Abstract
Fluorine-containing moieties show significant effects in improving the properties of functional molecules. Consequently, efficient methods for installing them into target compounds are in great demand, especially those enabled by metal-free catalysis. Here we show a diazaphospholene-catalyzed hydrodefluorination of trifluoromethylalkenes to chemoselectively construct gem-difluoroalkenes and terminal monofluoroalkenes by simple adjustment of the reactant stoichiometry. This metal-free hydrodefluorination features mild reaction conditions, good group compatibility, and almost quantitative yields for both product types. Stoichiometric experiments indicated a stepwise mechanism: hydridic addition to fluoroalkenes and subsequent β-F elimination from hydrophosphination intermediates. Density functional theory calculations disclosed the origin of chemoselectivity, regioselectivity and stereoselectivity, suggesting an electron-donating effect of the alkene-terminal fluorine atom.
Similar content being viewed by others
Introduction
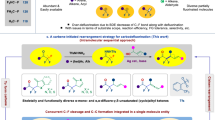
The introduction of fluorine-containing motifs is a commonly used strategy for improving the properties of target molecules in medicines, agrochemicals, and materials1,2,3,4,5,6,7,8, because of the specific characteristics of the fluorine atom, e.g., high lipophilicity, good absorbability, and strong electron-withdrawing ability5,6,9. Among these important fluorine-containing motifs, gem-difluoroalkenes10,11,12,13 and terminal monofluoroalkenes14,15,16,17, deemed, respectively, as mimics of carbonyl and amide groups, have attracted much attention in the modification of bioactive molecules (Fig. 1). For example, replacement of the carbonyl group in artemisinin by a gem-difluoroalkene can improve its antimalarial activity (Fig. 1a)10,11,12. In some cases, the introduced gem-difluoroalkene moiety reverses the regioselectivity of enzyme-catalyzed hydridic reduction, overriding conventional reduction12. Monofluoroalkenes with high stereoselectivities are also important fragments in bioactive compounds (Fig. 1b)18,19,20,21,22. These fluoroalkenes can also serve as versatile building blocks in the construction of other fluorine-containing molecules23,24,25. Consequently, the important functions of fluoroalkenes provoked a great demand for relevant synthetic strategies26,27,28,29,30,31,32.
As known, gem-difluoroalkenes could be conventionally constructed by functional-group interconversion, as represented by carbonyl olefination23,33 via Julia–Kocienski,34,35,36,37 Homer–Wadsworth–Emmons18 and Wittig reactions (Fig. 2a)37,38,39. However, these reactions usually involve the preparation of complicated fluorinated precursors and are usually conducted under harsh conditions, e.g., in strongly basic solutions, thus leading to a quite limited substrate scope. One other good alternative could be defluorination of polyfluoroalkenes via transition-metal catalysis40,41,42,43,44,45, photocatalysis46,47,48,49, or the classical SN2’ reactions50,51,52,53, delivering functionalized fluoroalkenes via alkenylation54, arylation42, borylation55,56, or hydrodefluorination (HDF) (Fig. 2b)45. Recently, Jubault, Poisson, and coworkers diastereoselectively synthesized monofluoroalkenes from the corresponding trifluoromethyl alkenes via successive dual-HDF with stoichiometric LiAlH4 (Fig. 2b)57. However, the use of the strong hydridic LiAlH4 made the reaction incompatible with some electron-deficient groups, and this leads to undesirable over-reduction. Until now, most of the reported methods have only furnished either gem-difluoroalkenes or terminal monofluoroalkenes. Very recently, Wang and coworkers developed an aluminum-catalyzed tunable halodefluorination of trifluoroalkyl-substituted alkenes via fluoride ion abstraction (Fig. 2c)58. In their system, an arbitrary number of fluorine atoms can be selectively replaced with chlorine or bromine atoms by modification of reaction conditions. However, the reaction suffered from drawbacks concerning excess use of one of the reactants (about 4 equiv.), moderate yields, unsatisfactory stereoselectivities, long reaction time (24–48 h) and high reaction temperatures (up to 120 °C).
In this work, we described a method for metal-free catalytic activation of C–F bonds in trifluoromethylalkenes under mild conditions. gem-Difluoroalkenes and terminal monofluoroalkenes can be chemoselectively produced in almost quantitative yields by simple adjustment of the amount of the terminal reductant PhSiH3 (Fig. 2c).
Results and discussion
Investigation of the reaction conditions
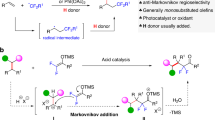
Building on our previous work59, we envisioned that diazaphospholenes of super hydricity60,61,62 may provide a good chance to realize HDF of trifluoromethylalkenes via an SN2’ path63. A preliminary attempt indicated that the reaction of α-trifluoromethyl-styrene 2a with diazaphospholene 1 primarily gave the hydrophosphination intermediate A. Only handful HDF products 3a and 1-F64 were obtained (Fig. 3a and Supplementary Figs. 1 and 2). However, the intermediate A was completely converted to 3a and 1-F at an elevated temperature (70 °C). This is the rare example of β-F elimination enabled by a non-metal neutral reductant, rather than by the well-established metal systems65. When a second equivalent of diazaphospholene 1 was used, the in situ-generated gem-difluoroalkene 3a was further hydrodefluorinated at 70 °C to afford the dual-HDF product 4a in an almost quantitative yield after 1 h (Fig. 3b and Supplementary Figs. 3 and 4). The excellent performance of diazaphospholene 1 in multiple-HDF prompted us to develop its chemoselective HDF of trifluoromethylalkenes for the synthesis of gem-difluoroalkenes and monofluoroalkenes. The successful regeneration of diazaphospholene 1 with fluorophilic PhSiH3 via σ-bond metathesis suggested the possibility of a catalytic version of our design (Fig. 3c and Supplementary Fig. 5)66.
Scope of mono-HDF reactions
As expected, mono-HDF of trifluoromethylalkenes 2 in CH3CN with a 5 mol% catalyst loading and 0.33 equiv. of PhSiH3 (i.e., 1 equiv. of Si–H bonds) proceeded smoothly to give gem-difluoroalkenes 3. The high-polarity solvent CH3CN favors polar hydride transfer. Other solvents, like toluene and THF, gave the mono-HDF products in <10% yields. The reaction showed a wide substrate scope, as shown in Fig. 4. Generally, reductions performed with either electron-rich or -deficient substrates all occurred facilely to give almost quantitative yields. Substrate 2a furnished gem-difluoroalkene 3a in 99% yield after 12 h at 70 °C. Phenyl-substituted 2b and non-substituted 2c were efficiently reduced at lower temperatures. The reactions of substrates with electron-donating groups such as methoxy (2d), methylthio (2e), and dimethylamino (2 f) also worked well, but needed slightly higher reaction temperatures and longer reaction times. Substrates bearing electron-withdrawing groups (2g–2 l) showed high reactivities and gave the corresponding gem-difluoroalkenes 3g–3 l in 91–99% yields. Notably, several functional groups (2j–2 l) that are incompatible with the strong bases used in Wittig, Julia–Kocienski, and Homer–Wadsworth–Emmons reactions, or with strong nucleophiles in SN2’-type reactions, are well tolerated in our system. The known reduction67 of aryl ketones by diazaphospholenes was completely depressed by the higher electrophilicity of the trifluoromethyl group in 2j. Substrate 2 m with a susceptible acetal moiety furnished the product 3 m quantitatively (99%) in a prolonged reaction time. The reaction was also applicable to the naphthalene analog 2n. The excellent performance in heterocyclic systems (3o–3r) shows that this reaction can chemoselectively reduce the trifluoromethyl moiety while leaving other unsaturated structures intact68,69. For tri-substituted alkenes, only the Z isomer of 2 s is applicable (the E isomer of 2 s did not work, see SI for details), and 3 s is produced in 91% yield. This is probably because of a steric effect in the initial hydride transfer. The low efficiency of the reaction of the endocyclic alkene 2t is probably also ascribable to a steric effect. Aliphatic trifluoromethylalkenes did not work in our conditions due to the low electrophilicity.
General reaction conditions: 2 (0.3 mmol), 1 (5 mol%), PhSiH3 (0.33 equiv.) and CH3CN (1 mL) were mixed in a tube under Ar. Isolated yields were given. [a] Determined by 19F NMR spectroscopy. [b] Isolated yields for gram-scale synthesis: 2j or 2 l (5.0 mmol), 1 (5 mol%), PhSiH3 (0.33 equiv.), CH3CN (5 mL), 50 oC, 3 h.
Scope of dual-HDF reactions
Because of the super-hydricity of the catalyst 1, the produced gem-difluoroalkenes 2 continued to react with the hydride 1. This explains why addition of a further 0.33 equiv. of PhSiH3 gave dual-HDF products. Such a result indicates that chemoselective HDF can be achieved by simply adjusting the stoichiometry of the reactants. Accordingly, the preparation of monofluoroalkenes 4 by dual-HDF with 0.7 equiv. of PhSiH3 (i.e., approximately 2 equiv. of Si–H bonds) was investigated. The results are summarized in Fig. 5. Overall, the reduction showed prominent chemoselectivity for most CF3-containing substrates 2, and monofluoroalkenes 4 were generated quantitatively with good to excellent stereoselectivities, although the slightly elevated temperature (80 °C) was necessary for several electron-rich substrates. For examples, substrates 2a–2c gave monofluoroalkenes 4a–4c quantitatively with good E/Z stereoselectivities. Electron-donating groups (2d–2 f) did not significantly depress successive C–F activations, and 4d–4 f were obtained in good to excellent yields (75%–99%). Various electron-withdrawing groups (2g–2 l) were also well tolerated (4g–4 l, 92–99% yields). Notably, the acetal moiety of 2 m was not sensitive to the dual-HDF conditions. Naphthalene 2n gave 4n in an excellent yield and with good diastereoselectivity. The heterocyclic trifluoromethylalkenes 2o–2r were also compatible and gave products 4o–4r in 64–99% yields with moderate to good E/Z stereoselectivities. Similarly to mono-HDF, only the Z isomer of 2 s showed reactivity in dual-HDF. The exocyclic trifluoromethylalkene 2t did not react at all. Note that further increase of the amount of PhSiH3 could remove the third fluoride from some substrates with electron-withdrawing groups.
General reaction conditions: 2 (0.3 mmol), 1 (5 mol%), PhSiH3 (0.7 equiv.), and CH3CN (1 mL) were mixed in a tube under Ar. Isolated yields were given. The E/Z ratios were determined by 19F NMR spectroscopy. [a] Determined by 19F NMR spectroscopy. [b] Isolated yield for gram-scale synthesis: 2 l (5.0 mmol), 1 (5 mol%), PhSiH3 (0.7 equiv.), CH3CN (5 mL), 50 oC, 3 h.
Synthetic applications
To show the versatility of the present system, we used its potential for modifying drug molecules. Indometacin is a commonly used drug, which has significant antipyretic, anti-inflammatory, and antirheumatic activities70. As shown in Fig. 6, under our reaction conditions the indometacin derivative 5 is effectively transformed into either the mono-HDF product 6 in 85% yield or the dual-HDF product 7 in 30% yield, depending on the amount of the reductant PhSiH3.
Mechanistic investigations
Density functional theory (DFT) calculations were used to gain mechanistic insights into the outstanding catalytic performance of diazaphospholene 1 in HDF. The calculations were performed at the (SMD)-M06-2X/6-311 + +G(2df,2p)//(SMD)-M06-2X/6-31 + G(d) level of theory71,72 with trifluoromethylalkene 2c as the template substrate. The results are shown in Fig. 7. During the first HDF, hydride transfer from diazaphospholene 1 to 2c proceeds via TS1, with a Gibbs activation barrier of 18.9 kcal mol−1, to generate the hydrophosphination intermediate A, in line with our room-temperature reaction conditions for initial hydrophosphination (Fig. 3a). Exothermic cis-β-F elimination from intermediate A furnishes the mono-HDF product 3c via TS2, with a 20.7 kcal mol−1 barrier. This suggests a need for elevated temperatures.
The possible paths for the second HDF are more complicated because the hydride can be transferred to either the C1 or C2 site of 3c, to produce intermediates B or B’, respectively (Fig. 8a). Intuitively, the C2 site is preferred, because of the strong electron-withdrawing ability of the fluorine atom. However, our experimental and DFT results uniformly led to good regioselectivity for the hydride transfer to the C1 site, which proceeds via TS3 with an energy barrier about 15 kcal mol−1 lower than the transfer to the C2 site via TS3′. This result is primarily attributed to the following two factors: (1) the stabilizing effect of the aromatic ring on the incipient benzyl carbanion during hydride addition at the C1 site, and (2) the repulsive interaction between the fluorine lone pairs of electrons and π-electrons, which makes the C1 site relatively electron deficient (Fig. 8b). Our results suggest that the alkene terminal fluorine atom has an electron-donating effect rather than the conventional electron-withdrawing effect. The NPA (natural population analysis) analysis also supported the regioselectivity in the second hydride transfer process.
The energy profile for the second HDF is given in Fig. 9. As shown, hydride transfer from 1 to 3c via TS3 has a higher Gibbs activation barrier (24.0 kcal mol−1) than that of TS1 in the first HDF (18.9 kcal mol−1). This 5.1 kcal mol−1 difference guarantees excellent chemoselectivity for mono- and dual-HDF. A second cis-β-F elimination from B can give two isomers of the monofluoroalkene 4c. Based on the difference between TS4 and TS5 (0.9 kcal mol−1), the E-isomer of the monofluoroalkene, E-4c, is preferentially formed. This stereoselectivity can be explained by using Newman projections of the conformers involved in cis-β-F elimination (Fig. 10a). The preferred conformer, which results in the E isomer being the major product, clearly diminishes electronic repulsion between the fluorine atom and the aromatic ring. Indeed, the stereoselectivity (E/Z = 82/18) calculated from the Gibbs activation energies for both β-F elimination steps is in good agreement with the experimental result (E/Z = 85/15).
A plausible mechanism for this HDF process is outlined in Fig. 10b. First, hydride transfer from the catalyst 1 to trifluoromethylalkenes 2 furnishes the hydrophosphination intermediate A. Subsequent β-F elimination gives the mono-HDF products 3 and 1-F. After complete consumption of 2, the excess PhSiH3 regenerates catalyst 1, which renders a second HDF to yield monofluoroalkenes 4.
In summary, we have developed a method for diazaphospholene-catalyzedchemoselective C–F bond activation of trifluoromethylalkenes, which enables the convenient construction of gem-difluoroalkenes and terminal monofluoroalkenes under metal-free conditions with PhSiH3 as the terminal reductant. NMR spectroscopic studies showed a hydrophosphination intermediate, which subsequently underwent β-F elimination at elevated temperatures. This metal-free strategy is applicable to a broad range of trifluoromethylalkenes. It shows good functional group tolerance and gives almost quantitative yields of both mono- and di-hydrodefluorinated products. DFT calculations suggested that the good chemoselectivity between mono- and dual-HDF stems from differences in the substrate electrophilicities, and the regioselectivity for hydride transfer to gem-difluoroalkenes is partly attributed to the electron-donating ability of the alkene terminal fluorine atoms. Other diazaphospholene-catalyzed HDF reactions are currently being investigated in our laboratory.
Methods
General information
Catalyst 1 has been synthesized and characterized in our previous work59,73,74. Trifluoromethyl alkenes 2 were synthesized according to references (see Supplementary Information for details). Other reagents and solvent were purchased from J&K or TCI Chemicals and used without further purification unless specified otherwise. Acetonitrile was purchased from J&K Chemical (99.9%, Extra dry, water <10 ppm, J&K seal) and degassed and distilled by standard methods. Reaction temperature refers to the temperature of an aluminum heating block or a silicon oil bath, which was controlled by an electronic temperature modulator from IKA.
Reactions
All hydrodefluorination reactions were carried out in dry glass wares under an argon atmosphere using Schlenk technique throughout the reaction procedures.
Analytics
1H and 13C NMR, 19F NMR spectra were recorded in CDCl3 (δ = 7.26 for 1H NMR, δ = 77.16 ppm for 13C NMR) on 400 MHz NMR instrument at Center of Basic Molecular Science (CBMS) of Tsinghua University.
DFT calculations
Geometry optimizations and frequency computations were performed using Gaussian 0975 at the M06-2X71,76/6-31 + G(d) level of theory, in conjunction with the SMD72 model to account for the solvation effect of acetonitrile. To obtain more accurate electronic energies, single point energy calculations were performed at the SMD-M06-2X/[6-311 + +G(2df, 2p)] level with the SMD-M06-2X/[6-31 + G(d)] structures.
Data availability
The authors declare that all the data supporting the findings of this work are available within the article and its Supplementary Information files.
References
Yoder, N. C. & Kumar, K. Fluorinated amino acids in protein design and engineering. Chem. Soc. Rev. 31, 335–341 (2002).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008).
Kirk, K. L. Fluorination in medicinal chemistry: methods, strategies, and recent developments. Org. Process Res. Dev. 12, 305–321 (2008).
Nie, J., Guo, H.-C., Cahard, D. & Ma, J.-A. Asymmetric construction of stereogenic carbon centers featuring a trifluoromethyl group from prochiral trifluoromethylated substrates. Chem. Rev. 111, 455–529 (2011).
Alonso, C., Martínez de Marigorta, E., Rubiales, G. & Palacios, F. Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 115, 1847–1935 (2015).
Liu, Q., Ni, C. & Hu, J. China’s flourishing synthetic organofluorine chemistry: innovations in the new millennium. Natl. Sci. Rev. 4, 303–325 (2017).
Tsui, G. C. & Hu, J. Organofluorine chemistry. Asian J. Org. Chem. 8, 566–567 (2019).
Marsh, E. N. G. Fluorinated proteins: from design and synthesis to structure and stability. Acc. Chem. Res. 47, 2878–2886 (2014).
Leriche, C., He, X., Chang, C.-W. T. & Liu, H.-W. Reversal of the apparent regiospecificity of NAD(P)H-dependent hydride transfer: the properties of the difluoromethylene group, a carbonyl mimic. J. Am. Chem. Soc. 125, 6348–6349 (2003).
Magueur, G., Crousse, B., Ourévitch, M., Bonnet-Delpon, D. & Bégué, J.-P. Fluoro-artemisinins: when a gem-difluoroethylene replaces a carbonyl group. J. Fluor. Chem. 127, 637–642 (2006).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Yanai, H. & Taguchi, T. Synthetic methods for fluorinated olefins. Eur J. Org. Chem. 2011, 5939–5954 (2011).
Couve-Bonnaire, S., Cahard, D. & Pannecoucke, X. Chiral dipeptide mimics possessing a fluoroolefin moiety: a relevant tool for conformational and medicinal studies. Org. Biomol. Chem. 5, 1151–1157 (2007).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Drouin, M., Laxio Arenas, J. & Paquin, J.-F. Incorporating a monofluoroalkene into the backbones of short peptides: evaluating the impact on local hydrophobicity. ChemBioChem 20, 1817–1826 (2019).
Drouin, M. et al. Monofluoroalkene-isostere as a 19F-NMR label for the peptide backbone: synthesis and evaluation in membrane-bound PGLa and (KIGAKI)3. Chem. Eur. J. 26, 1511–1517 (2020).
McCarthy, J. R. et al. Stereospecific method to (E) and (Z) terminal fluoroolefins and its application to the synthesis of 2’-deoxy-2’-fluoromethylenenucleosides as potential inhibitors of ribonucleoside diphosphate reductase. J. Am. Chem. Soc. 113, 7439–7440 (1991).
Shimizu, M., Ohno, A. & Yamada, S. (10Z)- and (10E)-19-fluoro-1α: 25-dihydroxyvitamin D3: an improved synthesis via 19-Nor-10-oxo-vitamin D. Chem. Pharm. Bull. 49, 312–317 (2001).
Ohno, A., Shimizu, M. & Yamada, S. Fluorinated vitamin D analogs to probe the conformation of vitamin D in its receptor complex: 19F-NMR studies and biological activity. Chem. Pharm. Bull. 50, 475–483 (2002).
Asahina, Y., Iwase, K., Iinuma, F., Hosaka, M. & Ishizaki, T. Synthesis and antibacterial activity of 1-(2-Fluorovinyl)-7-substituted-4-quinolone-3-carboxylic acid derivatives, conformationally restricted analogues of fleroxacin. J. Med. Chem. 48, 3194–3202 (2005).
Taverna, P. et al. Tezacitabine enhances the DNA-directed effects of fluoropyrimidines in human colon cancer cells and tumor xenografts. Biochem. Pharmacol. 73, 44–55 (2007).
Chelucci, G. Synthesis and metal-catalyzed reactions of gem-dihalovinyl systems. Chem. Rev. 112, 1344–1462 (2012).
Ahrens, T., Kohlmann, J., Ahrens, M. & Braun, T. Functionalization of fluorinated molecules by transition-metal-mediated C–F bond activation to access fluorinated building blocks. Chem. Rev. 115, 931–972 (2015).
Zhang, X. & Cao, S. Recent advances in the synthesis and CF functionalization of gem-difluoroalkenes. Tetrahedron Lett. 58, 375–392 (2017).
Fuchibe, K., Hatta, H., Oh, K., Oki, R. & Ichikawa, J. Lewis acid promoted single C–F bond activation of the CF3 group: SN1′-type 3,3-difluoroallylation of arenes with 2-trifluoromethyl-1-alkenes. Angew. Chem. Int. Ed. 56, 5890–5893 (2017).
Zhang, Z. et al. Reaction of diazo compounds with difluorocarbene: an efficient approach towards 1,1-difluoroolefins. Angew. Chem. Int. Ed. 55, 273–277 (2016).
Hu, M., Ni, C., Li, L., Han, Y. & Hu, J. gem-difluoroolefination of diazo compounds with TMSCF3 or TMSCF2Br: transition-metal-free cross-coupling of two carbene precursors. J. Am. Chem. Soc. 137, 14496–14501 (2015).
Liu, Q., Shen, X., Ni, C. & Hu, J. Stereoselective carbonyl olefination with fluorosulfoximines: facile access to Z or E terminal monofluoroalkenes. Angew. Chem. Int. Ed. 56, 619–623 (2017).
Ma, X. & Song, Q. Recent progress on selective deconstructive modes of halodifluoromethyl and trifluoromethyl-containing reagents. Chem. Soc. Rev. 49, 9197–9219 (2020).
Andrella, N. O., Xu, N., Gabidullin, B. M., Ehm, C. & Baker, R. T. Selective copper complex-catalyzed hydrodefluorination of fluoroalkenes and allyl fluorides: a tale of two mechanisms. J. Am. Chem. Soc. 141, 11506–11521 (2019).
Maekawa, Y., Nambo, M., Yokogawa, D. & Crudden, C. M. Alkyltriflones in the Ramberg–Bäcklund reaction: an efficient and modular synthesis of gem-difluoroalkenes. J. Am. Chem. Soc. 142, 15667–15672 (2020).
Burton, D. J., Yang, Z.-Y. & Qiu, W. Fluorinated Ylides and related compounds. Chem. Rev. 96, 1641–1716 (1996).
Zhu, L., Ni, C., Zhao, Y. & Hu, J. 1-tert-Butyl-1H-tetrazol-5-yl Fluoromethyl Sulfone (TBTSO2CH2F): a versatile fluoromethylidene synthon and its use in the synthesis of monofluorinated alkenes via Julia–Kocienski olefination. Tetrahedron 66, 5089–5100 (2010).
Prakash, G. K. S. et al. Synthesis of monofluoroalkenes via Julia–Kocienski reaction. J. Fluor. Chem. 131, 1192–1197 (2010).
Gao, B., Zhao, Y., Hu, M., Ni, C. & Hu, J. gem-difluoroolefination of diaryl ketones and enolizable aldehydes with difluoromethyl 2-pyridyl sulfone: new insights into the Julia–Kocienski reaction. Chem. Eur. J. 20, 7803–7810 (2014).
Cox, D. G., Gurusamy, N. & Burton, D. J. Surprising stereochemical control of Wittig olefination involving reaction of fluorine-containing phosphoranium salt and aldehydes. J. Am. Chem. Soc. 107, 2811–2812 (1985).
Watterson, A. C. & Shama, S. A. Photochemistry of acid hydrazides. Determination of modes of reaction and identification of photoproducts. J. Org. Chem. 40, 19–24 (1975).
Nowak, I. & Robins, M. J. New methodology for the deoxygenative difluoromethylenation of aldehydes and ketones; unexpected formation of tetrafluorocyclopropanes. Org. Lett. 7, 721–724 (2005).
Hu, J., Han, X., Yuan, Y. & Shi, Z. Stereoselective synthesis of Z fluoroalkenes through copper-catalyzed hydrodefluorination of gem-difluoroalkenes with water. Angew. Chem. Int. Ed. 56, 13342–13346 (2017).
Trost, B. M. & Debien, L. Palladium-catalyzed trimethylenemethane cycloaddition of olefins activated by the σ-electron-withdrawing trifluoromethyl group. J. Am. Chem. Soc. 137, 11606–11609 (2015).
Huang, Y. & Hayashi, T. Rhodium-catalyzed asymmetric arylation/defluorination of 1-(trifluoromethyl)alkenes forming enantioenriched 1,1-difluoroalkenes. J. Am. Chem. Soc. 138, 12340–12343 (2016).
Chen, F., Xu, X., He, Y., Huang, G. & Zhu, S. NiH-catalyzed migratory defluorinative olefin cross-coupling: trifluoromethyl-substituted alkenes as acceptor olefins to form gem-difluoroalkenes. Angew. Chem. Int. Ed. 59, 5398–5402 (2020).
Lan, Y., Yang, F. & Wang, C. Synthesis of gem-difluoroalkenes via nickel-catalyzed allylic defluorinative reductive cross-coupling. ACS Catal. 8, 9245–9251 (2018).
Yao, C., Wang, S., Norton, J. & Hammond, M. Catalyzing the hydrodefluorination of CF3-substituted alkenes by PhSiH3. H• transfer from a nickel hydride. J. Am. Chem. Soc. 142, 4793–4799 (2020).
Lang, S. B., Wiles, R. J., Kelly, C. B. & Molander, G. A. Photoredox generation of carbon-centered radicals enables the construction of 1,1-difluoroalkene carbonyl mimics. Angew. Chem. Int. Ed. 56, 15073–15077 (2017).
Phelan, J. P. et al. Open-air alkylation reactions in photoredox-catalyzed DNA-encoded library synthesis. J. Am. Chem. Soc. 141, 3723–3732 (2019).
Du, H.-W. et al. Synthesis of monofluoroalkenes through visible-light-promoted defluorinative alkylation of gem-difluoroalkenes with 4-alkyl-1,4-dihydropyridines. Org. Lett. 22, 1542–1546 (2020).
Xiao, T., Li, L. & Zhou, L. Synthesis of functionalized gem-difluoroalkenes via a photocatalytic decarboxylative/defluorinative reaction. J. Org. Chem. 81, 7908–7916 (2016).
Kobayashi, O., Uraguchi, D. & Yamakawa, T. Synthesis of α-trifluoromethylstyrene derivatives via Ni-catalyzed cross-coupling of 2-bromo-3,3,3-trifluoropropene and aryl Grignard reagents. J. Fluor. Chem. 130, 591–594 (2009).
Yang, J. et al. A simple base-mediated synthesis of diverse functionalized ring-fluorinated 4h-pyrans via double direct C–F substitutions. Chem. Commun. 51, 8326–8329 (2015).
Fuchibe, K., Takahashi, M. & Ichikawa, J. Substitution of two fluorine atoms in a trifluoromethyl group: regioselective synthesis of 3-fluoropyrazoles. Angew. Chem. Int. Ed. 51, 12059–12062 (2012).
Bergeron, M., Johnson, T. & Paquin, J.-F. The use of fluoride as a leaving group: SN2′ displacement of a C-F bond on 3,3-difluoropropenes with organolithium reagents to give direct access to monofluoroalkenes. Angew. Chem. Int. Ed. 50, 11112–11116 (2011).
Ichitsuka, T., Fujita, T. & Ichikawa, J. Nickel-catalyzed allylic C(sp3)–F bond activation of trifluoromethyl groups via β-fluorine elimination: synthesis of difluoro-1,4-dienes. ACS Catal. 5, 5947–5950 (2015).
Liu, Y., Zhou, Y., Zhao, Y. & Qu, J. Synthesis of gem-difluoroallylboronates via FeCl2-catalyzed boration/β-fluorine elimination of trifluoromethyl alkenes. Org. Lett. 19, 946–949 (2017).
Xu, W., Jiang, H., Leng, J., Ong, H.-W. & Wu, J. Visible-light-induced selective defluoroborylation of polyfluoroarenes, gem-difluoroalkenes, and trifluoromethylalkenes. Angew. Chem. Int. Ed. 59, 4009–4016 (2020).
Poutrel, P., Pannecoucke, X., Jubault, P. & Poisson, T. Stereoselective synthesis of terminal monofluoroalkenes from trifluoromethylated alkenes. Org. Lett. 22, 4858–4863 (2020).
Liu, Z., Tu, X.-S., Guo, L.-T. & Wang, X.-C. Aluminum-catalyzed tunable halodefluorination of trifluoromethyl- and difluoroalkyl-substituted olefins. Chem. Sci. 11, 11548–11553 (2020).
Zhang, J., Yang, J.-D. & Cheng, J.-P. A nucleophilicity scale for the reactivity of diazaphospholenium hydrides: structural insights and synthetic applications. Angew. Chem. Int. Ed. 58, 5983–5987 (2019).
Reed, J. H. & Cramer, N. 1,3,2-diazaphospholenes catalyze the conjugate reduction of substituted acrylic acids. ChemCatChem 12, 4262–4266 (2020).
Speed, A. W. H. Applications of diazaphospholene hydrides in chemical catalysis. Chem. Soc. Rev. 49, 8335–8353 (2020).
Reed, J. H., Klett, J., Steven, C. & Cramer, N. Stay positive: catalysis with 1,3,2-diazaphospholenes. Org. Chem. Front. 7, 3521–3529 (2020).
Huchenski, B. S. N., Robertson, K. N. & Speed, A. W. H. Functionalization of bis-diazaphospholene P–P bonds with diverse electrophiles. Eur. J. Org. Chem. 2020, 5140–5144 (2020).
Gudat, D., Haghverdi, A., Hupfer, H. & Nieger, M. Stability and electrophilicity of phosphorus analogues of Arduengo carbenes—an experimental and computational study. Chem. Eur. J. 6, 3414–3425 (2000).
Fujita, T., Fuchibe, K. & Ichikawa, J. Transition-metal-mediated and -catalyzed C−F bond activation by fluorine elimination. Angew. Chem. Int. Ed. 58, 390–402 (2019).
Chong, C. C. & Kinjo, R. Hydrophosphination of CO2 and subsequent formate transfer in the 1,3,2-diazaphospholene-catalyzed N-formylation of amines. Angew. Chem. Int. Ed. 54, 12116–12120 (2015).
Chong, C. C., Hirao, H. & Kinjo, R. Metal-free σ-bond metathesis in 1,3,2-diazaphospholene-catalyzed hydroboration of carbonyl compounds. Angew. Chem. Int. Ed. 54, 190–194 (2015).
Hynes, T., Welsh, E. N., McDonald, R., Ferguson, M. J. & Speed, A. W. H. Pyridine hydroboration with a diazaphospholene precatalyst. Organometallics 37, 841–844 (2018).
Rao, B., Chong, C. C. & Kinjo, R. Metal-free regio- and chemoselective hydroboration of pyridines catalyzed by 1,3,2-diazaphosphenium triflate. J. Am. Chem. Soc. 140, 652–656 (2018).
Lucas, S. The pharmacology of indomethacin. Headache 56, 436–446 (2016).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Gudat, D., Haghverdi, A. & Nieger, M. Umpolung of P−H bonds. Angew. Chem. Int. Ed. 39, 3084–3086 (2000).
Burck, S., Gudat, D., Nieger, M. & Du Mont, W.-W. P-hydrogen-substituted 1,3,2-diazaphospholenes: molecular hydrides. J. Am. Chem. Soc. 128, 3946–3955 (2006).
Frisch, M. J. T. et al. Gaussian 09,Revision D.01, Gaussian, Inc., Wallingford, CT (2013).
Zhao, Y. & Truhlar, D. G. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 502, 1–13 (2011).
Acknowledgements
We are grateful for the financial support from National Natural Science Foundation of China (Nos. 21973052, 21933008, 91745101) and Tsinghua University Initiative Scientific Research Program (No. 20181080083).
Author information
Authors and Affiliations
Contributions
J.-P.C. and J.-D.Y. conceived and supervised the project. The synthetic experiments and characterizations were carried out by J.Z. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Alexander Speed and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Yang, JD. & Cheng, JP. Chemoselective catalytic hydrodefluorination of trifluoromethylalkenes towards mono-/gem-di-fluoroalkenes under metal-free conditions. Nat Commun 12, 2835 (2021). https://doi.org/10.1038/s41467-021-23101-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-23101-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.