiS3 photocathode exhibited a poor photocurrent density that was even <1 mA/cm2. As a buffer layer, a chemical bath deposited (CBD) CdS layer was modified onto the Cu3BiS3 layer to enhance the PEC performance of the photocathodes, which was usually applied onto various absorber layers, such as CZTS and GeSe, to form heterojunctions in our previous work25,43. Accompanied by the modification of the CBD-CdS layer, the photocurrent density and onset potential of the Pt-CdS/Cu3BiS3 photocathodes were substantially improved to ~2 mA/cm2 (at 0 VRHE) and ~0.75 VRHE, respectively. The enhancement of the PEC performance was attributed to the heterojunction that was formed at the interface of CdS/Cu3BiS3, which can efficiently promote the separation of charge carriers and reduce surface recombination 25,43.
(a) Chopped photocurrent density-potential curves of the Pt-Cu3BiS3, Pt-CdS/Cu3BiS3 and Pt-TiO2/CdS/Cu3BiS3 photocathodes. (b) ABPE curves, (c) IPCE spectra and (d) photocurrent density-time curves of the Pt-CdS/Cu3BiS3 and Pt-TiO2/CdS/Cu3BiS3 photocathodes. (e) Hydrogen and oxygen evolution amounts of the Pt-TiO2/CdS/Cu3BiS3 photocathode with various illumination times and (f) photograph of the Pt-TiO2/CdS/Cu3BiS3 photocathode under working conditions. All measurements were carried out in a 0.2 mol/dm3 Na2HPO4/NaH2PO4 solution (pH = 6.5) under solar simulated AM 1.5 G light irradiation. Source data are provided as a Source Data file.
Nevertheless, the photocurrent density of the Pt-CdS/Cu3BiS3 photocathode was less than half of the initial value after the 3 h stability test, as shown in Fig. 3d, due to the oxidative self-photocorrosion of the CdS layer25. Consequently, a protective TiO2 layer deposited by atomic layer deposition (ALD) was modified onto the surface of the CdS layer to achieve relatively stable photocathodes in this work, which is a conventional strategy to enhance the stability of previously reported photocathodes such as CZTS44, Cu2BaSn(S, Se)445, Cu(In,Ga)Se213, GeSe43, Sb2Se341 and Cu2O31. To our surprise, as shown in Fig. 3a and d, not only was the stability of the photocathodes significantly improved to over 10 h but also the photocurrent density dramatically increased from ~2 mA/cm2 to ~7 mA/cm2 (at 0 VRHE), and the onset potential increased to above ~0.9 VRHE with the modification of the TiO2 layer. Meanwhile, there were no obvious corrosive metamorphisms at the surface of the finished photocathodes by comparing the cross-sectional SEM images before and after the 10 h stability test (Fig. S8), further confirming the great protective effect of the pinhole-free ALD-TiO2 layer. It should be emphasized that such a relatively high onset potential of ~0.9 VRHE provides the conditions for the realization of efficient unbiased water splitting cells in contact with appropriate photoanodes such as BiVO4 and Fe2O329,30. Moreover, the finished photocathode achieved the highest ABPE of 1.7% at 0.4 VRHE, which was more than four times that of the Pt-CdS/Cu3BiS3 photocathode, as shown in Fig. 3b.
The protective effect of the TiO2 layer can be referenced to previous related works13,31,41,43,45, and the underlying mechanism of the increased PEC performance of the inserted TiO2 layer was further understood through a series of PEC characterizations and band schema. Figure 3c shows the spectra of the incident photocurrent conversion efficiency (IPCE) undertaken at 0 VRHE and the corresponding integrated photocurrent density of the Pt-CdS/Cu3BiS3 and Pt-TiO2/CdS/Cu3BiS3 photocathodes. Accompanied by the importation of the TiO2 layer, the increase in the IPCE in the low wavelength range was more obvious than that in the relatively long wavelength range (the IPCE value increased from 15 to 44% at 450 nm), indicating that the inserted TiO2 layer greatly enhanced the light harvesting and conversion efficiency in the low wavelength range of the finished photocathode. It was observed that the efficiency dropped suddenly in the wavelength range of 490–540 nm with a long absorption tail after 590 nm for the finished photocathodes, suggesting that the interfacial recombination of photoexcited carriers in the Pt-TiO2/CdS/Cu3BiS3 photocathode was still obvious. Whether the TiO2 layer was introduced or not, the integrated photocurrent density practically matched the photocurrent density measured by LSV, demonstrating the uniformity of LSV measurements and IPCE results. In addition, the reflection characterizations (Fig. S9) of our Cu3BiS3-based photocathodes with and without TiO2 layer also demonstrated that the TiO2 layer can be used as an antireflection layer to enhance the light absorption for the Cu3BiS3-based photocathodes.
Furthermore, intensity-modulated photovoltage spectroscopy (Fig. S10) and intensity-modulated photocurrent spectroscopy (Fig. S11) of the three electrodes (Pt-Cu3BiS3, Pt-CdS/Cu3BiS3, and Pt-TiO2/CdS/Cu3BiS3) were performed to understand the surface/interfacial recombination or transfer of photogenerated carriers in the electrodes. Through the calculation (the detailed computational procedures are given in the Supporting Information), the Pt-TiO2/CdS/Cu3BiS3 photocathode exhibited a higher carrier lifetime (τn) and a lower charge transfer time (τd) than those of the Pt-Cu3BiS3 and Pt-CdS/Cu3BiS3 photocathodes, which indicated that the transfer efficiency of the photoexcited carriers was enhanced and the interfacial recombination rate was decreased with the importation of the TiO2/CdS overlayer 46.
The band diagram of the TiO2/CdS/Cu3BiS3 electrode was further investigated, as shown in Fig. S12. There was a conspicuous cliff-like conduction band offset (0.7 eV) at the interface of CdS/Cu3BiS3, which would result in a recombination center at the interface and be unbeneficial for the transfer of the photogenerated electrons33. This is also one of the reasons why the previously reported Cu3BiS3-based photovoltaic devices presented a poor conversion efficiency buffered with a CdS layer35,37. Accompanied by the inserted ALD-TiO2 layer, the photogenerated electrons and holes can be separated more effectively due to the relatively low valence band position of TiO2. Moreover, the photogenerated electrons can also be selectively transferred through the TiO2 layer directly into the electrolyte and participate in the hydrogen evolution reaction24. Based on the above discussion, these factors all led to a higher PEC performance for the Pt-TiO2/CdS/Cu3BiS3 photocathode than the photocathode modified with a single CdS layer.
Figure 3e shows the H2 and O2 production amounts of the finished photocathode biased at 0 VRHE with various illumination times. H2 gas was produced with a constant rate of 141 µmol cm−2 h−1, indicating the highly stable overall solar water splitting properties of the Pt-TiO2/CdS/Cu3BiS3 photocathode. Notably, the H2 and O2 production amounts of the finished photocathode biased at more positive potentials (such as 0.3 VRHE and 0.6 VRHE) were also determined, and as shown in Fig. S13, H2 gas can be produced steadily with constant rates of 95.7 µmol cm−2 h−1 and 35.2 µmol cm−2 h−1, respectively, when the finished photocathode was biased at 0.3 VRHE and 0.6 VRHE under simulated solar irradiation, which further identified the authenticity of such a high onset potential (~0.9 VRHE) of the Pt-TiO2/CdS/Cu3BiS3 photocathode. Meanwhile, according to our calculation, the faradaic efficiency of this Pt-TiO2/CdS/Cu3BiS3 photocathode biased at any potential (0 VRHE, 0.3 VRHE and 0.6 VRHE) were all close to 90% (Fig. S14). Figure 3f is a photograph of the Pt-TiO2/CdS/Cu3BiS3 photocathode under working conditions.
The thickness of the CdS buffer was found to significantly influence the PEC properties of the Cu3BiS3-based photocathode, and the statistical results are shown in Fig. S15. It was found that the optimum thickness of the CdS buffer was ~80 nm (i.e., 15 min CBD). A thinner CdS buffer (i.e., <80 nm) may not efficiently passivate the surface of Cu3BiS3, while a thicker CdS buffer (higher than 80 nm) would increase the resistance and absorb solar light without passing through the Cu3BiS3 photoabsorber (Fig. S16). In addition, a CdS layer that is too thick may increase the photoexcited carrier recombination efficiency, as shown in the transit photocurrent spectra (Fig. S17). On the basis of these studies, the optimum deposition time of the CBD-CdS layer was determined to be 15 min in this work, and the thickness of the CBD-CdS layer deposited for 15 min can reach ~80 nm (Fig. S7)
Scalable Cu3BiS3-BiVO4 tandem cell for unbiased solar water splitting
It should be noted here that the Cu3BiS3-based photocathode presented a very promising solar water splitting photovoltage >0.9 VRHE, and such a high onset potential is very important and suitable to assemble efficient tandem of photocathode-photoanode cells for standalone devices. In this work, we prepared a tandem cell of Cu3BiS3-BiVO4. Figure 4a shows the current-potential curves obtained from a Cu3BiS3-based photocathode and a BiVO4 photoanode; thus, a two-electrode tandem PEC cell was assembled by placing a BiVO4/FTO photoanode (0.73 cm2) in front of a Pt-TiO2/CdS/Cu3BiS3 photocathode (0.45 cm2) followed by exposure to simulated AM 1.5 G solar radiation, as shown in Fig. S18. The details of the synthesis of the efficient BiVO4 photoanode were illustrated in our previous paper29. There was an obvious duplication between the J–V curves of the Cu3BiS3 photocathode and BiVO4 photoanode under a photovoltage from 0.35 VRHE to 0.9 VRHE, and the crossing point and operation point were observed at 1.66 mA/cm2 (Jop) and 0.54 VRHE, respectively. We showed the video of the Cu3BiS3-BiVO4 tandem cell under working with simulated sunlight irradiation (Movie S1) in Supporting Information, many H2/O2 bubbles were evolved from the tandem cell for the overall solar water splitting reaction. Meanwhile, the IPCE spectra (Fig. S19) measured under the operating bias (0.54 VRHE) for both electrodes show complimentary spectral responses. The energy band diagrams of the Cu3BiS3-BiVO4 tandem cell (Fig. S20) show the photogenerated carrier transfer path and solar hydrogen evolution reaction process. Based on the STH efficiency calculation equation:
where P is the power of the illuminating light, the STH of this Cu3BiS3-BiVO4 tandem cell achieved a remarkable efficiency of 2.04%. This 2.04% STH efficiency is the first reported and recorded unbiased STH efficiency for Cu3BiS3-BiVO4 tandem cells, which is very close to the record STH efficiency from Cu2O-BiVO4 tandem cells reported by the Gratzel group (Table 1) 31.
(a) J–V curves of the Cu3BiS3 photocathode, BiVO4 photoanode and Cu3BiS3 photocathode behind the BiVO4 photoanode. (Jop: the operation point photocurrent density, STH: solar to hydrogen efficiency). (b) Photocurrent density-time curve, (c) working diagram and (d) hydrogen and oxygen evolution amount-time curves of the Cu3BiS3-BiVO4 tandem device. (e) Photographs of the 5 × 5 cm2 Cu3BiS3 photocathode, 5 × 5 cm2 BiVO4 photoanode and large area (5 × 5 cm2) Cu3BiS3-BiVO4 tandem device and diagram of the large area (5 × 5 cm2) Cu3BiS3-BiVO4 tandem device. (f) Working photograph of the large area (5 × 5 cm2) Cu3BiS3-BiVO4 tandem device under solar simulated AM 1.5 G irradiation. (g) Photocurrent density-time curve of the large area (5 × 5 cm2) Cu3BiS3-BiVO4 tandem device. All measurements were carried out in a 0.2 mol/dm3 Na2HPO4/NaH2PO4 solution (pH = 6.5) under solar simulated AM 1.5 G irradiation. Source data are provided as a Source Data file.
Stability is another important issue to evaluate the real application potential, and we characterized the long-term photocurrent stability of this Cu3BiS3-BiVO4 tandem cell over 20 h of irradiation (AM 1.5 G). The data shown in Fig. 4b demonstrate that the tandem cell possessed a very high long-term stability, and the photocurrent after 20 h of irradiation maintained ~90% of its initial value. The working diagram of this tandem cell is shown in Fig. 4c. In addition, Fig. 4d shows that the Cu3BiS3-BiVO4 tandem cell possessed stable H2 gas evolution with a constant ratio of 18 µmol/h.
The appreciable efficiency and high stability of the Cu3BiS3 photocathode and the Cu3BiS3-BiVO4 tandem cell are very suitable for practical utilizations. However, the device size is another key point. Fortunately, large Cu3BiS3 and BiVO4 photoelectrodes are not difficult to obtain. We prepared 5 × 5 cm2 Cu3BiS3 and BiVO4 and assembled them into a tandem device in this work, and their photos and the model diagram are shown in Fig. 4e. The 5 × 5 cm2 size Cu3BiS3 photocathode not only presented an appreciable photocurrent of about 90 mA under 0 VRHE (active area: 21 cm2) but also possessed an excellent working stability (Fig. S21). Significant number of H2 bubbles were observed to be continuously produced from the surface of Cu3BiS3 photocathode under simulated sunlight irradiation (Movie S2, Fig. S22). It should be noted here that 5 × 5 cm2 is the largest size we can currently prepare due to the limit of our apparatus, and much larger sizes, such as 10 × 10 cm2 or 20 × 20 cm2, should be able to be prepared when we update our apparatus. Figure 4f and Movie S3 show a photograph and a video of this 5 × 5 cm2 Cu3BiS3-BiVO4 tandem device under simulated AM 1.5 G solar light illumination, respectively. Many H2 and O2 bubbles can be observed on the surface of the Cu3BiS3 photocathode and BiVO4 photocathode, respectively. As shown in Fig. 4g, the photocurrent of this 5 × 5 cm2 Cu3BiS3-BiVO4 tandem device did not obviously decrease even over the 60 h stability test, indicating that the long-term operation of the large Cu3BiS3-BiVO4 tandem device is promising.
Although the photocurrent of the Cu3BiS3-based photocathodes in this work still has much room for improvement, the photovoltage and stability of the Cu3BiS3 photocathode presented here are very close to the record of the Cu2O photocathode31 but already higher than those of various typical photocathodes, such as CZTS25, CIGS12, Sb2Se318, Si10 and so on (Table 1). We can expect large potential for the improvement in the PEC properties of Cu3BiS3-based photocathodes. Compared with Cu2O, we found that the Cu3BiS3 film is relatively stable in neutral buffer solutions and that the stable Cu3BiS3 film is much easier to obtain than Cu2O31. We believe that the Cu3BiS3-based photocathode will attract much attention and be significantly improved in the near future. The over 2% STH efficiency of the Cu3BiS3-BiVO4 tandem devices and over 20 h of stability presented indicate great potential for solar water splitting. In comparison with the previously reported typical tandem cell (Fig. 5), it was found that the STH efficiency of our Cu3BiS3-BiVO4 tandem cell was competitive among them. Nevertheless, it should be noted that the research of the Cu3BiS3 photoelectrode is almost at the starting point now, and the presented appreciable STH efficiency (2.04%) and stability (20 h and 60 h) indicate great potential for Cu3BiS3 photoelectrodes. We believe that the PEC efficiency/stability of the Cu3BiS3 photoelectrode and Cu3BiS3-BiVO4 tandem cell will be significantly improved and even higher than those of other famous CIGS-, Cu2O- and CZTS-based photoelectrodes in the near future. This work provides a promising starting point for the development of Cu3BiS3-based photoelectrodes, and we believe that one milestone after another will be achieved in the future.
Efficiency and stability benchmarks of the previously reported photocathode-photoanode tandem cells for standalone water splitting. The statistical PEC performance parameters are shown in Table 1.
Discussion
In this work, the Cu3BiS3-based photocathode exhibited excitation at a set potential over 0.9 VRHE and an excellent PEC current density of ~7 mA/cm2 under 0 VRHE with 10 h long-term high stability in neutral water solutions. Further fabricated Cu3BiS3-BiVO4 photocathode-to-photoanode tandem cells presented a good unbiased STH conversion efficiency as high as 2.04%. However, such tandem devices also presented superior high stability over 20 h. Finally, a 5 × 5 cm2 large Cu3BiS3-BiVO4 tandem device module was fabricated for scalable overall solar water splitting device applications, and the stable photocurrent of this larger module over 60 h of operation under sustained solar light irradiation was detected. The appreciable PEC properties, such as the high onset potential, good photocurrent, over 60 h of high stability, ideal STH efficiency (2.04%) and scalable device module (5 × 5 cm2) for the Cu3BiS3-based photocathode that were presented in this work, all indicate its remarkable application value and great potential for solar water splitting.
Methods
Preparation of the Cu3BiS3 films
The Cu3BiS3 films (with a Cu/3:Bi = 1 mole ratio) were prepared by spray pyrolysis method. The sprayed precursor solution was mixed by 15 mL CuCl-Tu (excess) DMSO solution and 15 mL BiCl3- Tu (excess) DMSO solution. Specifically, the Cu-Tu stock solution was formed by 2.97 g CuCl (2 M) and excess thiourea (Tu) both dissolved in 15 mL DMSO, and the Bi-Tu stock solution was formed by 3.15 g BiCl3 (0.666 M) and excess thiourea (Tu) both dissolved in 15 mL DMSO. Then, the two stock solutions were both separately stirred until clear and transparent before their mix. Furthermore, the mixing CuCl (1 M), BiCl3 (0.333 M) and excess Tu precursor solution was also stirred for 5 h, ultimately obtaining a yellow clear sprayed solution for Cu3BiS3 films preparation. The precursor solution was subsequently sprayed onto a cleaned Mo-coated soda-lime glass substrate preheated to 380 °C for about 3 min.
Surface mdification with TiO2/CdS overlayer
First, a CdS layer was deposited under the Cu3BiS3 layer by the chemical bath deposition method (CBD). The prepared Cu3BiS3 layer was dipped into an aqueous solution containing 12.5 mM CdSO4, 0.22 mM SC(NH2)2, and 11 M NH4OH at 60 °C for 15 min.
Furthermore, a TiO2 layer was deposited under the CdS/Cu3BiS3 double layer by ALD method. The TiO2 layer was grown by using titanium tetrakis (dimethylamide) as titanium source and H2O as oxygen source. Based on our empirical value, the growth rate was estimated to be about 0.054 nm per cycle and the TiO2 film was grown for 926 cycles at 120 °C, and the corresponding thicknesses of the TiO2 layer was 50 nm, ultimately obtaining a TiO2/CdS/Cu3BiS3 electrode.
Deposition of Pt particles
The Pt particles deposition was performed by using a three-electrode system consisting of TiO2/CdS/Cu3BiS3 as a working electrode, a Pt wire as a counter electrode, and Ag/AgCl as a reference electrode. These electrodes were put in 0.1 M Na2SO4 solution containing 1 mM H2PtCl6, and the deposition was performed with a constant potential of −0.1 VAg/AgCl by using CHI660E electrochemical measurement unit. During the deposition process, the working electrode was illuminated by simulated AM 1.5 G solar irradiation.
Preparation of the BiVO4 photoanodes
The BiVO4 films were also prepared by spray pyrolysis method. The sprayed solution was prepared by dissolving Bi(NO3)3·5H2O in acetic acid and VO(AcAc)2 in absolute ethanol. The Bi stock solution was then added into the V stock solution, and the mixture was diluted to 4 mM with excess ethanol. The mixed precursor solution was immediately sprayed onto a cleaned FTO-coated glass substrate preheated to 450 °C, and the spray nozzle was placed 25 cm above the heating plate. The sprayed solution was driven by an over pressure of 0.5 bar of N2 gas.
Photoelectrochemical measurements
An online gas chromatography system (Shimadazu GC-2014 gas analyzer equipped with a MS-5A column and a thermal conductivity detector) was used to detect H2 and O2 during the PEC water splitting. PEC H2 generation from photocathodes was examined in a pH 6.5 phosphate buffer solution (0.2 M Na2HPO4/NaH2PO4) by using the above-mentioned solar simulator as a light source. The PEC cell was covered by a water jacket to maintain the temperature at 293 K. The two-electrode setup composed of the Pt-TiO2/CdS/Cu3BiS3 photocathode and a BiVO4 photoanode in series connection was also employed to examine water splitting under the bias-free condition. Furthermore, the phosphate buffer solution was updated per 20 h stability test to keep the concentration (0.2 M) and pH (6.5) unchanged. The intensity of simulated sunlight was also calibrated per 20 h stability test. Potentials referred to the Ag/AgCl electrode were converted to reversible hydrogen electrode using the Nernst equation:
ABPE was determined from the current density−potential response of the photocathodes by using the following equation:
Where J is the photocurrent density (mA/cm2), V is the applied potential (VRHE), and P is the intensity of simulated sunlight (100 mW/cm2).
Structural characterization
Crystalline structures of the films were determined by XRD, Raman spectroscopy and TEM using a Rigaku Mini Flex X-ray diffractometer, a Jasco NRC 3100 laser Raman spectrophotometer and JEOL JEM-2100HR microscope respectively. Surface and cross section morphology were observed by scanning electron microscope (SEM) using Hitachi S-4800 micros.
Data availability
The data that support the plots and other findings of within this paper are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Hisatomi, T. & Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2, 387–399 (2019).
Larcher, D. & Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Lewis, N. S. Toward cost-effective solar energy use. Science 315, 798–801 (2007).
Kamat, P. V. Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J. Phys. Chem. C. 111, 2834–2860 (2007).
Yang, Y. et al. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv. Energy Mater. 7, 1700555 (2017).
Niu, F. J. et al. Hybrid photoelectrochemical water splitting systems: from interface design to system assembly. Adv. Energy Mater. 10, 1900399 (2020).
Hou, Y., Zhuang, X. D. & Feng, X. L. Recent advances in earth-abundant heterogeneous electrocatalysts for photoelectrochemical water splitting. Small Methods 1, 1700090 (2017).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Chen, S. et al. Semiconductor-based photocatalysts for photocatalytic and photoelectrochemical water splitting: will we stop with photocorrosion? J. Mater. Chem. A 8, 2286–2322 (2020).
Vijselaar, W. et al. Spatial decoupling of light absorption and catalytic activity of Ni-Mo-loaded high-aspect-ratio silicon microwire photocathodes. Nat. Energy 3, 185–192 (2018).
Lin, Y. J. et al. Amorphous Si thin film based photocathodes with high photovoltage for efficient hydrogen production. Nano Lett. 13, 5615–5618 (2013).
Kobayashi, H. et al. Development of highly efficient CuIn0.5Ga0.5Se2-based photocathode and application to overall solar driven water splitting. Energy Environ. Sci. 11, 3003–3009 (2018).
Chen, M. X. et al. Spatial control of cocatalysts and elimination of interfacial defects towards efficient and robust CIGS photocathodes for solar water splitting. Energy Environ. Sci. 11, 2025–2034 (2018).
Zhao, J. et al. Enhancement of solar hydrogen evolution from water by surface modification with CdS and TiO2 on porous CuInS2 photocathodes prepared by an electrodeposition-sulfurization method. Angew. Chem. Int. Ed. 53, 11808–11812 (2014).
Tay, Y. F. et al. Solution-processed Cd-substituted CZTS photocathode for efficient solar hydrogen evolution from neutral water. Joule 2, 537–548 (2018).
Jiang, F. et al. Pt/In2S3/CdS/Cu2ZnSnS4 thin film as an efficient and stable photocathode for water reduction under sunlight radiation. J. Am. Chem. Soc. 137, 13691–13697 (2015).
Zhang, L. et al. Solution-processed CuSbS2 thin film: a promising earth-abundant photocathode for efficient visible-light-driven hydrogen evolution. Nano Energy 28, 135–142 (2016).
Yang, W. et al. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat. Commun. 11, 861 (2020).
Su, J., Minegishi, T. & Domen, K. Efficient hydrogen evolution from water using CdTe photocathodes under simulated sunlight. J. Mater. Chem. A 5, 13154–13160 (2017).
DeAngelis, A. D., Horsley, K. & Gaillard, N. Wide band gap CuGa(S,Se) thin films on transparent conductive fluorinated tin oxide substrates as photocathode candidates for tandem water splitting devices. J. Phys. Chem. C. 122, 14304–14312 (2018).
Muzzillo, C. P. et al. Low-cost, efficient, and durable H2 production by photoelectrochemical water splitting with CuGa3Se5 photocathodes. ACS Appl. Mater. Interfac. 10, 19573–19579 (2018).
Kim, J. H. et al. Overall photoelectrochemical water splitting using tandem cell under simulated sunlight. ChemSusChem 9, 61–66 (2016).
Yokoyama, D. et al. H2 evolution from water on modified Cu2ZnSnS4 photoelectrode under solar light. Appl. Phys. Express 3, 101202 (2019).
Yang, W. et al. Molecular chemistry-controlled hybrid ink derived efficient Cu2ZnSnS4 photocathodes for photoelectrochemical water splitting. ACS Energy Lett. 1, 1127–1136 (2016).
Huang, D. et al. Over 1% efficient unbiased stable solar water splitting based on a sprayed Cu2ZnSnS4 photocathode protected by a HfO2 photocorrosion-resistant film. ACS Energy Lett. 3, 1875–1881 (2018).
Feng, K. et al. MoSx-CdS/Cu2ZnSnS4-based thin film photocathode for solar hydrogen evolution from water. Appl. Catal. B: Environ. 268, 118438 (2020).
Tay, Y. F. et al. Improving the interfacial properties of CZTS photocathodes by Ag substitution. J. Mater. Chem. A 8, 8862–8867 (2020).
Li, L. et al. Surface plasmon resonance effect of a Pt-nano-particles-modified TiO2 nanoball overlayer enables a significant enhancement in efficiency to 3.5% for a Cu2ZnSnS4-based photocathode used for solar water splitting. Chem. Eng. J. 396, 125264 (2020).
Kuang, Y. et al. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy 2, 16191 (2016).
Jang, J. W. et al. Enabling unassisted solar water splitting by iron oxide and silicon. Nat. Commun. 6, 7447 (2015).
Pan, L. et al. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 1, 412–420 (2018).
Paracchino, A., Laporte, V., Sivula, K., Gratzel, M. & Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10, 456–461 (2011).
Whittles, T. J. et al. Band alignments, band gap, core levels, and valence band states in Cu3BiS3 for photovoltaics. ACS Appl. Mater. Interfac. 11, 27033–27047 (2019).
Liu, S., Wang, X., Nie, L., Chen, L. & Yuan, R. Spray pyrolysis deposition of Cu3BiS3 thin films. Thin Solid Films 585, 72–75 (2015).
Hussain, A. et al. P-type Cu3BiS3 thin films for solar cell absorber layer via one stage thermal evaporation. Appl. Surf. Sci. 505, 144597 (2020).
Viezbicke, B. D. & Birnie, D. P. III Solvothermal synthesis of Cu3BiS3 enabled by precursor complexing. ACS Sustain. Chem. Eng. 1, 306–308 (2013).
Li, J. J. et al. One-step synthesis of Cu3BiS3 thin films by a dimethyl sulfoxide (DMSO)-based solution coating process for solar cell application. Sol. Energy Mater. Sol. Cells 174, 593–598 (2018).
Kamimura, S., Beppu, N., Sasaki, Y., Tsubota, T. & Ohno, T. Platinum and indium sulfide-modified Cu3BiS3 photocathode for photoelectrochemical hydrogen evolution. J. Mater. Chem. A 5, 10450–10456 (2017).
Li, J. J., Han, X. X., Wang, M., Zhao, Y. & Dong, C. Fabrication and enhanced hydrogen evolution reaction performance of a Cu3BiS3 nanorods/TiO2 heterojunction film. N. J. Chem. 42, 4114–4120 (2018).
Yin, J. & Jia, J. Synthesis of Cu3BiS3 nanosheet films on TiO2 nanorod arrays by a solvothermal route and their photoelectrochemical characteristics. Crystengcomm 16, 2795–2801 (2014).
Yu, X. L. et al. Biomolecule-assisted self-assembly of CdS/MoS2/graphene hollow spheres as high-efficiency photocatalysts for hydrogen evolution without noble metals. Appl. Catal. B: Environ. 182, 504–512 (2016).
Tan, J. et al. Fullerene as a photoelectron transfer promoter enabling stable TiO2-protected Sb2Se3 photocathodes for photo-electrochemical water splitting. Adv. Energy Mater. 9, 1900179 (2019).
Wang, K. et al. Promising GeSe nanosheet-based thin-film photocathode for efficient and stable overall solar water splitting. ACS Catal. 9, 3090–3097 (2019).
Rovelli, L., Tilley, S. D. & Sivula, K. Optimization and stabilization of electrodeposited Cu2ZnSnS4 photocathodes for solar water reduction. ACS Appl. Mater. Interfac. 5, 8018–8024 (2013).
Zhou, Y. H. et al. Efficient and stable Pt/TiO2/CdS/Cu2BaSn(S,Se)4 photocathode for water electrolysis applications. ACS Energy Lett. 3, 177–183 (2018).
Zhang, Y. P. et al. Improvement of BiVO4 photoanode performance during water photo-oxidation using rh-doped SrTiO3 perovskite as a co-catalyst. Adv. Funct. Mater. 29, 1902101 (2019).
Bornoz, P. et al. A bismuth vanadate-cuprous oxide Tandem cell for overall solar water splitting. J. Phys. Chem. C. 118, 16959–16966 (2014).
Kornienko, N. et al. Growth and photoelectrochemical energy conversion of wurtzite indium phosphide nanowire arrays. ACS Nano 10, 5525–5535 (2016).
Song, A. G. et al. Assessment of a W:BiVO4-CuBi2O4 tandem photoelectrochemical cell for overall solar water splitting. ACS Appl. Mater. Interfac. 12, 13959–13970 (2020).
Moore, E. E., Andrei, V., Zacarias, S., Pereira, I. A. C. & Reisner, E. Integration of a hydrogenase in a lead halide perovskite photoelectrode for tandem solar water splitting. ACS Energy Lett. 5, 232–237 (2020).
Andrei, V. et al. Scalable triple cation mixed halide perovskite-BiVO4 tandems for bias-free water splitting. Adv. Energy Mater. 8, 1801403 (2018).
Kaneko, H., Minegishi, T., Kobayashi, H., Kuang, Y. & Domen, K. Suppression of poisoning of photocathode catalysts in photoelectrochemical cells for highly stable sunlight-driven overall water splitting. J. Chem. Phys. 150, 041713 (2019).
Huang, D. et al. 3.17% efficient Cu2ZnSnS4–BiVO4 integrated tandem cell for standalone overall solar water splitting. Energy Environ. Sci. 14, 1480–1489 (2021).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 61704060); Scientific and Technological Plan of Guangdong Province (No. 2021A0505030061; No. 2019B090905005; No. 2020A1414010058); “Outstanding Young Talent Project” of South China Normal University, Characteristic Innovation Research Project of the University Teachers in Guangdong Province (No. 2020XCC07) and the Innovation Project of Graduate School of South China Normal University.
Author information
Authors and Affiliations
Contributions
F.J. conceived the content and idea of this work, analyzed the data and wrote the paper. D.H. did the experiments and characterizations of the Cu3BiS3-based photocathode and the Cu3BiS3-BiVO4 tandem cell. L.L. and K.W. participated into some experiments of the preparation of the BiVO4 photoanode. Y.L and K.F. did some photoelectrochemical characterizations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Jae Sung Lee and other, anonymous reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, D., Li, L., Wang, K. et al. Wittichenite semiconductor of Cu3BiS3 films for efficient hydrogen evolution from solar driven photoelectrochemical water splitting. Nat Commun 12, 3795 (2021). https://doi.org/10.1038/s41467-021-24060-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-24060-5
This article is cited by
-
A photoelectrochemical aptasensing platform assembled at the heterojunction interface of Cu3BiS3 sensitized CuV2O6 for bisphenol A
Microchimica Acta (2024)
-
Hydrogen-substituted graphdiyne encapsulated cuprous oxide photocathode for efficient and stable photoelectrochemical water reduction
Nature Communications (2022)
-
Rapid Microwave Irradiation Synthesis and Photoelectrochemical Performance of Cu3BiS3 Nanoparticles
Electronic Materials Letters (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.