Abstract
The light-harvesting-reaction center complex (LH1-RC) from the purple phototrophic bacterium Thiorhodovibrio strain 970 exhibits an LH1 absorption maximum at 960 nm, the most red-shifted absorption for any bacteriochlorophyll (BChl) a-containing species. Here we present a cryo-EM structure of the strain 970 LH1-RC complex at 2.82 Å resolution. The LH1 forms a closed ring structure composed of sixteen pairs of the αβ-polypeptides. Sixteen Ca ions are present in the LH1 C-terminal domain and are coordinated by residues from the αβ-polypeptides that are hydrogen-bonded to BChl a. The Ca2+-facilitated hydrogen-bonding network forms the structural basis of the unusual LH1 redshift. The structure also revealed the arrangement of multiple forms of α- and β-polypeptides in an individual LH1 ring. Such organization indicates a mechanism of interplay between the expression and assembly of the LH1 complex that is regulated through interactions with the RC subunits inside.
Similar content being viewed by others
Introduction
Photosynthetic organisms have evolved a diversity of light-harvesting strategies to absorb specific portions of the electromagnetic spectrum and thereby enable their growth in different habitats. Whereas oxygenic phototrophs synthesize chlorophylls as the major pigments that have long-wavelength absorption bands (Qy transition) in the relatively narrow range of 680–750 nm in vivo, the evolutionarily more ancient anoxygenic phototrophic bacteria produce a suite of bacteriochlorophylls (BChl) that extend their light absorption range from as low as 700 nm to beyond 1000 nm1,2,3. In purple phototrophic bacteria, the core light-harvesting complexes (LH1) usually display absorption maxima ~870 nm for BChl a-containing species and 1020 nm for the BChl b-containing species. However, among the BChl a-containing phototrophs, several species exhibit a red-shifted LH1-Qy to a range of 890–963 nm4,5,6,7. These organisms represent model systems for revealing the mechanism that phototrophic purple bacteria have evolved to avoid competition with other phototrophic species while at the same time avoiding spectral overlap with water molecules that absorb strongly near 980 nm.
The most thoroughly investigated LH1 complex showing a red-shifted Qy band is that from the thermophilic purple sulfur bacterium Thermochromatium (Tch.) tepidum, a phototroph isolated from a hot spring microbial mat in Yellowstone National Park (USA) and capable of growth up to 57 °C4. The Tch. tepidum LH1 complex, purified in a reaction center (RC)-associated form, revealed a Qy at 915 nm and enhanced thermostability compared with those from mesophilic counterparts8. Both the LH1-Qy redshift and increased thermostability of the Tch. tepidum LH1-RC are due to the binding of calcium ions to the LH1 polypeptides9,10,11. Crystal structures of the Tch. tepidum LH1-RC complex confirmed these findings and identified sixteen Ca-binding sites in the LH1 complex near the BChl a coordination sites12. Each Ca2+ is ligated by three residues from an α-polypeptide, one from a β-polypeptide, and two water molecules, forming a double-pyramid coordination structure13. As a consequence, the LH1-α(inner) and β(outer) rings form a tight network connected by Ca2+. This leads to a more rigid structure for the entire LH1 complex and highly restricts molecular motions around the BChl a-binding domain causing inhomogeneous narrowing in the spectroscopy14,15. Collectively, these structural features explain the observed LH1-Qy redshift and elevated thermostability of the Tch. tepidum LH1-RC complex.
Based on the work on Tch. tepidum LH1-RC, we have examined the LH1 complex from a spectrally unique mesophilic purple sulfur bacterium, Thiorhodovibrio (Trv.) strain 970; this phototroph shows an LH1-Qy at 960 nm (Supplementary Fig. 1), the most red-shifted Qy of all BChl a-possessing species6. As for the thermophilic Tch. tepidum, calcium ions were responsible for this ultra-redshift; removal of Ca2+ resulted in a blue-shift of the LH1-Qy to 875 nm, whereas the subsequent addition of Ca2+ restored 960-nm absorbance16. Although this process required Ca2+ and was freely reversible, the structural basis behind these Ca2+-induced spectral changes remained unsolved. In addition to the large redshift of the LH1-Qy, the special pair BChl a dimer in the RC of Trv. strain 970 also exhibits a red-shifted absorption band at 920 nm6, the longest wavelength reported for all BChl a-containing RCs (which typically range from 865 nm to 890 nm). Although “uphill” energy transfer is common in bacterial photosynthesis17, the energy gap (425 cm−1) between LH1 and RC in Trv. strain 970 is the largest among those reported for wild-type purple bacteria (130–350 cm−1) including the BChl b-containing species6. Despite the large uphill nature, a time constant of 65 ps was observed for the trapping of excitation energy from LH1 by the RC in Trv. strain 970, a value typical for other purple bacteria6. Elucidating the structural basis for this ultra-redshift of the special pair would reveal how RCs have evolved to coordinate absorbance with their LH1 partner in order to maintain an appropriate energy gap for energy transfer.
We present here the cryo-EM structure of the LH1-RC from Trv. strain 970. Our results provide the first structural insights into the mechanisms that underlie the unique absorbance properties of both the LH1 and the RC of this spectrally unusual purple bacterium, mechanisms that may have relevance for other purple bacteria with red-shifted LH-RC complexes as well. The highly detailed density map of the Trv. strain 970 LH1-RC complex obtained in our work is the first to unravel the structural arrangement of multiple forms of α- and β-polypeptides in the LH rings along with the multiple detergent-binding sites that exist in purified LH1 complexes.
Results
Structural overview
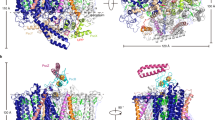
The cryo-EM structure of Trv. strain 970 LH1-RC was determined at 2.82 Å resolution (Supplementary Table 1 and Supplementary Figs. 2–4), and its overall structure draws both parallels and contrasts with that of the Tch. tepidum LH1-RC13. The LH1 subunits of strain 970 are uniformly distributed around the RC forming a closed, slightly elliptical double cylinder composed of 16 pairs of helical α(inner)β(outer)-polypeptides (αβ-dimer), 32 BChls a, 16 carotenoids (3,4,3′,4′-tetrahydrospirilloxanthin)16, and 16 Ca ions (Fig. 1 and Supplementary Figs. 5 and 6). The pigment and Ca2+ stoichiometry are consistent with those determined by biochemical analysis16. The long and short dimensions of the outer LH1 β-polypeptide ring are 114 Å and 107 Å (distances between the outer edges of opposite helices), respectively. The RC is accommodated in the LH1 ellipsoid and fits the shape of the inner LH1 α-ring with the L- and M-subunits in close proximity to the LH1 α2- and α4-polypeptides, respectively, in the transmembrane region (Fig. 1b, Supplementary Table 2, and Supplementary Fig. 5). Structures of the Trv. strain 970 RC subunits are nearly identical to those of corresponding subunits in the Tch. tepidum RC with smaller root-mean-square deviations (RMSD) for the integral membrane protein subunits (L and M) and larger RMSD for the membrane-associated protein subunits (C and H; Supplementary Fig. 7). Cofactors in the RC include four BChls a, two bacteriopheophytins (BPhe) a, one 15-cis-carotenoid, a menaquinone (MQ)-8 at the QA site, and a ubiquinone (UQ)-8 at the QB site.
a Side view of the LH1-RC parallel to the membrane plane with the periplasmic C-subunit above and the cytoplasmic H-subunit below. b Top view of the LH1-RC from the periplasmic side of the membrane. c Side view of the cofactor arrangement along the membrane plane with the periplasm above and the cytoplasm below. d Top view of the cofactor arrangement from the periplasmic side of the membrane. Hemes in the C-subunit are omitted for clarity. Color scheme: LH1 α, green; LH1 β, slate blue; L-subunit, magenta; M-subunit, blue; C-subunit, cyan; H-subunit, orange; Ca2+, gold ball; BChl a, red sticks; 3,4,3′,4′-tetrahydrospirilloxanthin, yellow sticks; DDM, black sticks. Phospholipids are omitted for clarity. Cofactors in L- and M-subunits of RC are colored in magenta and blue, respectively.
BChl a molecules in the strain 970 LH1 are aligned with the RC special pair (BChl aL and BChl aM) on the same plane that parallels the membrane surface (Fig. 1c). The bacteriochlorins of the special pair point approximately toward the middle of the long and short axes of the LH1 BChl a ring (Fig. 1d). The non-heme iron and the head groups of MQ and UQ in the RC are aligned along a line parallel to the long axis of the LH1 BChl a ring. The LH1 BChls a are ligated by histidine residues (α-His37 and β-His36, Supplementary Fig. 8) with markedly longer coordination lengths (Supplementary Table 3) compared with those of the light-harvesting complexes from other species. The 32 BChl a molecules in LH1 form an elliptical and partially overlapping ring-shaped arrangement with average Mg–Mg distances of 9.0 Å within a dimer and 8.8 Å between dimers (Fig. 1d). Carotenoids in strain 970 LH1 spanned the transmembrane region between the α- and β-polypeptides and showed no apparent association with the polypeptides or BChl a.
The Ca2+- and BChl a- binding sites
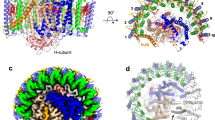
Of the 16 Ca ions present in the Trv. strain 970 LH1 C-terminal region between the inner and outer rings (Fig. 2a), each is coordinated by the main chain oxygen atoms of α-Trp47 and α-Ile52, the sidechain carboxyl group of α-Asp50, and the main chain oxygen atom of β-Trp45 in the adjacent subunit (Fig. 2b). Water molecules may also participate in the ligation, as occurs in the Tch. tepidum LH113, although their densities were not identified. All of the Ca2+-ligating residues are conserved in the expressed LH1 polypeptides (Fig. 2e and Supplementary Fig. 8). As a result, the Ca ions connect the α-inner and β-outer rings and therefore stabilize the entire LH1 structure. Because the Ca2+-binding network is close to the LH1 BChl a molecules, both static and dynamic properties of the coupled pigments are likely modified through the nearby Ca2+-bound α-Trp47 and β-Trp45 residues whose sidechains are hydrogen-bonded to the C3-acetyl oxygen atoms of BChls a (Fig. 2c, d). This Ca2+-binding effect has been demonstrated to contribute to the large redshifts of the LH1-Qy transitions in both Tch. tepidum9,14,18,19,20 and Trv. strain 97016.
a Top view of the LH1-RC from the periplasmic side of the membrane. Color scheme: LH1 α, green; LH1 β, slate blue; L- and M-subunits, gray; Ca2+, orange balls. b A typical Ca2+-binding site in LH1. Color scheme: LH1 α, green; LH1 β, light-blue; Ca2+, yellow ball. Density map is shown in magenta mesh around the Ca2+ at a contour level of 2.5σ. c Hydrogen bonding site around BChl a C3-acetyl group (light-blue sticks) in LH1 α-polypeptide (green). The Ca2+ (magenta ball) is ligated by the main chain oxygen atom of α-Trp47. d Hydrogen bonding site around BChl a C3-acetyl group (light-blue sticks) in LH1 β-polypeptide (cyan). The Ca2+ (magenta ball) is ligated by the main chain oxygen atom of β-Trp45. e Sequence scheme showing the relative positions of the Ca2+- and BChl a-binding sites. The LH1 αβ-polypeptides are aligned relative to the BChl a-coordinating histidine residues (magenta letters with vertical dashed line). Underlined region represents presumed membrane-spanning domain. All distances are in ångströms.
A unique feature of the Trv. strain 970 LH1 is that another histidine residue (His48) in the LH1 α-polypeptide, a residue absent from other purple bacterial LH1 α-polypeptides (Supplementary Fig. 8a)21, also participates in hydrogen bonding with the C3-acetyl group of BChls a (Fig. 2c) in addition to Trp47 that is conserved in all LH1 α-polypeptides. Furthermore, the His48 also shows π–π interaction with Trp47 (Fig. 2c). The additional hydrogen bond between His48 and BChl a, together with an interacting triangle formed with BChl a–Trp47–His48–BChl a, further strengthens interactions between the pigment and surrounding polypeptides and stabilizes the conformation of the BChl a molecules. This structure is in good agreement with resonance Raman results on the Trv. strain 970 LH1-RC complex16 that showed a markedly red-shifted C3-acetyl C=O stretching band and indicate even stronger hydrogen bonding for the carbonyl oxygen than occurs in Tch. tepidum LH1. This enhanced hydrogen bonding between BChl a and polypeptides likely contributes significantly to the ultra-redshift of the LH1-Qy transition observed in Trv. strain 970.
Arrangement of the LH1 multiple polypeptides
The sequence of the Trv. strain 970 genome (GenBank assembly accession: GCA_000228725.3) revealed five and four putative genes encoding the LH1 α- and β-polypeptides, respectively (Supplementary Fig. 8). Expressions of four forms of the α-polypeptides (α1–4) and two forms of the β-polypeptides (β1 and β4) were identified in the purified LH1-RC by TOF/MS and HPLC (Supplementary Fig. 9). These β-polypeptides correspond to the gene products of pufB1 and pufB2 reported previously21. All of the expressed LH1 polypeptides were assigned in the cryo-EM structure (Fig. 3a, b and Supplementary Fig. 5). Six copies of the α1- and eight copies of the α3-polypeptides form two groups and align on the two sides of the LH1 ellipse. The α1- and α3-polypeptides only differ in the C-terminal regions where the α3-polypeptide is four residues longer than the α1-polypeptide (Supplementary Fig. 8b). The α3-polypeptides have weak interactions with the RC C-subunit through their slightly longer C-terminal domains, whose solvent-exposed entity is largely tilted toward the α3-crescent.
a Top view of the LH1-RC from periplasmic side of the membrane. Color scheme: α1, green; α2, red; α3, orange; α4, magenta; β1, black; β4, blue. The RC L-, M-, and C-subunits are shown in gray cartoon. b Illustration of the arrangement of the LH1 multiple polypeptides bound with Ca ions. Same color scheme as in a. c Side view of the LH1 α2- and α4-polypeptides with RC L-, M-, and C-subunits shown by transparent orange, blue, and cyan cartoons, respectively (Note: the color schemes for L- and M-subunits are different from those in Fig. 1). The C-terminal domains of the two polypeptides marked by the dashed boxes are illustrated to show the interactions with surrounding proteins by dashed lines.
One α2- and one α4-polypeptide were identified in the cryo-EM structure (Fig. 3a, b and Supplementary Fig. 5b). These two polypeptides have longer C-terminal domains and a higher sequence similarity than other α pairs, except for α1/α3 (Supplementary Fig. 8c). The α2- and α4-polypeptides are located at unique positions between the α1- and α3-crescents and interact in the transmembrane domains with the RC L- and M-subunits, respectively, through sidechains of Arg residues (α2-Arg19, α2-Arg20, and α4-Arg20) that are specific for the α2- and α4-polypeptides. The solvent-exposed C-terminal domain of the α2-polypeptide forms a rectangular-shaped conformation on the periplasmic side of the membrane and is stabilized by interactions with surrounding RC proteins through the N- and C-terminal domains of the C-subunit and the C-terminal domain of the M-subunit (Fig. 3c and Supplementary Fig. 5b). The long C-terminal domain of the α4-polypeptide forms a large loop spanning the presumed periplasmic membrane surface and interacts with both RC C- and M-subunits (Fig. 3c and Supplementary Fig. 5b).
Three and thirteen copies of the LH1 β1- and β4-polypeptides, respectively, were assigned in the cryo-EM structure. The two β isomers have high sequence similarity with slight differences in the N-terminal domains. The three β1-polypeptides are located at triangle positions in the LH1 outer ring (Fig. 3a, b) with their N-terminal domains in the vicinities of the RC H- and M-subunits. This indicates that the subtle modifications in the N-terminal sequence of the β-polypeptides likely facilitate interactions with the RC subunits on the cytoplasmic membrane surface.
Structural features for the special pair of RC
In addition to the major redshift of the LH1 of Trv. strain 970, the special pair BChl a dimer in the RC of this phototroph displays a largely red-shifted absorption band at 920 nm6, the longest wavelength among all BChl a-containing RCs reported. The Trv. strain 970 RC structure from our study provides further insights into the structure-function relationships of this unusual feature. Most notably, the coordination lengths of the His residues to the special pair BChls a are markedly longer (Fig. 4a and Supplementary Table 3) than in the RCs of Tch. tepidum13, Rhodobacter (Rba.) sphaeroides22, and the BChl b-containing Blastochloris (Blc.) viridis23. This is likely due to relatively strong hydrogen bonds formed between the imidazole groups of the coordinating His residues and nearby main chain oxygen atoms.
a Top view of the BChl a dimer whose central Mg atoms are coordinated by the His residues in the L- and M-subunits. b Superposition of the residues in the RC L-subunits of Trv. strain 970 (magenta), Tch. tepidum (gray), and Rba. sphaeroides (gray) that form hydrogen bonds or interact with the BChl a molecule. c Superposition of the residues in the RC M-subunits of Trv. strain 970 (blue), Tch. tepidum (gray), and Rba. sphaeroides (gray) that form hydrogen bonds or interact with the BChl a molecule. All distances are in ångströms.
Key residues that participate in hydrogen bonding with the L-subunit-bound BChl aL, along with a partially overlapping Phe (L-Phe168), are all conserved in the L-subunits of the BChl a-containing Trv. strain 970, Tch. tepidum and Rba. sphaeroides (Fig. 4b and Supplementary Fig. 10). In contrast, differences exist in the residues interacting with the M-subunit-bound BChl aM. In Trv. strain 970, the M-Tyr197 forms a hydrogen bond with the BChl aM C3-acetyl group (Fig. 4c and Supplementary Fig. 10). This is also true of Tch. tepidum but differs from that of Rba. sphaeroides, where the corresponding residue is Phe, which does not form a hydrogen bond. The most striking two features are that (i) a partially BChl aM-overlapping Phe (M-Phe196) exists only in the Trv. strain 970, whereas it is a Leu in the BChl a-containing Tch. tepidum and Rba. sphaeroides, and (ii) a Ser residue (M-Ser280) forms an additional hydrogen bond with the BChl aM C132-ester group in strain 970, whereas the corresponding residue is Ala in Tch. tepidum and Gly in Rba. sphaeroides (Fig. 4c and Supplementary Fig. 10). It should be noted that a Phe in the M-subunit of the BChl b-containing Blc. viridis is also located at the same position of the M-Phe196 in Trv. strain 970. These residues have been demonstrated to affect the spectral behavior of the special pair, and the increased number of hydrogen bonds appears to contribute to the further redshifts of the Trv. strain 970 special pair absorption band (see Discussion section)24,25,26.
Detergents, lipids, and quinones
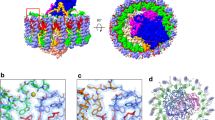
Ordered densities were identified on the periplasmic side between the Trv. strain 970 LH1 β-polypeptides and can be best fit with molecules of the detergent DDM (Fig. 5a). The head groups of the DDM lie on the presumed membrane surface and interact with LH1 αβ-polypeptides (Fig. 5b) leading to stabilization of both DDM and the LH1 ring. The alkyl chains of the DDM are inserted into the transmembrane regions. The putative DDM molecules are located at similar positions to those of the LH1 γ-polypeptides of Blc. viridis (Supplementary Fig. 11)23. Residual densities at the same positions were also observed in the Tch. tepidum LH1-RC structure and were tentatively assigned with unknown alkyl chains13.
a Tentatively assigned DDM molecules (green sticks) in the LH1 outer ring. b Expanded view of a putative DDM-binding site showing the interactions with the residues in LH1 α- (gray) and β- (blue) polypeptides. All distances are in ångströms. c Phospholipid distribution in LH1-RC viewed from the periplasmic side of the membrane with the special pair (sp) positioned at the center. CL: cyan; PG: magenta. The pigment and quinone (UQ and MQ) molecules in the RC (hemes in the C-subunit were omitted for clarity) and the LH1 polypeptides are colored in gray. d Side view of the phospholipid distribution along the presumed membrane planes. Same color scheme as in c. The LH1 complex was omitted for clarity.
31P-NMR measurement of the phospholipids extracted from purified Trv. strain 970 LH1-RC yielded approximately 47 phosphatidylglycerol (PG), 12 cardiolipin (CL), and 17 phosphatidylethanolamine (PE; Supplementary Fig. 12a). A total of 21 phospholipids (18 PG and 3 CL) were tentatively assigned in the cavities between the RC and LH1 based on the density map (Fig. 5c, d). The different head groups of PG and PE could not be distinguished at the current resolution. All of the CL molecules were oriented toward the cytoplasmic side of the membrane with their head groups aligned on the membrane surface, whereas the PG molecules were distributed on both cytoplasmic and periplasmic sides. In addition, a diacylglycerol is covalently bound to the N-terminus of the RC C-subunit. These lipid distribution features are similar to those observed in the Tch. tepidum LH1-RC13,27.
Biochemical analysis revealed approximately four UQ-8 and one MQ-8 in the purified Trv. strain 970 LH1-RC (Supplementary Fig. 12b). Only one UQ-8 could be assigned in the cryo-EM structure in addition to the UQ-8 at the QB site. The head group of the additional UQ-8 appears to interact with sidechains of the RC L-Gln88 and L-Thr191 in the transmembrane region. Channels exist between every adjacent pair of the LH1 αβ-polypeptides (Supplementary Fig. 13), as is also observed in the Tch. tepidum LH1-RC12,13. The channel openings nicely fit the size and shape of the ubiquinone head group, suggesting that these channels may serve as quinone paths. The putative ubiquinone channels seem to be mainly defined by the LH1 α-polypeptides because they form the narrowest portion of the openings with highly hydrophobic residues.
Discussion
Our structural determination of a Ca2+-bound LH1-RC from the purple sulfur phototrophic bacterium Trv. strain 970 here provides the second example of this particular arrangement, the first being that of Tch. tepidum12,13. However, the effect of Ca2+ on the redshift of the LH1-Qy transition is more dramatic in Trv. strain 970 than in Tch. tepidum16. Much of the insight gained from the thoroughly investigated Tch. tepidum LH1-RC9,10,11,14,15,18,20,28,29,30 has paved the way for understanding the structural basis of the spectroscopic behavior of the Trv. strain 970 LH1-RC because the Ca2+-binding sites are similar in the two complexes. Both static and dynamic properties on the pigment–pigment and pigment–protein interactions are known to influence the excitation energy of the BChl a molecules14,19,31,32,33. Among the many factors, hydrogen bonding between BChl a and its surrounding proteins has a direct effect on the lowest-energy transition, and one of the most effective methods for detecting the hydrogen bonds is resonance Raman spectroscopy. There is a linear relationship between the redshift of the Raman band in the BChl a C3-acetyl stretching mode and the redshift in the LH absorption maximum7,18,34,35. The redshift of the Raman band observed at 1621 cm−1 for the Trv. strain 970 LH1 is the largest among all LH1 complexes reported16, indicating that an increased number of hydrogen bonds are present. In the Trv. strain 970 LH1 structure, the unique His residue (α-His48) present in all its LH1 α-polypeptides participates in hydrogen bonding with the BChl a C3-acetyl group and has π–π interaction with the Ca2+-coordinating α-Trp47. This forms a Ca2+-stabilized hydrogen bonding network (Figs. 2c and 3b) that tightly locks the LH1 inner and outer rings and contributes to the ultra-redshift of the Trv. strain 970 LH1-Qy transition. The markedly elongate coordination lengths of the His–BChl a in LH1 (Supplementary Table 3) may also influence the BChl a site energy (see discussion below for the RC special pair).
However, hydrogen bonding with pigments can explain only part of the extra redshifts of the LH1-Qy transition as seen in the Tch. tepidum LH1-RC19 because structural disorder and the dynamic property of the BChl a-binding environment also need to be taken into account9,14,15,28,29,36. This is supported by the fact that the full C-terminal domains of all LH1 α-polypeptides can be traced from the current density map (2.82 Å resolution) observed for the Trv. strain 970 LH1-RC, even though the chain lengths of the LH1 α-polypeptides of this phototroph are much longer (11–24 residues) than those of Tch. tepidum (Supplementary Fig. 8a). In contrast, a number of the C-terminal residues of the Tch. tepidum LH1 α-polypeptides were invisible in the 1.9-Å resolution structure due to their weak densities13. This indicates that the Trv. strain 970 LH1 complex is structurally more homogeneous and less mobile than the Tch. tepidum LH114,30,36, especially as regards the C-terminal domains that are close to the BChl a-binding sites. By contrast, the Trv. strain 970 LH1 β-polypeptides form hydrogen bonds through their C-terminal carboxyl group with the β-Tyr42 in the adjacent chain, further strengthening the connectivity between the LH1 αβ-subunits. Overall, the combined features of relatively ordered structure and restricted molecular motion highlight the structural integrity of the Trv. strain 970 LH1 complex, and this is likely an important factor affecting the significant redshift of this phototroph’s LH1-Qy transition.
The structure of the Trv. strain 970 LH1-RC has revealed the organization of multiple forms of a polypeptide in a single LH1 ring. Chemical cross-linking experiments37 and single-molecule spectroscopy38 have demonstrated that peripheral light-harvesting (LH2) complexes have a heterogeneous polypeptide composition in the individual LH2 rings. However, it remains a challenge for structural biology, such as crystallography or NMR, to detect the structural arrangement within a stochastically mixed complex composed of multiple polypeptides with very similar amino acid sequences. In our study, all of the four LH1 α-polypeptides expressed in Trv. strain 970 were identified from the density map. They form the LH1 inner ring and are arranged in a highly characteristic way that can be understood in terms of their interactions with the RC subunits: the six α1-polypeptides with the shortest chain length are aligned on one side of the LH1 ellipse without apparent interaction with the RC; the eight α3-polypeptides are aligned on the other side with their slightly longer C-terminal domains interacting with the solvent-exposed portion of the tilted RC C-subunit; the single α2- and α4-polypeptides, each of which is located between the α1- and α3-crescents, interact with the RC L- and M-subunits in the transmembrane regions, and also interact with the RC C-subunit with their long C-terminal domains on the periplasmic side. These results suggest that a mechanism exists for expression and assembly of the LH1 complex that is regulated through interactions with the RC subunits inside the ring. It is unclear whether the results of our LH1 study are applicable to the LH2 complex that also contains multiple forms of αβ-polypeptides because LH2 complexes apparently lack any protein and pigment inside their ring structure.
The ultra Qy-redshift of the special pair BChl a dimer in the Trv. strain 970 RC may be explained by a combination of at least three structural features: (i) the elongate coordination lengths of the His residues to BChls a, (ii) an aromatic residue (Phe196) in the RC M-subunit that is in close proximity to BChl aM, and (iii) an additional hydrogen bond formed between the Ser280 in the RC M-subunit and the BChl aM. The relatively longer coordination lengths of the His–BChl a in the strain 970 RC special pair (Supplementary Table 3) indicate a reduced degree of electron donation from the His imidazole group to the BChl a macrocycles, leading to a relatively electron-deficient (more positively charged) state in the BChl a dimer. This likely contributes to its red-shifted absorption because it is known that the oxidized special pair dimer shows a largely red-shifted absorbance at 1250 nm26,39,40.
In the Trv. strain 970 RC, an aromatic residue (Phe196) in the M-subunit is in close proximity to BChl aM, and another aromatic residue is conserved at the symmetry-related position in the L-subunit (L-168, Fig. 4b); Phe for the BChl a-containing RCs and Trp for the BChl b-containing RC (Supplementary Fig. 10). The aromatic plane (L-Phe168) and the bacteriochlorin plane are partially overlapped with ~25° inclined toward each other, and therefore have π–π interaction. Mutation of the Phe to Leu in the Rba. sphaeroides RC L-subunit resulted in a 10-nm blue-shift of the Qy-band of the special pair along with an increased initial electron transfer time constant, decreased charge recombination time constant, and an increased redox potential of the RC25, indicating significant roles for this aromatic residue. Based on this result, a redshift of the special pair Qy-band would be expected by changing the Leu196 in the RC M-subunits of Tch. tepidum and Rba. sphaeroides to Phe as present in Trv. strain 970. The Phe196 aromatic plane of Trv. strain 970 is inclined by about 34° toward the bacteriochlorin plane (Fig. 4c), indicating a possible π–π interaction. It is of interest to point out that a Phe residue exists at the corresponding position with similar conformation in the M-subunit of the BChl b-containing Blc. viridis (Supplementary Fig. 10) whose special pair shows an absorption band at 955 nm41. It is unique for the Trv. strain 970 to have an additional hydrogen bond formed between the Ser280 in the RC M-subunit and the BChl aM C132-ester group (Fig. 4c). Introducing additional hydrogen bonds to the BChl a ring V of the special pair was demonstrated to result in a 10-nm redshift of the Rba. sphaeroides Qy-band at 20 K along with slower forward electron transfer rates, faster charge recombination rates, and increased redox potentials of the RC24. All of the structural features of the Trv. strain 970 RC reveal a more symmetrical configuration along the pseudo-C2 symmetry axis, a characteristic more similar to that of the Blc. viridis RC than the RCs of Tch. tepidum and Rba. sphaeroides in terms of how key interacting residues are arranged around the special pair of BChl molecules. These features contribute not only to the ultra-redshifted Qy-band of the Trv. strain 970 RC but also to its electron transfer rate, charge recombination rate, and RC redox potential.
In summary, our cryo-EM structure of the Trv. strain 970 LH1-RC provides a structural foundation for the unusual spectroscopic properties of this unique phototroph’s LH1 and RC complexes. The characteristic organization of the multiple forms of LH1 polypeptides in an individual LH1 ring reveals a mechanism for the expression and assembly of the LH1 complex that is strictly regulated through extensive interactions with the RC core inside the ring. Moreover, our study highlights the dramatic diversity of light-harvesting strategies in bacterial photosynthesis and a fine-tuned mechanism that has evolved for the sustainable growth of photosynthetic organisms in a changing environment.
Methods
Preparation and characterization of the LH1-RC complex
The Trv. strain 970 cells were cultivated phototrophically (anoxic/light) at 22 °C for 7 days under incandescent light (60 W). Preparation of the Trv. strain 970 LH1-RC followed the previous procedure16 with minor modifications. The chromatophores were suspended in a buffer containing 20 mM Tris-HCl (pH 8.5) and 10 mM CaCl2 and were treated with 0.7% n-dodecyl β-d-maltopyranoside (DDM) at a concentration of OD963 = 30 to extract the LH1-RC. The crude LH1-RC was loaded on a DEAE column (Toyopearl 650 S, TOSOH) equilibrated with 20 mM Tris-HCl (pH 7.5) and 0.05% DDM at 7 °C. LH1-RC components were eluted by a linear gradient of CaCl2 from 20 mM to 100 mM, and fractions with A959/A280 > 1.8 were collected for the subsequent measurements (Supplementary Fig. 1), then assessed by negative-stain EM using a JEM-1011 instrument (JEOL). Masses and composition of the LH1 polypeptides were measured by matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF/MS) and reversed-phase HPLC, respectively, using methods described elsewhere42. Phospholipid and quinone contents in the purified LH1-RC were analyzed (Supplementary Fig. 10) as previously describe27,43.
Cryo-EM data collection
Proteins for cryo-EM were concentrated to ~3 mg/ml. Three microliters of the protein solution were applied on a glow-discharged holey carbon grids (200 mesh Quantifoil R2/2 molybdenum), which had been treated with H2 and O2 mixtures in a Solarus plasma cleaner (Gatan, Pleasanton, USA) for 30 s and then blotted, and plunged into liquid ethane at −182 °C using an EM GP2 plunger (Leica, Microsystems, Vienna, Austria). The applied parameters were a blotting time of 6 s at 80% humidity and 4 °C. Data were collected at OIST on a Titan Krios (Thermo Fisher Scientific, Hillsboro, USA) electron microscope at 300 kV equipped with a Falcon 3 camera (Thermo Fisher Scientific). Movies were recorded using EPU software (Thermo Fisher Scientific) at a nominal magnification of 96 k in counting mode and a pixel size of 0.840 Å at the specimen level with a dose rate of 0.92 e- per physical pixel per second, corresponding to 1.3 e- per Å2 per second at the specimen level. The exposure time was 30.6 s, resulting in an accumulated dose of 40 e- per Å2. Each movie includes 40 fractioned frames.
Image processing
All of the stacked frames were subjected to motion correction with MotionCor244. Defocus was estimated using CTFFIND445. A total of 153,004 particles were selected from 1701 micrographs using the EMAN2 suite (Supplementary Figs. 2 and 3a)46. The initial 3-D model was generated with 15,721 particles from 55 selected micrographs with underfocus values ranging between 2 and 3 μm using EMAN2. The particles were further analyzed with RELION3.047, and 123,728 particles were selected by 2-D classification and divided into four classes by 3-D classification resulting in only one good class containing 106,529 particles. The 3-D auto refinement without any imposed symmetry (C1) produced a map at 2.96 Å resolution after contrast transfer function refinement, Bayesian polishing, masking, and post-processing. Then, 1609 micrographs were further chosen based on the number of particles, the estimated image resolution, and the quality of Thon ring fitting. The selected 105,234 particle projections were subjected to subtraction of the detergent micelle density followed by 3-D auto refinement to yield the final map with a resolution of 2.82 Å according to the gold-standard Fourier shell correlation using a criterion of 0.143 (Supplementary Fig. 3)48. The local resolution maps were calculated on RESMAP49.
Model building and refinement of the LH1-RC complex
The atomic model of the Tch. tepidum LH1-RC (PDB code 5Y5S) was fitted to the cryo-EM map obtained for the Trv. strain 970 LH1-RC using Chimera50. Amino acid substitutions and real space refinement for the peptides and cofactors were performed using COOT51. The C-terminal regions of the LH1 α-subunit were modeled ab-initio based on the density. The manually modified model was real space refined on PHENIX52, and the COOT/PHENIX refinement was iterated until the refinements converged. The final model was further refined using REFMAC5 in the CCP-EM suite53. Finally, the statistics calculated using MolProbity54 were checked. Figures were drawn with the Pymol Molecular Graphic System (Schrödinger)55 and UCSF Chimera50.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Map and model have been deposited in the EMDB and PDB with the accession codes: EMD-30314 and PDB-7C9R [https://doi.org/10.2210/pdb7C9R/pdb]. Other data are available from the corresponding authors upon reasonable request.
References
Croce, R. & van Amerongen, H. Natural strategies for photosynthetic light-harvesting. Nat. Chem. Biol. 10, 492–501 (2014).
Reimers, J. R. et al. Challenges facing an understanding of the nature of low-energy excited states in photosynthesis. Biochim. Biophys. Acta 1857, 1627–1640 (2016).
Mirkovic, T. et al. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 117, 249–293 (2017).
Madigan, M. T. A novel photosynthetic bacterium isolated from a Yellowstone hot spring. Science 225, 313–315 (1984).
Glaeser, J. & Overmann, J. Selective enrichment and characterization of Roseospirillum parvum, gen. nov. and sp. nov., a new purple nonsulfur bacterium with unusual light absorption properties. Arch. Microbiol. 171, 405–416 (1999).
Permentier, H. P., Neerken, S., Overmann, J. & Amesz, J. A bacteriochlorophyll a antenna complex from purple bacteria absorbing at 963 nm. Biochemistry 40, 5573–5578 (2001).
Kimura, Y. et al. Effects of calcium ions on the thermostability and spectroscopic properties of the LH1-RC complex from a new thermophilic purple bacterium Allochromatium tepidum. J. Phys. Chem. B 121, 5025–5032 (2017).
Suzuki, H. et al. Purification, characterization and crystallization of the core complex from thermophilic purple sulfur bacterium Thermochromatium tepidum. Biochim. Biophys. Acta 1767, 1057–1063 (2007).
Kimura, Y. et al. Calcium ions are involved in the unusual red shift of the light-harvesting 1 Qy transition of the core complex in thermophilic purple sulfur bacterium Thermochromatium tepidum. J. Biol. Chem. 283, 13867–13873 (2008).
Kimura, Y., Yu, L.-J., Hirano, Y., Suzuki, H. & Wang, Z.-Y. Calcium ions are required for the enhanced thermal stability of the light-harvesting-reaction center core complex from thermophilic purple sulfur bacterium Thermochromatium tepidum. J. Biol. Chem. 284, 93–99 (2009).
Yu, L.-J., Kato, S. & Wang, Z.-Y. Examination of the putative Ca2+-binding site in the light-harvesting complex 1 of thermophilic purple sulfur bacterium Thermochromatium tepidum. Photosynth. Res. 106, 215–220 (2010).
Niwa, S. et al. Structure of the LH1-RC complex from Thermochromatium tepidum at 3.0 Å. Nature 508, 228–232 (2014).
Yu, L.-J., Suga, M., Wang-Otomo, Z.-Y. & Shen, J.-R. Structure of photosynthetic LH1-RC supercomplex at 1.9 Å resolution. Nature 556, 209–213 (2018).
Ma, F., Yu, L.-J., Wang-Otomo, Z.-Y. & van Grondelle, R. The origin of the unusual Qy red shift in LH1-RC complexes from purple bacteria Thermochromatium tepidum as revealed by Stark absorption spectroscopy. Biochim. Biophys. Acta 1847, 1479–1486 (2015).
Ma, F., Yu, L.-J., Hendrikx, R., Wang-Otomo, Z.-Y. & van Grondelle, R. Direct observation of energy detrapping in LH1-RC complex by two-dimensional electronic spectroscopy. J. Am. Chem. Soc. 139, 591–594 (2017).
Imanishi, M. et al. A dual role for Ca2+ in expanding the spectral diversity and stability of light-harvesting 1 reaction center photocomplexes of purple phototrophic bacteria. Biochemistry 58, 2844–2852 (2019).
Tan, L.-M. et al. New insights into the mechanism of uphill excitation energy transfer from core antenna to reaction center in purple photosynthetic bacteria. J. Phys. Chem. Lett. 9, 3278–3284 (2018).
Kimura, Y. et al. Metal cations modulate the bacteriochlorophyll-protein interaction in the light-harvesting 1 core complex from Thermochromatium tepidum. Biochim. Biophys. Acta 1817, 1022–1029 (2012).
Yu, L.-J., Kawakami, T., Kimura, Y. & Wang-Otomo, Z.-Y. Structural basis for the unusual Qy red-shift and enhanced thermostability of the LH1 complex from Thermochromatium tepidum. Biochemistry 55, 6495–6504 (2016).
Kimura, Y. et al. Spectroscopic and thermodynamic characterization of the metal-binding sites in the LH1-RC complex from thermophilic photosynthetic bacterium Thermochromatium tepidum. J. Phys. Chem. B 120, 12466–12473 (2016).
Rücker, O., Köhler, A., Behammer, B., Sichau, K. & Overmann, J. Puf operon sequences and inferred structures of light-harvesting complexes of three closely related Chromatiaceae exhibiting different absorption characteristics. Arch. Microbiol. 194, 123–134 (2012).
Koepke, J. et al. pH Modulates the quinone position in the photosynthetic reaction center from Rhodobacter sphaeroides in the neutral and charge separated states. J. Mol. Biol. 371, 396–409 (2007).
Qian, P., Siebert, C. A., Wang, P., Canniffe, D. P. & Hunter, C. N. Cryo-EM structure of the Blastochloris viridis LH1-RC complex at 2.9 Å. Nature 556, 203–208 (2018).
Williams, J. C. et al. Effects of mutations near the bacteriochlorophylls in reaction centers from Rhodobacter sphaeroides. Biochemistry 31, 11029–11037 (1992).
Murchison, H. A. et al. Mutations designed to modify the environment of the primary electron donor of the reaction center from Rhodobacter sphaeroides: phenylalanine to leucine at L167 and histidine to phenylalanine at L168. Biochemistry 32, 3498–3505 (1993).
Mattioli, T. A., Lin, X., Allen, J. P. & Williams, J. C. Correlation between multiple hydrogen bonding and alteration of the oxidation potential of the bacteriochlorophyll dimer of reaction centers from Rhodobacter sphaeroides. Biochemistry 34, 6142–6152 (1995).
Nagatsuma, S. et al. Phospholipid distributions in purple phototrophic bacteria and LH1-RC core complexes. Biochim. Biophys. Acta Bioenerg. 1860, 461–468 (2019).
Jakob-Grun, S., Radeck, J. & Braun, P. Ca2+-binding reduces conformational flexibility of RC-LH1 core complex from thermophilic Thermochromatium tepidum. Photosynth. Res. 111, 139–147 (2012).
Ma, F., Yu, L.-J., Hendrikx, R., Wang-Otomo, Z.-Y. & van Grondelle, R. Excitonic and vibrational coherence in the excitation relaxation process of two LH1 complexes as revealed by two-dimensional electronic spectroscopy. J. Phys. Chem. Lett. 8, 2751–2756 (2017).
Ma, F. et al. Metal cations induced ab-BChl a heterogeneity in LH1 as revealed by temperature-dependent fluorescence splitting. ChemPhysChem 18, 2295–2301 (2017).
Cogdell, R. J., Howard, T. D., Isaac, N. W., McLuskey, K. & Gardiner, A. T. Structural factors which control the position of the Qy absorption band of bacteriochlorophyll a in purple bacterial antenna complexes. Photosynth. Res. 74, 135–141 (2002).
Cogdell, R. J., Gall, A. & Köhler, J. The architecture and function of the light-harvesting apparatus of purple bacterial: from single molecules to in vivo membranes. Quart. Rev. Biophys. 39, 227–324 (2006).
Robert, B. In The purple phototrophic bacteria (eds Hunter, C. N., Daldal, F. & Beatty, J. T.) 199–212 (Springer, 2009).
Sturgis, J. N. & Robert, B. Pigment binding-site and electronic properties in light-harvesting proteins of purple bacteria. J. Phys. Chem. 101, 7227–7231 (1997).
Uyeda, U., Williams, J. C., Roman, M., Mattioli, T. A. & Allen, J. P. The influence of hydrogen bonds on the electronic structure of light-harvesting complexes from photosynthetic bacteria. Biochemistry 49, 1146–1159 (2010).
Zerlauskiene, O. et al. Static and dynamic protein impact on electronic properties of light-harvesting complex LH2. J. Phys. Chem. B 112, 15883–15892 (2008).
Mascle-Allemand, C., Duquesne, K., Lebrun, R., Scheuring, S. & Sturgis, J. N. Antenna mixing in photosynthetic membranes from Phaeospirillum molischianum. Proc. Natl Acad. Sci. USA 107, 5357–5362 (2010).
Brotosudarmo, T. H. P. et al. Single-molecule spectroscopy reveals that individual low-light LH2 complexes from Rhodopseudomonas palustris 2.1.6. have a heterogeneous polypeptide composition. Biophys. J. 97, 1491–1500 (2009).
Dutton, P. L., Kaufmann, K. J., Chance, B. & Rentzepis, P. M. Picosecond kinetics of the 1250 nm band of the Rps. sphaeroides reaction center: the nature of the primary photochemical intermediary state. FEBS Lett. 60, 275–280 (1975).
Kobayashi, M. et al. Reconstitution and replacement of bacteriochlorophyll a molecules in photosynthetic reaction centers. J. Biochem. 136, 363–369 (2004).
Philipson, K. D. & Sauer, K. Comparative study of the circular dichroism spectra of reaction centers from several photosynthetic bacteria. Biochemistry 12, 535–539 (1973).
Sekine, F. et al. Gene sequencing and characterization of the light-harvesting complex 2 from thermophilic purple sulfur bacterium Thermochromatium tepidum. Photosynth. Res. 111, 9–18 (2012).
Kimura, Y. et al. Characterization of the quinones in purple sulfur bacterium Thermochromatium tepidum. FEBS Lett. 589, 1761–1765 (2015).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Nicholls, R. A., Tykac, M., Kovalevskiy, O. & Murshudov, G. N. Current approaches for the fitting and refinement of atomic models into cryo-EM maps using CCP-EM. Acta Crystallogr. D Struct. Biol. 74, 492–505 (2018).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
DeLano, W. L. The PyMOL Molecular Graphics System (DeLano Scientific, LCC, San Carlos, CA USA, 2004).
Acknowledgements
We thank Anika Methner and Franziska Klann (Leibniz Institute DSMZ) for providing excellent technical assistance. This research was partially supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Numbers JP20am0101118 (support number 1758) and JP20am0101116 (support number 1878), 17am0101116j0001, 18am0101116j0002, and 19am0101116j0003. R.K., M.H., and B.M.H. acknowledge the generous support of the Okinawa Institute of Science and Technology and the Japanese Cabinet Office. This work was supported in part by JSPS KAKENHI Grant Numbers JP16H04174, JP18H05153, JP20H05086, and JP20H02856, Takeda Science Foundation, and the Kurata Memorial Hitachi Science and Technology Foundation, Japan, and the National Key R&D Program of China (No. 2019YFA0904600).
Author information
Authors and Affiliations
Contributions
Z.-Y.W.-O. and K.T. designed the work, J.O. and M.T.M. provided materials and comments, K.T., R.K., Y.M., M.H., M.T., and M.I. performed the experiments, K.T., R.K., L.-J.Y., Y.K., A.M., B.M.H., and Z.-Y.W.-O. analyzed data, Z.-Y.W.-O. and K. T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Dmitry Semchonok and Rienk van Grondelle for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tani, K., Kanno, R., Makino, Y. et al. Cryo-EM structure of a Ca2+-bound photosynthetic LH1-RC complex containing multiple αβ-polypeptides. Nat Commun 11, 4955 (2020). https://doi.org/10.1038/s41467-020-18748-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-18748-3
This article is cited by
-
High-resolution structure and biochemical properties of the LH1–RC photocomplex from the model purple sulfur bacterium, Allochromatium vinosum
Communications Biology (2024)
-
Enhancing solar spectrum utilization in photosynthesis: exploring exciton and site energy shifts as key mechanisms
Scientific Reports (2023)
-
Atomic force microscopic analysis of the light-harvesting complex 2 from purple photosynthetic bacterium Thermochromatium tepidum
Photosynthesis Research (2023)
-
Structural basis for the assembly and quinone transport mechanisms of the dimeric photosynthetic RC–LH1 supercomplex
Nature Communications (2022)
-
An LH1–RC photocomplex from an extremophilic phototroph provides insight into origins of two photosynthesis proteins
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.