Abstract
The one-step electrochemical synthesis of H2O2 is an on-site method that reduces dependence on the energy-intensive anthraquinone process. Oxidized carbon materials have proven to be promising catalysts due to their low cost and facile synthetic procedures. However, the nature of the active sites is still controversial, and direct experimental evidence is presently lacking. Here, we activate a carbon material with dangling edge sites and then decorate them with targeted functional groups. We show that quinone-enriched samples exhibit high selectivity and activity with a H2O2 yield ratio of up to 97.8 % at 0.75 V vs. RHE. Using density functional theory calculations, we identify the activity trends of different possible quinone functional groups in the edge and basal plane of the carbon nanostructure and determine the most active motif. Our findings provide guidelines for designing carbon-based catalysts, which have simultaneous high selectivity and activity for H2O2 synthesis.
Similar content being viewed by others
Introduction
Hydrogen peroxide is widely used as a green oxidant in disinfectants, bleaching agents, sanitizing agents, chemical synthesis, and even as a potential energy carrier1,2,3,4,5,6,7,8,9,10,11,12,13,14. According to a report by Global Industry Analysts, Inc.15, the global consumption of H2O2 is projected to reach 6.0 million metric tons by 2024. Approximately 99% of all H2O2 is currently produced using the multi-step anthraquinone process. However, this process is energy intensive, and can only be performed in a centralized factory1,5,10. The inherent complexity of the anthraquinone process has driven many researchers to investigate one-step methods, which can continuously produce H2O2 on-site at small scale using a simple device1,5. Among these, the electrochemical synthesis approach is one of the best choices to meet the above demands.
Metal-free oxidized carbon materials have received particular attention for this application because they offer a simple synthetic procedure, cost-effectiveness, as well as high activity and selectivity16,17,18,19,20,21,22,23,24,25,26,27,28,29. They allow the incorporation of a variety of oxygen functional groups, with the potential to widely tune performance, and to optimize active sites. For example, Lu et al. recently used nitric acid to oxidize carbon nanotubes and demonstrated that the resulting oxidized carbon-material was highly active for oxygen reduction to hydrogen peroxide (ORHP)16. They considered the active sites to be the carbon atoms adjacent to carboxylic acid and etheric groups (–COOH and C–O–C). Kim et al. used few-layer mildly reduced graphene oxide electrodes, by partially removing oxygen from graphene oxide using hydrothermal heating without a reducing agent, and showed that sp2-hybridized carbon near ring ethers along the sheet edges were the most active sites for ORHP17.
All of these pioneering studies apply harsh oxidation conditions to cleave the strong sp2 C–C bonds16,30 and incorporate oxygen functional groups, and this in turn makes it difficult to functionalize the carbon materials with targeted groups. As a result, the oxygen functional groups inevitably saturate the dangling bonds on the edges with sophisticated multi-components, which hinders systematic experimental study. It becomes inherently difficult to accurately identify the most active functional groups, because the resolution of ordinary characterization methods is not high enough to distinguish between similar groups.
From the industrial anthraquinone process, we know that quinones are the champion catalytically active oxygen functional groups towards H2O2 synthesis10,31. Herein we demonstrate a facile synthesis method to incorporate quinone functional groups in carbon nanostructures. For comparison, we also employ a pre-activated method to build carbonyl-enriched and etheric ring-enriched graphitic nanoplatelets (denoted as GNPC=O and GNPC–O–C, respectively). Using fine characterization methods, such as soft XANES, XPS, FTIR, and CV, we show that each of our samples have targeted and desired functional groups (etheric ring, carboxyl and quinone). Electrochemical measurements reveal that the sample with abundant quinone functional group (GNPC=O,1) exhibits high selectivity, with a H2O2 yield ratio of 97.8% at 0.75 V, which is superior to previously reported etheric ring and carboxylic acid groups.
We further demonstrate the activity of the quinone functional groups by examining standalone quinone molecular systems. Finally, we use density functional theory (DFT) calculations to model different possible quinone functional groups and examine their activity toward ORHP. We find that the quinone groups exhibit very high activity with negligible overpotential.
Results
Synthesis and structure characterization
We adopted a two-step method to prepare edge-oxygenated graphitic nanoplatelets. The free edge sites were first created by simultaneously crushing and exfoliating graphite mechanochemically. Then, the reactive edge sites were saturated with mild oxidants of CO2 or diluted O2. We found that when CO2 was applied as an oxidant, carbonyl-related groups were easily formed. In this case, the as-prepared product is designated GNPC=O. Even dilute O2 could also be selected as an oxidant. Because the electronic ground state of O2 and carbon are different, the triplet O2 cannot react with singlet defect-free graphitic carbon spontaneously, due to the energy barrier present. This allows selective oxidation of the reactive edge sites with a high degree of controllability. Etheric ring (C–O–C)-functionalized graphitic nanoplatelets (GNPC–O–C) were obtained in dilute O2 atmosphere.
We first characterized the morphology of the above samples using field emission scanning electron microscopy (FESEM, Supplementary Fig. 1) and transmission electron microscopy (TEM, Supplementary Fig. 2). This analysis showed that GNPC=O and GNPC–O–C had morphologies typical of graphitic nanoplatelets and nanoparticles, respectively. The successful nanosizing and functionalization were further verified by Raman spectra and X-ray powder diffraction (XRD) patterns. As shown in Supplementary Fig. 3, the strong D band (usually as an indicator of defective edges) and broad (002) facets (derived according to the Scherrer equation), together indicate the existence of plenty of edge sites in the nanosized GNPs. The Brunauer–Emmett–Teller (BET, Supplementary Fig. 4) analysis determined that the specific surface areas of GNPC=O,1, GNPC=O,2, and GNPC–O–C, are 450, 753, and 657 m2 g−1, respectively.
Next, we measured sample oxygen content using three different methods, elemental analysis (EA), energy dispersive spectroscopy (EDS), and XPS. The results (summarized in Supplementary Table 1 and Figs. 5–7) agreed with each other well. Here, we highlight the EA results because it has higher accuracy than the other two methods. The amount of oxygen content was calculated to be 10.5 at% for GNPC=O,1, 20.6 at% for GNPC=O,2, and 10.0 at% for GNPC–O–C. A possible iron remnant was checked by time-of-flight secondary ion mass spectrometry (TOF-SIMS), which has a detection limit as low as ppm. The results (Supplementary Fig. 6) showed that there was no detectable iron in the samples.
Finally, to carefully unravel the nature of the oxygen functional groups we used a combination of techniques, including soft XANES, XPS, FTIR, and CV. Among them, soft XANES is one of the most powerful tools for characterizing graphitic materials, and can provide important information about bonding configurations with high resolution32,33,34.
As shown in Fig. 1a, the C K-edge includes unoccupied π* (peak A–C) and excited σ* (peak D, E) states. Here, we only discuss the fingerprint region of π* in detail. The peak A around 285.4 eV is assigned to 1s – π* from sp2 C=C32,33,34,35,36. The peak B results from the charge transfer induced by the O in the etheric ring. The GNPC–O–C and partially reduced graphene oxide (pRGO, Supplementary Methods) exhibit a minor shoulder at 287.2 eV (Peak B1), which corresponds to the out-of-plane etheric ring (C–O–C)32,34,35. Since the in-plane C–O bonding of C–O–C is much stronger than the out-of-plane, the peak at 288.2 eV is supposed to be in-plane C–O–C at the edge of the GNP. Thus, the GNPC–O–C is mainly composed of in-plane C–O–C, and partly by out-of-plane C–O–C. In pRGO there is only out-of-plane C–O–C. The peak C around 288.6 eV is attributed to π*(C=O)32,35, which should be determined in combination with other characterizations.
a C K-edge. A, 1s – π* from sp2 C=C; B, 1s – π* from the etheric ring, B1, the out-of-plane C–O-C, B2, the in-plane C–O-C; C, 1s – π* from ketone or carboxylic acid; D, 1s – σ* from sp2 C=C; D, 1s – σ* from C=O. b O K-edge. A, 1s – π* from C=O, A1, organic carbonate, A2, quinone, A3, ketone or carboxylic acid; B, the 1s – π* excitations due to the charge transfer between C and O, including C=O and C–O; C, 1s – σ* from C–O, C1, etheric ring (C–O–C), C2, the C–O in COOH; C, 1s – σ* from C=O. The soft XANES were collected in the total electron yield (TEY) mode.
The soft O K-edge XANES (Fig. 2b) provides further information about the groups. Here, the pRGO shows an exclusive A1 peak (530.1 eV), which is assigned to the π*(C=O) of the organic carbonate. The peak A2 (531.0 eV) is assigned to quinone contributions33. The results of the O K-edge demonstrate that the GNPC=O,1 is enriched with quinone. The presence of a peak A2 shoulder indicates the GNP C=O,2 and pRGO also contain less quinone groups. Peak A3 (531.8 eV) may be composed of ketones or/and carboxylic acid32,33,34. Fortunately, peak A3 can be identified by peak C2 (539.6 eV), which originates from the σ*(C–O) in carboxylic acid (COOH)32,33,34,36. Peaks A3 and C2 are dominant in GNPC=O,2 and pRGO, which indicates that they are mainly composed of COOH.
a Fourier transform infrared (FTIR) spectra. b The high-resolution O 1s of the X-ray photoelectron spectra (XPS). OI, 531.35 ± 0.05 eV, C=O, quinone, or ketone; OII, 532.8 eV, C–O-C, or COOH; OIII, 534.0 eV, C–O(H); OIV, 535.6 eV, adsorbed H2O and O2. c The cyclic voltammetry. The CV curves were measured in Ar-saturated 0.5 M H2SO4 at a scan rate of 50 mV s−1. The quinone redox reaction were recorded in the potential window from 0.3 to 0.8 V.
We can draw the conclusion that the quinone was easily formed in the short reaction time (GNPC=O,1). However, carboxylic acid was the main group after the prolonged reaction time (GNPC=O,2). The contribution of π* peak B is complicated. It originates from the resonance transition of quinone, or the charge transfer due to the influence of the neighboring O in the etheric ring33. The GNPC–O–C gives rise to a σ* resonance at 536.3 eV (peak C1), which is attributed to C–O–C34. Here, we noticed that GNPC–O–C contains peak A3, and a small shoulder of π*(C=O) in the C K-edge, which indicates that some carbonyl-related groups are present in the GNPC–O–C.
FTIR is another powerful tool for characterizing functional groups, and was employed here. As shown in Fig. 2a, all of the spectra exhibit a broad band at around 3400 cm−1, which is mainly assigned to the stretching vibration (υ OH) of the adsorbed moisture in KBr. Another common band, located at 1610 cm−1, is attributed to the asymmetric stretching of sp2-hybridized C–C bonds (υ C=C). Its shifting and intensity are intimately related to the oxygenated groups37,38.
The fingerprint region, ranging from 1750 to 1000 cm−1, was scrutinized to identify the functional groups present. The well-documented pRGO was analyzed first. Consistent with other reports38,39,40,41,42, the functional groups in the pRGO were assigned to ketones (υ C=O, the shoulder at 1654 cm−1), carboxylic acid (υ C=O at 1715 cm−1, δ OH at 1402 cm−1, υ C–OH at 1214 cm−1), organic carbonate (υ C=O at 1715 cm−1, δ OH at 1384 cm−1), ethers (υ C–O at 1084 cm−1), and aromatic hydrocarbons (δ C–H at 740 cm−1)38,39,40,41,42.
Both GNPC=O,1 and GNPC=O,2 produced a typical fingerprint band of carboxylic acid (υ C=O in COOH, 1716 cm−1). The COOH is formed via hydrolysis in acid43,44. GNPC=O,1 and GNPC=O,2 also displayed a peak and a shoulder, respectively, at 1632 cm−1, which was thought to be the quinone based on the XANES results. The GNPC–O–C displayed a broad band from 1380 to1050 cm−1, which was attributed to the asymmetric C–O-C stretching vibration (in-plane υ C–O–C) of the etheric rings. This is caused by an unusual absorption mechanism40.
The XPS was then performed (Supplementary Fig. 7). It is known that that the C 1s typically exhibits a pronounced asymmetric tail at higher binding energy45, making it difficult to obtain accurate deconvolution results, especially considering the controversial assignment. Here, we only focused on the O 1s spectroscopy.
The high-resolution O 1s are shown in Fig. 2b. After careful deconvolution with the same standard rules, four distinct regions were identified. To easily distinguish them, we labelled them OI (531.35 ± 0.05 eV, C=O related groups)46,47, OII (532.8 eV, C–O–C or COOH)46,47, OIII (534.0 eV, C–O(H))46, and OIV (535.6 eV, physically adsorbed H2O and O2)41,42. Because the different groups overlap, it was difficult to determine the contents of the groups by O 1s alone. However, we can still draw some useful information. All samples had a very small OIII region, which indicates that very little phenolic -OH is present. Based on the XANES and FTIR results, it was determined that the OII regions in GNPC=O,1, GNPC=O,2, and pRGO mainly result from COOH. However, the OII region in GNPC-O-C originates from C–O-C. GNPC=O,1 had a higher percentage of OI relative to GNPC=O,2 due to the higher content of quinone. The GNPC-O-C exhibited the smallest OI region. In contrast, the pRGO exhibited the highest OI region. However, the ketone and quinone in the OI region still could not be distinguished.
Fortunately, quinone is sensitive to cyclic voltammetry (CV). Since the quinone redox couple is the only reaction that can be detected by CV, we used CV to characterize the content of quinone in the samples (Supplementary Fig. 8)10,31. As shown in Fig. 2c, the quinone content was 4.2%, 7.4%, 0.0%, and 2.3% for GNPC=O,1, GNPC=O,2, GNPC–O–C, and pRGO, respectively. The absence of quinone redox indicates that the OI region in GNPC–O–C is attributed to the presence of ketone. The reduced quinone redox content in pRGO demonstrates that most of the OI region is composed of COOH. The functional groups of the samples are summarized in Supplementary Table 2.
Oxygen reduction to hydrogen peroxide
The oxygen reduction to hydrogen peroxide (ORHP) performance of the synthesized samples with different oxygen groups was evaluated using a rotating ring-disk electrode (RRDE). Since H2O2 can upshift the potential of the reference electrode because of its strong oxidizing nature (Supplementary Fig. 9), a salt bridge must be used to separate the electrochemical cell and reference electrode (Supplementary Fig. 10). For a fair comparison of activity, all of the tested samples were tuned to the same capacitance by changing the volume of drop-cast ink. We used a reversible hydrogen electrode (RHE, Supplementary Fig. 11) for all our analyses.
The polarization curves were measured in O2-saturated 0.1 M aq. KOH solution at a scan rate of 1600 rpm. To eliminate the contributions of capacitance, we averaged the current of the forward and backward scans. The H2O2 current (JH2O2) was collected at the ring electrode (JR) with an applied potential of 1.15 V, and the collection efficiency was 37% (Supplementary Fig. 12). The four-electron byproducts of H2O were calculated using the relationship: JH2O = JD – JR.
As shown in Fig. 3a and Supplementary Table 3, the carbonyl-enriched samples, GNPC=O,1 (0.826 V), GNPC=O,2 (0.815 V), and pRGO (0.810 V), exhibited a higher onset potential than GNPC–O–C (0.805 V). Here, the onset potential is defined as the potential measured at a current density of 0.15 mA cm−2 (5% of the theoretical limiting current) for ORHP. The result indicates that the sample with the in-plane etheric ring had lower activity than the carbonyl-enriched samples.
a The polarization curves of H2O2 current (JR or JH2O2), disk current (JD), and H2O current (JH2O). The curves were measured in O2-saturated 0.1 M KOH solution at a scan rate of 10 mV s−1 by RRDE with a rotation speed of 1600 rpm. The applied potential of the ring was 1.15 V. The current was the average of the forward and backward scans. b The corresponding Tafel plot of kinetic current of H2O2 (JK,H2O2). c The JK,H2O2 comparison at 0.65 and 0.75 V. d The corresponding H2O2 yield ratio. e Stability tests in four fresh 0.1 M KOH electrolytes, which were measured in a H-type cell, with 400 ppm MgSO4 as stabilizer to suppress the decomposition of H2O2. The applied potential was 0.65 V.
It is noteworthy that all of the onset potentials were even higher than the thermodynamic equilibrium potential (O2 + H2O + 2 e ⇌ HO2− + OH−, 0.75 V vs RHE). This counterintuitive phenomenon has been widely reported16,48. Bao and his colleagues attributed this to a Nernst-related potential shift due to the low concentration of H2O2 in the electrolyte, and/or a possible pH-related change48. Although H2O2 can upshift the potential of the reference electrode, its influence can be easily ruled out by employing a salt bridge and refreshing the electrolyte for every measurement. This abnormal phenomenon did not exist with a planar electrode, which further confirms that the potential shifting is unrelated to the yield of H2O2. We suggest this abnormal phenomenon only results from the localized pH-related change in the mesoporous electrode due to the constraint of mass transport.
The kinetic current of H2O2 (JK,H2O2) was calculated according to the Koutecky-Levich equation: 1∕JH2O2 = 1∕JK,H2O2 + 1∕JL,H2O2, where JK,H2O2 is the measured current of the H2O2 yield, JL,H2O2 is the theoretical limiting current of ORHP23,49. JL,H2O2 was obtained from the Levich equation and determined to be 2.9 mA cm−2. The calculated results are shown in the Tafel plot in Fig. 3b. GNPC=O,1, and GNPC=O,2 showed the smallest Tafel slope (48 mV dec−1), which was superior to the C–O–C-enriched GNPC–O–C (55 mV dec−1). The same Tafel slope indicates similar active sites. Although the pRGO was also carbonyl-enriched, its Tafel slope was only 63 mV dec−1. This is possibly due to the considerable N content, which is inevitably introduced during the preparation of pRGO. The doped N is known to enhance the yield of 4e product H2O50.
The JK,H2O2 at 0.65 and 0.75 V were selected to compare activity (Fig. 3c). We note that GNPC=O,1 had the highest activity (25.1 mA cm−2) at 0.65 V. However, the most active sample was GNPC=O,2 (3.6 mA cm−2) at a higher potential of 0.75 V. This phenomenon is caused by the samples′ different selectivity (the H2O2 yield ratio, Fig. 3d), which plays a major role in the low potential region. The GNPC=O,1 exhibited higher selectivity (the H2O2 yield ratio, 97.8% at 0.75 V) with an electron transfer number of nearly 2 (Supplementary Fig. 13), which is the highest ratio reported so far (Supplementary Table 3)16,18,19,21,22,25,26,27,28,29,48. The pRGO exhibited the poorest JK,H2O2 (1.2 mA cm−2 at 0.75 V) and the lowest H2O2 yield ratio (83.4% at 0.75 V) because of the presence of graphitic N (Supplementary Fig. 14), which is reported to be the active sites for 4e ORR50.
The H2O2 yield ratio of COOH-enriched GNPC=O,2 decreased relative to the quinone-enriched GNPC=O,1. The possible reason is that COOH has a negative impact on selectivity. The H2O2 yield ratio of all of the carbonyl-enriched samples decreased as we lowered the potential. This may have occurred due to the reduction of quinone to hydroquinone at low potential. Distinctively, the GNPC–O–C displayed a tendency opposite to the others, due to the absence of quinone.
The ORHP performance was also evaluated in neutral (0.05 M Na2SO4) and acidic (0.1 M HClO4) media. The results are shown in Supplementary Fig. 15. Both results in neutral and acidic media demonstrated that the quinone-enriched sample (GNPC=O,1) had the highest selectivities, which were 95% (0.6 V in neutral) and 85% (0.2 V in acid), respectively. In the neutral medium, the onset potential is the same with the thermodynamic equilibrium potential (0.70 V), which indicates the absence of potential shift, which is caused by the localized pH-related change. In the acidic medium, the activity toward ORHP is poor. The result agrees well with the previous reports16,17,18.
Besides selectivity and activity, stability is also one of the three indispensable factors for catalysts, and it affects their economical durability. The samples′ stability was measured in an H-type cell, which was separated by a Nafion 115 membrane (Supplementary Fig. 16). It is known that the alkaline H2O2 solution is unstable, and spontaneous decomposition will occur1,51,52. Approximately 30% of H2O2 was decomposed during a 30 h stability test (Supplementary Fig. 17). Therefore, MgSO4, a popular stabilizer in alkaline solution, was selected to suppress the decomposition51.
As shown in Fig. 3e, the current exhibited a slow decline as the time increased. The possible reason is that the increasing H2O2 concentration enhances electrolyte viscosity. The increasingly sluggish diffusion of O2 in the H2O2 solution subsequently deteriorates the ORHP current53. This speculation was further verified by the observation that there was no current drop after changing to a fresh electrolyte. Even after 120 h, there was no evident decline, which demonstrates that the catalyst has good stability.
The concentration of generated H2O2 was determined using the KMnO4 titration method (Supplementary Movie 1)28. With an applied potential of 0.65 V in 90 mL electrolyte, a typical concentration of H2O2 after 30 h reaction is about 6.1 mM, with a Faraday efficiency11,23 of 95%.
Determination of active sites
So far, we have shown that the carbonyl-enriched groups possess higher activity than the etheric ring groups. However, the nature of the active carbonyl groups remains elusive. To elaborate the nature of the active carbonyl groups, we designed a special experiment.
We tuned the content of quinone and carboxylic acid by leaching the sample of GNPC=O,2 in an acidified concentrated H2O2 solution. The leached sample was labelled GNPC=O,3. As shown in Fig. 4a, FTIR determined that the carboxylic acid content evidently increased after leaching. However, the quinone content was reduced from 7.4% to 4.5% (Fig. 4b). This change in groups was also recorded by XPS (Supplementary Fig. 18). The active sites can be easily identified by comparing the ORHP performance of GNPC=O,2 and GNPC=O,3.
a FTIR. The COOH is increased. b Cyclic voltammetry, which was measured in Ar-saturated 0.5 M H2SO4 at a scan rate of 50 mV s−1. The quinone is decreased. c The polarization curves of JH2O2, JD, and JH2O. The curves were measured in O2-saturated 0.1 M KOH solution at a scan rate of 10 mV s−1 by RRDE with a rotation speed of 1600 rpm. The current was the average of the forward and backward scan. d The JK,H2O2 as a function of quinone content.
The polarization curves are shown in Fig. 4c. The GNPC=O,3 had both a lower onset potential of 14 mV and JK,H2O2 (3.1 mA cm−2, Supplementary Fig. 19) than GNPC=O,2. The relation of JK,H2O2 and quinone content was further checked, and the results are shown in Fig. 4d. JK,H2O2 increased as the quinone content increased. Because of the influence of other groups, it did not follow a linear relation. These results confirmed that the active sites were quinone.
To further verify these results, we investigated several standalone molecules with quinone, carboxylic acid, and etheric ring groups, such as phenanthrenequinone, anthraquinone, naphthalenetetracarboxylic dianhydride, perylenetetracarboxylic dianhydride, dibenzodioxin, and dibenzofuran. The polarization curves are shown in Fig. 5. Except for phenanthrenequinone and anthraquinone, the other four molecules did not show activity towards ORHP; the activity was inferior to blank glass carbon (GC). The phenanthrenequinone was superior to anthraquinone in both the JK,H2O2 (0.7 vs 0.5 mA cm−2 at 0.65 V) and Tafel slope results (45 vs 48 mV dec−1, Supplementary Fig. 20). These molecular chemistry results further confirm that the quinones are the active sites.
Phenanthrenequinone, anthraquinone, naphthalenetetracarboxylic dianhydride, perylenetetracarboxylic dianhydride, dibenzodioxin, dibenzofuran, and blank glass carbon (GC) were compared. The curves were measured in O2-saturated 0.1 M KOH solution at a scan rate of 10 mV s−1 by RRDE with a rotation speed of 1600 rpm. The applied potential of the ring was 1.15 V. The current was the average of the forward and backward scans.
Theoretical investigation
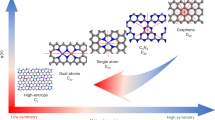
To gain atomistic insights about the nature of the active quinone motifs, we next used density functional theory (DFT) calculations. We examined a variety of model structures (Fig. 6a) to study the different possible quinone groups on the edge and basal planes. These model structures were used to model the ORHP reaction pathway (Eqs. 1 and 2)5,16:
where the O2 molecule adsorbs at the carbon surface and is reduced through the first proton-electron transfer to form OOH* (Eq. 1). The second electron transfer results in the formation of HO2–, which is desorbed from the surface (Eq. 2). The key intermediate OOH* plays a pivotal role in the ORHP. Its adsorption in Eq. 1 and desorption in Eq. 2 jointly determines the activity, according to the Sabatier principle1,16. The adsorption energy of OOH* (ΔGOOH*) is therefore the best descriptor to capture the trends in activity for different oxygen functional groups1,16.
a The atomic structures of the examined oxygen functional groups. Color code: carbon, gray; oxygen, red; hydrogen, white. The corresponding examined active sites are marked with a dashed green circle in each model structure. b Theoretical ORHP activity volcano plot. Horizontal dashed line corresponds to the thermodynamic equilibrium potential for ORHP (U0 = 0.70 V). The activity of alloys and edge COOH are adapted from refs. 1,16, respectively.
We also calculated the barrier of proton transfer to the oxygen atom of adsorbed OOH* to be zero. This means that there is a close connection between the thermodynamic and kinetic formulations for two-electron oxygen reduction reaction. Therefore, we only focus on thermodynamic analysis, which has played an essential role in providing insights on the nature of active sites and guiding the design and optimization of various catalysts.
In line with previous reports1,16, we used the calculated limiting potential (UL) as the indicator of activity towards ORHP, which is defined as the maximum potential at which the above two reaction steps are downhill in free energy. The ORHP results in this work on quinone functional groups are summarized along with the previous report of oxygen functional groups by Lu, et al.16 in an activity volcano plot in Fig. 6b. The vertex of the activity volcano corresponds to the thermodynamic equilibrium potential (U0 = 0.70 V) for the ORHP. In theory, an ideal catalyst should have a ΔGOOH* of 4.22 (±0.1) eV, which provides the highest activity. Based on this analysis, while the quinone functional groups on the edge (Q-edge 5) are comparable to the reported catalysts for ORHP1,16, the Q-basal 2-2 displayed the highest activity. Of note, the formation of quinone functional groups on the edge seems more feasible than in the basal area, because the formation of Q-Basal groups significantly interrupts the sp2 network and requires a lot of energy input. However, the edge-located structures of Q-Edge 4 and 5 are easily formed, because the sp2 C–C bond breaking is lower. Thus, the Q-Edges are the most likely sites.
Furthermore, a different mechanism similar to industrial anthraquinone process was considered. The results of the anthraquinone mechanism shown in Supplementary Figs. 21 and 22 reveals that the formation of anthrahydroquinone (AHQ) is uphill by 3.64 eV. In addition, the next step, which is transferring proton from of AHQ to O2 molecule and forming radical OOH, is even more exergonic around 4.93 eV. Both of them are far beyond the energy capacity for the two-electron ORR. Therefore, the anthraquinone mechanism is not a possible competitive pathway for our catalyst system.
Discussion
In summary, we adopted a pre-activated method to decorate the dangled edges of graphitic materials with targeted groups (ether, carboxyl and quinone). The functional groups were then characterized by a combination of soft XANES, XPS, FTIR and CV. Our results confirmed a new class of quinone-edged groups, which exhibited higher selectivity than previously reported oxygenated groups with similar onset potential. The quinone-enriched samples (GNPC=O,1) exhibited a H2O2 yield ratio of 97.8% at 0.75 V. The results were further verified using standalone molecular chemistry and theoretical analysis. These findings will be beneficial for understanding active sites in ORHP, and will be a guide to designing high ORHP catalysts.
Methods
Preparation of graphitic nanoplatelets
The pre-activated method was used for the preparation of the graphitic nanoplatelets (GNP). We first cleaved graphite using the mechanochemical method. The graphite crushing and exfoliation into nanosized particles were accomplished at the same time. The freshly broken edges are free and reactive. Then, the activated edges were reacted with target molecules, such as CO2 and O2. The groups were edge-enriched with CO2 and O2, and the resulting as-prepared GNPs were designated as GNPC=O and GNPC–O–C, respectively.
GNPC=O,1: the mechanochemical ball-milling method was employed to activate and saturate the graphite at the same time. The preparation was conducted on a planetary ball-milling device (Pulverisette 6, Fritsch GmbH) in a rotation speed of 500 rotation per minutes (rpm). In brief, graphite (15 g, Alfa Aesar, 100 mesh, 99.9995%, product number: 14735), dry ice (100 g, Hanyu Chemical Inc.), and hardened steel balls with a diameter of 3 mm (500 g) were placed into a ball-mill container (250 mL). Then, the air in the container was completely pumped out by five repeated argon (Ar) charging/discharging cycles for 15 min and ball-milled under reduced pressure for 15 h. Finally, the as-prepared GNPC=O was leached in 1 M aq. H2SO4 solution for 24 h to completely remove possible contamination from unbound Fe debris, followed by rinsing with ultra-pure water (18.2 MΩ cm, Direct-Q® 3UV, Millipore Corporation) more than 6 times and freeze-drying in tert-butyl alcohol. Finally, the samples were further dried in vacuum oven at 80 °C for 10 h.
GNPC=O,2: the amount of carbonyl-related group loading was controlled by varying the graphite loading amounts and ball-milling conditions. GNPC=O,2 was prepared with more carbonyl-related groups by ball-milling graphite (10 g) and dry ice (100 g) with hardened steel balls (500 g, Φ = 5 mm) for 40 h.
GNPC=O,3: the different contents of carbonyl-related groups was obtained by H2O2 leaching in acid. The leaching process was conducted by immersing GNPC=O,2 in 20 mL 3.5 M aq. H2O2 and 1.0 M acetic acid mixture for 12 h. Here, acetic acid acted as a stabilizer to suppress the spontaneous decomposition of H2O2. Finally, the samples were further dried in a vacuum oven at 80 °C for 10 h before characterizations.
GNPC-O-C: the mechanochemical ball-milling method was first applied for activation. The experiment procedures were conducted in a planetary ball-milling device (Pulverisette 6, Fritsch GmbH) at a rotation speed of 500 rpm with the protection of 5 bar Ar (UHP, 99.999%, N50, KOSEM, Korea). In brief, graphite (15 g) and hardened steel balls (500 g, Φ = 3 mm) were charged in a ball-mill container (250 mL). Then, the container was filled with argon gas (5 bar), after five purging cycles with the aid of a vacuum pump to remove residual air.
After cooling to room temperature, the container was filled with an O2/Ar mixture (10 vol%) for 6 h at a flow rate of 250 standard cubic centimeters per minute (sccm). SAFETY NOTE: The concentration of O2 in the gas mixture should be lower than the burn-off point to avoid fire, which can be caused by violent oxidation.
Since the dangling edges activated by unzipping the graphitic framework tend to reconstruct spontaneously to reduce their surface energy, the cleavage process was exponentially reduced as the ball-milling was prolonged. Gas oxidation in the O2/Ar mixture was divided into 7 periods. The ball-milling time for each period was 20 min, 20 min. 20 min, 30 min, 30 min, 60 min, and 120 min, respectively. The total ball-milling time was 5 h.
Finally, to completely remove unbound Fe debris, the as-prepared GNPC–O–C was leached in 1 M aq. H2SO4 solution for 24 h, followed by rinsing with ultra-pure water more than six times and freeze-drying in tert-butyl alcohol. Finally, the samples were further dried in a vacuum oven at 80 °C for 10 h.
Structural characterization
The microstructures were characterized on a JEM-2100 transmission electron microscope (TEM) at an accelerating voltage of 200 kV, and by field emission scanning electron microscopy (FESEM, Nova NanoSEM, FEI), equipped with energy dispersive spectroscopy (EDS, EDAX, AMETEK). X-Ray diffraction (XRD) patterns were recorded on a D/max2500V (Rigaku, Japan) using Cu-Kα radiation (40 kV, 100 mA, λ = 1.5418 Å) in a 2θ range of 3°–60° at a scan rate of 4° min−1. The specific surface area was analyzed on a Micromeritics ASAP 2504 N by nitrogen adsorption-desorption isotherms using the Brunauer-Emmett-Teller (BET) method. The pore distributions were calculated by the non-local density functional theory (NLDFT) method.
Fourier transform infrared spectra (FTIR) were collected on a Perkin-Elmer Spectrum 100 with a resolution of ~1 cm−1, and the samples were tableted with KBr as support. The Raman spectra were characterized on a WITec Alpha300R with a laser wavelength of 532 nm.
The elemental analysis (EA) was conducted using a Flash 2000 CHNS/O Analyzers (Thermo Scientific). All samples were measured at least three times. The element of iron was detected by time-of-flight secondary ion mass spectrometry (TOF-SIMS, TOF.SIMS5, IONTOF GmbH, Germany) with a resolution of ppm). The primary ion species was Bi with a dose of 2.0 × 109, and the raster area was about 400 × 400 µm2. The X-ray photoelectron spectra (XPS) was recorded on a Thermo Fisher XPS spectrometer (K-alpha), which employed monochromatic Al Ka radiation as the X-ray source.
The soft X-ray absorption near edge structure (XANES) experiments were performed at the BL12B-A beamline in the National Synchrotron Radiation Laboratory (NSRL), University of Science and Technology of China (USTC), Hefei, P. R. China.
Electrochemical measurements
The electrochemical measurements were conducted in a three-electrode electrochemical cell on a workstation of CompactStat (Ivium Technologies B.V., Netherlands). A graphite rod (Alfa Aesar, Ultra purity, 99.9995 %) and an Ag/AgCl electrode were selected as the counter electrode and reference electrode, respectively. As-prepared GNPC=O,1, GNPC=O,2, GNPC=O,3, GNPC-O-C, and pRGO inks were drop-cast on glassy carbon (GC, 0.247 cm2) supports to prepare working electrodes.
To obtain reliable and reproducible measurements, the cleanness of the GC supports is particularly important. Before each measurement, the cleanness of blank GC was first checked by scanning CV at a scan rate of 50 mV s−1. The current curves should only exhibit the shape of capacitance with a current density less than an order of magnitude below 10−7 A. If it was not, the GC support was polished with alumina (0.05 μm), and then ultrasonically cleaned in ethanol and ultra-pure water.
Computational method
We used the Atomic Simulation Environment (ASE)54 to handle the simulation and the QUANTUM ESPRESSO55 program package to perform electronic structure calculations. The electronic wavefunctions were expanded in plane waves up to a cutoff energy of 500 eV, while the electron density is represented on a grid with an energy cutoff of 5000 eV. Core electrons were approximated using ultrasoft pseudopotentials56. To describe chemisorption properties on graphene structures, we used the PBE exchange-correlation functional with dispersion correction57. Graphene structures were modeled as one layer with a vacuum of 20 Å to decouple the periodic replicas. To model the quinone functional groups in the basal plane, we use a 5 × 5 super cell lateral size, and the Brillouin zone was sampled with (4 × 4 × 1) Monkhorst-Pack k-points. For the oxygen functional groups in the edge, we used a super cell with a lateral size 5 × 6 and the Brillouin zone was sampled with a (1 × 4 × 1) Monkhorst-Pack k-points.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013).
Liu, C. et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 11, 1098–1104 (2016).
Hooe, S. L. & Machan, C. W. Dioxygen reduction to hydrogen peroxide by a molecular Mn complex: mechanistic divergence between homogeneous and heterogeneous reductants. J. Am. Chem. Soc. 141, 4379–4387 (2019).
Sun, Y. et al. Activity–selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal–nitrogen–carbon catalysts. J. Am. Chem. Soc. 141, 12372–12381 (2019).
Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442–458 (2019).
Shen, R. et al. High-concentration single atomic Pt sites on hollow CuSx for selective O2 reduction to H2O2 in acid solution. Chem 5, 2099–2110 (2019).
Freakley, S. J. et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 351, 965–968 (2016).
Melchionna, M., Fornasiero, P. & Prato, M. The rise of hydrogen peroxide as the main product by metal‐free catalysis in oxygen reductions. Adv. Mater. 31, 1802920 (2019).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Campos-Martin, J. M., Blanco-Brieva, G. & Fierro, J. L. G. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 45, 6962–6984 (2006).
Yang, S. et al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS Catal. 8, 4064–4081 (2018).
Zheng, Z., Ng, Y. H., Wang, D.-W. & Amal, R. Epitaxial growth of Au-Pt-Ni nanorods for direct high selectivity H2O2 production. Adv. Mater. 28, 9949–9955 (2016).
Shiraishi, Y. et al. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 18, 985–993 (2019).
Melchionna, M. & Fornasiero, P. High Pt single-atom density for high-rate generation of H2O2. Chem 5, 1927–1928 (2019).
Hydrogen peroxide - market analysis, trends, and forecasts. (2018).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Kim, H. W. et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 1, 282–290 (2018).
Sa, Y. J., Kim, J. H. & Joo, S. H. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. 58, 1100–1105 (2019).
Chen, Z. et al. Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2. React. Chem. Eng. 2, 239–245 (2017).
Čolić, V., Yang, S., Révay, Z., Stephens, I. E. L. & Chorkendorff, I. Carbon catalysts for electrochemical hydrogen peroxide production in acidic media. Electrochim. Acta 272, 192–202 (2018).
Li, B., Zhao, C., Liu, J. & Zhang, Q. Electrosynthesis of hydrogen peroxide synergistically catalyzed by atomic Co–Nx–C sites and oxygen functional groups in noble‐metal‐free electrocatalysts. Adv. Mater. 31, 1808173 (2019).
Choi, C. H. et al. Unraveling the nature of sites active toward hydrogen peroxide reduction in Fe-N-C. catalysts. Angew. Chem. Int. Ed. 56, 8809–8812 (2017).
Fellinger, T. P., Hasché, F., Strasser, P. & Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 134, 4072–4075 (2012).
Byers, J. C., Güell, A. G. & Unwin, P. R. Nanoscale electrocatalysis: visualizing oxygen reduction at pristine, kinked, and oxidized sites on individual carbon nanotubes. J. Am. Chem. Soc. 136, 11252–11255 (2014).
Sun, Y. et al. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts. ACS Catal. 8, 2844–2856 (2018).
Han, L. et al. In-plane carbon lattice-defect regulating electrochemical oxygen reduction to hydrogen peroxide production over nitrogen-doped graphene. ACS Catal. 9, 1283–1288 (2019).
Chen, S. et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustain. Chem. Eng. 6, 311–317 (2018).
Iglesias, D. et al. N-doped graphitized carbon nanohorns as a forefront electrocatalyst in highly selective O2 reduction to H2O2. Chem 4, 106–123 (2018).
Thiyagarajan, N. et al. A carbon electrode functionalized by a tricopper cluster complex: overcoming overpotential and production of hydrogen peroxide in the oxygen reduction reaction. Angew. Chem. Int. Ed. 57, 3612–3616 (2018).
Yan, H. et al. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity. Nat. Commun. 9, 3197 (2018).
Yang, H.-H. & McCreery, R. L. Elucidation of the mechanism of dioxygen reduction on metal-free carbon electrodes. J. Electrochem. Soc. 147, 3420 (2000).
Jeong, H.-K. et al. X-ray absorption spectroscopy of graphite oxide. EPL 82, 67004 (2008).
Hopkins, R. J., Tivanski, A. V., Marten, B. D. & Gilles, M. K. Chemical bonding and structure of black carbon reference materials and individual carbonaceous atmospheric aerosols. J. Aerosol Sci. 38, 573–591 (2007).
Ganguly, A., Sharma, S., Papakonstantinou, P. & Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C. 115, 17009–17019 (2011).
Gao, Y. et al. Nitrogen-doped sp2-hybridized carbon as a superior catalyst for selective oxidation. Angew. Chem. Int. Ed. 52, 2109–2113 (2013).
Banerjee, S. et al. Ozonized single-walled carbon nanotubes investigated using NEXAFS spectroscopy. Chem. Commun. 772–773 (2004).
Fuente, E., Menéndez, J. A., Díez, M. A., Suárez, D. & Montes-Morán, M. A. Infrared spectroscopy of carbon materials: a quantum chemical study of model compounds. J. Phys. Chem. B 107, 6350–6359 (2003).
Acik, M. et al. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C. 115, 19761–19781 (2011).
Szabó, T. et al. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 18, 2740–2749 (2006).
Acik, M. et al. Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 9, 840–845 (2010).
Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007).
Bagri, A. et al. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2, 581–587 (2010).
Boehm, H.-P. Surface chemical characterization of carbons from adsorption studies. in Adsorption by Carbons 301–327 (Elsevier, 2008).
Jeon, I.-Y. et al. Edge-carboxylated graphene nanosheets via ball milling. Proc. Natl Acad. Sci. USA 109, 5588–93 (2012).
Desimoni, E., Casella, G. I., Morone, A. & Salvi, A. M. XPS determination of oxygen-containing functional groups on carbon-fibre surfaces and the cleaning of these surfaces. Surf. Interface Anal. 15, 627–634 (1990).
Lin, Y. et al. Electrocatalytic water oxidation at quinone-on-carbon: a model system study. J. Am. Chem. Soc. 140, 14717–14724 (2018).
Beamson, G. & Briggs, D. High resolution XPS of organic polymers: the scienta ESCA300 database. (John Wiley & Sons, Ltd., 1992).
Chen, S. et al. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide. J. Am. Chem. Soc. 140, 7851–7859 (2018).
Jirkovský, J. S. et al. Single stom hot-spots at Au–Pd nanoalloys for electrocatalytic H2O2 production. J. Am. Chem. Soc. 133, 19432–19441 (2011).
Jia, Y. et al. Identification of active sites for acidic oxygen reduction on carbon catalysts with and without nitrogen doping. Nat. Catal. 2, 688–695 (2019).
Wuorimaa, A., Jokela, R. & Aksela, R. Recent developments in the stabilization of hydrogen peroxide bleaching of pulps: an overview. Nord. Pulp Pap. Res. J. 21, 435–443 (2006).
Verdaguer-Casadevall, A. et al. Trends in the electrochemical synthesis of H2O2: Enhancing activity and selectivity by electrocatalytic site engineering. Nano Lett. 14, 1603–1608 (2014).
Ruiz-Ibanez, G., Bidarian, A., Davis, R. A. & Sandall, O. C. Solubility and diffusivity of oxygen and chlorine in aqueous hydrogen peroxide solutions. J. Chem. Eng. Data 36, 459–466 (1991).
Bahn, S. R. & Jacobsen, K. W. An object-oriented scripting interface to a legacy electronic structure code. Comput. Sci. Eng. 4, 56–66 (2002).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Ahmed Adllan, A. & Dal Corso, A. Ultrasoft pseudopotentials and projector augmented-wave data sets: application to diatomic molecules. J. Phys. Condens. Matter 23, 425501 (2011).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
This work was supported by the Creative Research Initiative (CRI, 2014R1A3A2069102), BK21 Plus (10Z20130011057), and Science Research Center (SRC, 2016R1A5A1009405) programs through the National Research Foundation (NRF) of Korea. S.S. acknowledges the support from the University of Calgary′s Canada First Research Excellence Fund Program, the Global Research Initiative in Sustainable Low Carbon Unconventional Resources.
Author information
Authors and Affiliations
Contributions
J-B.B. conceived the project and oversaw all the research phases. J-B.B. and G-F.H. designed the project. G-F.H. synthesized and characterized the samples. F.L., Z.F., Y.L., and W.Z. measured the soft XANES. S.S. and M.K. conducted the theoretical calculations. S-W.K. and Y.B. built the two-electrode device. S-K.K. and J-P.J. performed BET measurements. Data collection and analysis were conducted by J-B.B., S.S., and G-F.H. All the authors contributed to and commented on this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Gilberto Maia and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, GF., Li, F., Zou, W. et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat Commun 11, 2209 (2020). https://doi.org/10.1038/s41467-020-15782-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-15782-z
This article is cited by
-
Mesoporous carbon spheres with programmable interiors as efficient nanoreactors for H2O2 electrosynthesis
Nature Communications (2024)
-
Regulating oxygenated groups and carbon defects of carbon-based catalysts for electrochemical oxygen reduction to H2O2 by a mild and self-recycled modification strategy
Carbon Research (2024)
-
Recent Advances in Mechanistic Understanding of Metal-Free Carbon Thermocatalysis and Electrocatalysis with Model Molecules
Nano-Micro Letters (2024)
-
Electrosynthesis of chlorine from seawater-like solution through single-atom catalysts
Nature Communications (2023)
-
A polymeric hydrogel electrocatalyst for direct water oxidation
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.