Abstract
The ability to grow at moderate acidic conditions (pH 4.0–5.0) is important to Escherichia coli colonization of the host’s intestine. Several regulatory systems are known to control acid resistance in E. coli, enabling the bacteria to survive under acidic conditions without growth. Here, we characterize an acid-tolerance response (ATR) system and its regulatory circuit, required for E. coli exponential growth at pH 4.2. A two-component system CpxRA directly senses acidification through protonation of CpxA periplasmic histidine residues, and upregulates the fabA and fabB genes, leading to increased production of unsaturated fatty acids. Changes in lipid composition decrease membrane fluidity, F0F1-ATPase activity, and improve intracellular pH homeostasis. The ATR system is important for E. coli survival in the mouse intestine and for production of higher level of 3-hydroxypropionate during fermentation. Furthermore, this ATR system appears to be conserved in other Gram-negative bacteria.
Similar content being viewed by others
Introduction
Enteric bacteria such as Escherichia coli (E. coli) and Salmonella can colonize and cause disease in the human intestinal tract. They have to combat acidic environments during the whole process of invading the host. With pH values as low as 1.5–2.5, the stomach has been recognized as a natural antibiotic barrier1. With their passage into the small intestine, E. coli cells will encounter a less acidic environment (pH 4.0–6.0) with the presence of organic acids produced by the normal intestinal flora2.
E. coli has developed variable acidic stress response systems, including the acid resistance (AR) systems response to extreme acid stress and the acid tolerance response (ATR) system towards mild and moderate acid stress3,4. Up to now, five AR systems, AR1−AR5, are reported. The AR1 system is activated by alternative σ factor (RpoS) and cAMP receptor protein (CRP)5,6. Due to the involvement of CRP, the AR1 system is repressed by glucose. The AR2−AR5 systems are all dependent on a specific extracellular amino acid, and consist of an antiporter as well as a decarboxylase enzyme that is usually induced by low pH and extracellular amino acid3,7, except that AR2 can be induced at acidic pH in the absence of glutamate8. They confer acid resistance by consumption of intracellular protons in amino acid decarboxylation reaction to produce a less acidic internal pH, using glutamate, arginine, lysine and ornithine as their corresponding substrates, respectively1,5,9,10,11,12. All five AR systems can protect stationary phase cells from the extreme acidity and prolong survival, while only AR2 and AR3 were reported to function during the exponential phase5,13.
Among AR systems, AR2 is by far the most effective and the most complex. The glutamate decarboxylase isoforms, GadA and GadB, and the glutamate/γ-aminobutyric acid antiporter GadC are key components of AR2, and their regulation relies on the action of over 20 proteins and 3 small noncoding RNAs, including two-component systems EvgAS and PhoPQ; regulatory proteins RpoS, GadE, RcsB, GadX, GadW and HNS; protease ClpXP and Lon; and small RNAs DsrA, GadY and GcvB, which together form a regulatory network with high level of complexity (for a review, see refs. 3,7). The periplasmic chaperons HdeAB and their cytoplasmic counterpart Hsp31, which assist the refolding of denatured proteins during the acid stress7,14,15, are also induced as part of the AR2 regulon16,17.
The ATR system, though poorly understood, is induced by exposing E. coli cells to moderate acid stress (pH 4.5–5.8), and will protect cells from a subsequent challenge of extreme acid pH (pH 2.0–3.0)4,6. ATR can be activated during adaptation at mild acidic pH by the regulators Fur and PhoPQ in exponential phase cells and by RpoS and OmpR in stationary phase cells, but the stationary phase cells are much more tolerant to acid than the log phase cells3,4.
Benefited from the complicated AR and ATR systems, E. coli can survive without growth for several hours at pH 2.01,18,19,20, and the acid limit for growth of E. coli is pH 4.0 in rich medium, or pH 4.5 in minimal medium6,18,20,21,22. So, E. coli will experience the transition of pH from no-growth to growth conditions when passing through the stomach and entering the intestine. It is exceptionally important to elucidate how E. coli adapts to and grows at pH 4.0–5.0, because the capability of bacteria to outgrow hundreds of competing species in gut microbiome in this lower range of growth pH will determine which strain can colonize the gut18. Unfortunately, we still barely know that.
In this study, we challenged the exponentially growing cells of E. coli at pH 4.2, and characterized a regulatory circuit required for bacterial growth under moderate acidic conditions through modulation of the membrane lipid composition. The two-component system CpxRA directly senses acidification through protonation of the CpxA periplasmic histidine residues, and thus activates transcription of the essential genes fabA and fabB in biosynthesis of unsaturated fatty acids (UFAs) to enhance the UFAs content in membrane lipid. This mechanism enables E. coli to grow at acidic pH, and also functions in diverse bacterial species.
Results
UFAs are required for E. coli growth under acidic pH
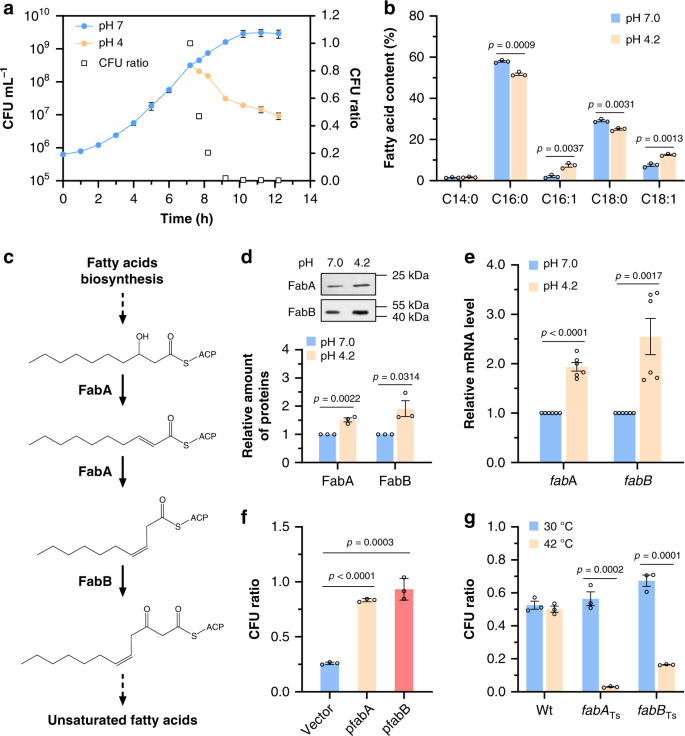
We carried out a screening to characterize an E. coli ATR system required for bacterial survival in exponential growth. At first, the overnight culture of E. coli BW25113 wild-type strain was directly inoculated into minimal medium E at pH 4.2 using glucose as sole carbon source without supplement of any amino acid. However, the cells were rapidly killed, even preadapted at pH 5.0 (Supplementary Fig. 1). Then the BW25113 strain was grown in medium E at pH 7.0 to a cell density of ≈3 × 108 CFU mL−1, and the cells were collected, washed and transferred into the same medium at pH 7.0 and 4.2, respectively. While the cells at pH 7.0 grew normally, the cell density at pH 4.2 decreased continuously, which led to reduction of the ratio of CFU at pH 4.2 vs. pH 7.0 to 0.21 ± 0.02 after 1 h of exposure at different pH (Fig. 1a). This result confirmed that exponentially growing E. coli was susceptible to this moderate acidic condition.
a Growth of E. coli BW25113 strain at different pH. The strain was grown to 3 × 108 CFU mL−1 in minimal medium at pH 7.0, and transferred into the same medium at pH 7.0 and pH 4.2 (n = 3 biologically independent samples). CFU ratio = CFU at pH 4.2/CFU at pH 7.0. b Membrane lipid composition of BW25113 strain after acidic challenge at pH 4.2 for 1 h. The compositions were determined by GC-MS, and fatty acid content is given as the relative peak area [(peak area of one fatty acid/total peak area) × 100%] (n = 3 biologically independent samples). c Biosynthetic pathway of unsaturated fatty acids, in which FabA and FabB, 3-hydroxyacyl-ACP dehydratase/isomerase and β-ketoacyl-ACP synthase play essential roles. d FabA-His6 and FabB-His6 protein level of BW25113 strain after 1 h of exposure to pH 7.0 and 4.2 determined by western blot. The relative amount of each protein was determined using ImageJ (n = 3 biologically independent samples). e Relative mRNA level of fabA and fabB in BW25113 strain after 1 h of exposure to pH 7.0 and 4.2 determined by qRT-PCR (n = 2 biologically independent samples with three technical repeats). f Tolerance of BW25113 strain carrying empty vector pTrcHis2B, pfabA or pfabB, respectively, after acidic challenge at pH 4.2 for 1 h (n = 3 biologically independent samples). g Tolerance of BW25113 strain, temperature-sensitive FabA and FabB mutants after acidic challenge at pH 4.2 at 30 or 42 °C for 0.5 h (n = 3 biologically independent samples). Error bars, mean ± standard error of mean (SEM). Two-tailed Student’s t tests were performed to determine the statistical significance for two group comparisons. The source data are provided as a Source Data file.
In previous studies, survival was used to measure the bacterial acid resistance, which is calculated by the formula, survival (%) = (CFU after acid challenge/CFU before acid challenge) × 100%. However, survival was determined under conditions E. coli can only survive without growth, and is not suitable here as we are trying to figure out how E. coli grows at moderate acidic pH. So, the CFU ratio (pH 4.2/pH 7.0) is calculated to represent the acid tolerance of exponentially growing E. coli because growth of E. coli at pH 7.0 is steady. A value of CFU ratio close to 1 indicates that E. coli grows at pH 4.2 to a cell density similar to that at pH 7.0.
Phospholipids were extracted from exponential phase cells after exposure of 1 h to pH 7.0 and 4.2, and analyzed as previously described23. The results showed that exposure to such acidic condition caused a change of membrane lipid composition in E. coli cells (Fig. 1b). Specifically, levels of the UFAs, including palmitoleic acid (C16:1) and oleic acid (C18:1), were elevated by 3.83- and 1.66-fold; meanwhile, the levels of palmitic acid (C16:0) and stearic acid (C18:0) were reduced. These five fatty acids shown in Fig. 1b represented more than 96% of total fatty acids in E. coli cells, consistent with those results reported previously24,25. As a consequence, the ratio of unsaturated to saturated fatty acids increased from 0.11 at pH 7.0 to 0.32 at pH 4.2. Comparable to this result in E. coli, a shift in the unsaturated/saturated ratio in response to acid stress was also observed in Streptococcus mutants26,27.
We also found that the expression of two essential genes required for UFAs biosynthesis, fabA and fabB (Fig. 1c), was significantly upregulated under the acidic condition since their protein and mRNA levels were elevated (Fig. 1d, e). A previous study showed that overexpression of fabA and fabB increased UFA contents in E. coli28. Therefore, it is plausible that overexpression of fabA and fabB may allow E. coli to grow under pH 4.2. To test this hypothesis, we cloned fabA and fabB into vector pTrcHis2B, and introduced them into BW25113 wild-type strain. The strain carrying empty vector presented a CFU ratio of 0.26 ± 0.03, similar with that of BW25113 wild-type strain, while either strain with fabA or fabB showed much higher acid tolerance at pH 4.2 (Fig. 1f).
In agreement with this result, strains harboring a temperature-sensitive mutant of fabA or fabB gene became much more susceptible to acid at 42 °C, whereas the wild-type strain showed similar acid tolerance at different temperatures (Fig. 1g). Taken together, these results demonstrated that elevating the biosynthesis of UFAs enhanced the acid tolerance of exponentially growing E. coli.
Transcription of fabA and fabB is activated by CpxRA system
To find out the possible regulator of these loci, we compared the DNA sequences of fabA and fabB promotor regions, and found a conserved sequence GTAAA-(5 nt)-GCAAA (Fig. 2a, b), which was similar to the identified CpxR recognition site29. This raised the hypothesis that the transcription of fabA and fabB is regulated by two-component system CpxRA, which consists of a sensor histidine kinase CpxA and a cytoplasmic response regulator CpxR. Since overexpression of outer membrane protein NlpE was identified as a Cpx-specific activation signal30,31, we cloned the nlpE gene and tested its effect on expression of fabA and fabB loci. As shown in Fig. 2c, d, excessive NlpE protein significantly enhanced the expression level of fabA and fabB at pH 7.0, and deletion of cpxR totally abolished this activating effect of NlpE. Besides those, NlpE overexpression also raised the UFAs content in E. coli membrane lipid at pH 7.0 (Fig. 2e) and E. coli acid tolerance at pH 4.2 (Fig. 2f) in a CpxR-dependent manner since cpxR knockout mutant displayed higher susceptibility to acidic challenge than wild-type strain even overexpressing NlpE (Fig. 2f). These observations collectively demonstrate that the CpxRA system activates transcription of fabA and fabB.
a Schematic diagram showing the genomic locations of fabA and fabB genes and corresponding CpxR boxes. CB CpxR box. Arrows show the transcription direction of fabA and fabB genes. b Alignment of CpxR binding sequence and the promoter sequences of fabA and fabB genes. The conserved CpxR binding sequence was highlighted in red. c Western blot analysis of FabA-His6 and FabB-His6 protein in BW25113 strains carrying empty vector or pnlpE, and cpxR mutant carrying pnlpE grown at pH 7.0. Overexpression of outer membrane protein NlpE was reported as a specific activating signal of CpxRA system. The relative amount of each protein was determined using ImageJ from two independent experiments. d Relative mRNA level of fabA and fabB in BW25113 strains carrying empty vector or pnlpE, and cpxR mutant carrying pnlpE grown at pH 7.0 determined by qRT-PCR (n = 2 biologically independent samples with three technical repeats). e Membrane lipid composition of BW25113 strains carrying empty vector or pnlpE, and cpxR mutant carrying pnlpE grown at pH 7.0 (n = 2 biologically independent samples). The compositions were determined by GC-MS, and fatty acid content is given as the relative peak area [(peak area of one fatty acid/total peak area) × 100%]. f Acid tolerance of BW25113 strains carrying empty vector or pnlpE, and cpxR mutant carrying vector or pnlpE after acidic challenge at pH 4.2 for 1 h (n = 3 biologically independent samples). Error bars, mean ± SEM. Two-tailed Student’s t tests were performed to determine the statistical significance for two group comparisons. The source data are provided as a Source Data file.
Transcription start site of fab genes controlled by CpxRA
We mapped the transcription starts of fabA and fabB using RACE (rapid-amplification of cDNA ends) experiment. As was previously reported, for fabA gene, two transcription initiation sites were detected under growth at pH 7.0: S1 positively regulated by FadR32 and S2 negatively regulated by FabR33. Interestingly, when the cell growth was at pH 4.2, our results indicated that the fabA gene has a third transcription initiation site located at the 203 bp upstream of the start codon, the S3 (summarized in Fig. 3a). For fabB gene, the transcription was initiated 37 bp upstream of the start codon in both conditions (summarized in Fig. 3b), consistent with previous reports34.
a, b DNA sequences of fabA and fabB promoter regions. The FabR box, FadR box and CpxR box sequences were underlined. Two previously reported transcription start sites of fabA, S1 and S2, and corresponding −35 and −10 regions were shown in blue and red, respectively. The transcription start site identified in this study and its −35 and −10 regions were shown in green. Numbering is from the start codon of each gene. c Western blot analysis of FabA-His6 and FabB-His6 in BW25113 wild-type strain at pH 7.0 and 4.2, and strain with CpxR site substitution at pH 7.0 and 4.2. n = 2 biologically independent samples. CB− substituted CpxR box. d Relative mRNA level of fabA and fabB determined by qRT-PCR in BW25113 wild-type strain at pH 7.0 and 4.2, and strain with CpxR site substitution at pH 7.0 and 4.2 (n = 2 biologically independent samples with three technical repeats). The fabA up primer sequences were shown in purple in (a). e Acid tolerance of BW25113 wild-type strain and mutants with substitution in fabA or fabB CpxR box after acidic challenge at pH 4.2 for 1 h (n = 3 biologically independent samples). f Gel-shift assay. 32P-labeled DNA fragments containing fabA and fabB promoter regions were incubated without and with His6-CpxR protein, shown in lanes 1–2. Lane 3 is the same as lane 2 but supplemented with “cold” DNA fragments. g DNase I footprinting analysis of fabA and fabB promoters with probes for the noncoding strand and increasing amount of His6-CpxR protein. His6-CpxR-DNA mixture were subjected to 5% polyacrylamide electrophoresis, and visualized by autoradiography. The regions protected by CpxR protein were shown in orange in (a, b). AG, DNA sequence ladder generated with the same primers using a Maxam and Gilbert A + G reaction. f and g are representative results from two independent experiments. Error bars, mean ± SEM. Two-tailed Student’s t tests were performed to determine the statistical significance for two group comparisons. The source data are provided as a Source Data file.
qRT-PCR result showed that the mRNA level of fabA and fabB was upregulated by NlpE overexpression in a fabR fadR double mutant strain, indicating that they had no obvious effect on fabA and fabB gene expression under CpxRA-dependent activation (Supplementary Fig. 2).
CpxR protein directly binds to the fabA and fabB promoters
To verify the putative CpxR recognition sites, two mutant strains were constructed by site-directed mutagenesis, in which the CpxR binding box on fabA or fabB promoter region was replaced by CATCT-(5 nt)-CATCT sequence, and expression of fabA and fabB was determined. Both western blot and qRT-PCR results showed that the activating effect of acid stress on expression of fabA and fabB was completely eliminated by substitution of CpxR site (Fig. 3c, d). Moreover, the acid tolerance of these mutants significantly decreased (Fig. 3e).
The function of CpxR site was further confirmed in vitro through gel-shift assay and DNase I footprinting analysis using purified His6-CpxR protein and 211- and 219-bp DNA fragments corresponding to the promoter regions of fabA and fabB, respectively. The purified His6-CpxR protein could shift those two DNA fragments (Fig. 3f), and protect the fabA promoter at the −287 to −255 region (numbering from the start codon) and the fabB promoter at the −119 to −94 region in the noncoding strand containing the putative CpxR binding site (Fig. 3g). All these results demonstrate that the CpxR protein enhances transcription of fabA and fabB by direct binding to their promoters.
CpxRA is directly activated by acidic environments
As a sensor histidine kinase in two-component system, CpxA spans the cell membrane and exposes its sensor domain into the periplasm. When CpxA detects a specific signal, it autophosphorylates and then transports the phosphate group to its cognate regulator CpxR, enabling the regulatory activity of CpxR. Without inducing signal, CpxA acts as a phosphatase to maintain CpxR in an inactive state35,36.
To monitor the expression and phosphorylation level of CpxA protein, we constructed a strain carrying chromosomal His6-tagged CpxA. As shown in Fig. 4a, acid shock significantly enhanced the amount of CpxA-His6 protein in exponentially growing cells. More importantly, increased level of phosphor-CpxA, the active form of CpxA, was detected at pH 4.2 (Fig. 4a).
a Western blot of CpxA-His6 protein in BW25113 strain after acidic challenge. For total CpxA protein, the same amount of total cellular proteins extracted from cells grown at different pH was applied to SDS-PAGE. For analysis of phosphorylated CpxA protein, the same amount of CpxA-His6 protein purified from cells grown at different pH was separated using SDS-PAGE containing Mn2+ and Phos-tag acrylamide, which retard the mobility of phosphoproteins. n = 3 biologically independent samples. b Schematic diagram of the proteoliposome system. c In vitro analysis of CpxA and CpxR phosphorylation induced by acidic pH, carried out using reconstituted proteoliposomes, which were preloaded with buffers at different pH and purified CpxA-His6 protein. To analyze phosphotransfer, purified His6-CpxR was incubated with CpxA-His6-containing proteoliposome in phosphorylation buffer. n = 2 biologically independent samples. d Design and construction of a PhoQ-CpxA fusion protein. PhoPQ is a known two-component system which can sense the acidic pH, and activate transcription of rstA and slyB genes. We replaced the sensor domain of PhoQ (amino acids 43–193) with the periplasmic domain of CpxA (amino acids 28–164). e Western blot of RstA and SlyB proteins in BW25113 with wild-type PhoQ or PhoQ-CpxA fusion at pH 7.0, at pH 4.2, with NlpE overexpression, and at pH 4.2 with NlpE overexpression. n = 2 biologically independent samples. f Western blot of FabA-His6 protein in BW25113 strains carrying wild-type CpxA or CpxA mutants in which the periplasmic histidine/glutamate was replaced with alanine at pH 4.2. n = 2 biologically independent samples. g Western blot of FabA-His6 protein in BW25113 strains carrying wild-type CpxA or CpxA mutants in which the histidine at positions 52 and 117 was replaced by alanine, lysine, and arginine, respectively. n = 2 biologically independent samples. Error bars, mean ± SEM. Two-tailed Student’s t tests were performed to determine the statistical significance for two group comparisons. The source data are provided as a Source Data file.
To demonstrate direct activation of CpxRA upon exposure to acidic pH, we used a reconstituted proteoliposome system, where a different internal and external pH can be stably maintained (Fig. 4b). The CpxA-His6 protein was purified from membranes and reconstituted into vesicles mainly in the inside-out orientation using the detergent-mediated method as described37. As shown in Fig. 4c, lowering the pH inside vesicles from 7.0 to 4.2 greatly enhanced the amount of phospho-CpxA. The phosphoryl group was transferred to regulator CpxR when the pH inside vesicles was 4.2, while phosphorylated CpxR could be hardly detected with neutral lumen pH (Fig. 4c) or without the sensor kinase CpxA. These results suggested that CpxA is capable of activating CpxR upon direct exposure to acidic environments.
To further verify the sensitivity of CpxA periplasmic domain to acidification, a chromosomal PhoQ-CpxA fusion strain was constructed, in which the sensor domain of PhoQ (amino acids 43–193) was replaced by the periplasmic domain of CpxA (amino acids 28–164) (Fig. 4d). PhoPQ is a known two-component system that can sense the acidic pH, Mg2+ depletion and antimicrobial peptides, and activate transcription of genes including rstA and slyB38,39. Western blot results showed that both low pH and overexpression of NlpE promoted expression of RstA and SlyB in strain carrying the PhoQ-CpxA fusion protein, while only acidic environments increased the protein level of RstA and SlyB in wild-type strain (Fig. 4e). On the other hand, when the CpxA periplasmic domain was replaced with that from another kinase AtoS which senses the presence of acetoacetate40, neither could the protein level of FabA-His6 and FabB-His6 be enhanced by acidic conditions in vivo, nor could the CpxR protein be phosphorylated at pH 4.2 in reconstituted proteoliposome (Supplementary Fig. 3), indicating that CpxA periplasmic domain is sensitive to acidification. All these results collectively demonstrated that CpxRA system is activated by direct exposure of CpxA periplasmic domain to acidic environments.
CpxA H52 and H117 are required for sensing of acidic pH
As reported, the histidine and glutamic acid residues were regarded as sensors detecting mild acidic pH because they can change their protonation state upon variation in the surrounding pH3,41,42,43. So, we hypothesized that the histidine and glutamate residues in CpxA sensor domain might be required for pH sensing. To test this hypothesis, we constructed a series of plasmids encoding CpxA mutants in which the periplasmic histidine/glutamate residue was replaced with alanine, respectively. Strains expressing the mutant CpxA proteins could express fabA in response to NlpE overexpression normally (Supplementary Fig. 4), indicating that mutations in residues of the periplasmic domain of CpxA protein do not impair the kinase activity of CpxA cytoplasmic domain. As shown in Fig. 4f, only two mutants (H52A and H117A) resulted in the loss of CpxA capability to upregulate fabA expression at pH 4.2, while the other mutations in glutamate residues did not change the expression level of fabA. To confirm that protonation of H52 and H117 is responsible for pH-mediated gene regulation, we also mutated these two residues to other basic amino acids, lysine and arginine, respectively. It was discovered that the substitution of H52 and H117 by lysine and arginine had no effect on the response to acidic pH (Fig. 4g). Moreover, the strain carrying CpxA variant with a basic amino acid residue at positions 52 or 117 presented a higher level of FabA protein even at pH 7.0, when compared with strain with CpxA H52A or H117A mutant (Supplementary Fig. 5), probably due to the protonation of basic amino acid residue at pH 7.0. These results demonstrate that protonation of residues at positions 52 and 117 is required to maintain the response of CpxA to acidic pH, but the presence of a histidine at those positions is not a specific requirement for activation.
Physiological effects of changes in lipid composition
To learn how changed content in membrane lipid affect E. coli cell functions and physiology, several assays were carried out using cells carrying empty vector as control, pfabA, or pfabB, after acidic challenge at pH 4.2. The membrane fluidity was determined by analyzing the fluorescence anisotropy of 1,6-diphenyl-1,3,5-hexatriene (DPH), which is negatively correlated to fluidity44. As shown in Fig. 5a, the strains with overexpression of fabA or fabB presented much less cell membrane fluidity than strain carrying empty vector. The membrane permeability was measured by detecting the leakage of OD260 materials (predominantly nucleotides), and there was no significant difference observed despite the varied UFA contents in membrane (Supplementary Fig. 6). F0F1-ATPase spans the cell membrane and transports periplasmic protons to cytoplasm with production of ATP, and lipids are required for its optimal functioning45. The activity of F0F1-ATPase was measured, and results suggested that the enhancement of UFAs content repressed its activity (Fig. 5b). Then the intracellular pH was monitored using ratiometric pH-sensitive GFP pHluorin246. When growing under pH 5.0–9.0, E. coli can maintain the intracellular pH between pH 7.4 and 7.947,48. Upon exposure to pH 4.2, the internal pH of control strain decreased to 6.82 ± 0.06, and the homeostasis of pH in E. coli cells with overexpression of fabA or fabB was good (Fig. 5c), probably due to the lowered membrane fluidity and F0F1-ATPase activity.
The membrane fluidity (a), F0F1-ATPase activity (b), and intracellular pH (c) of BW25113 strains carrying empty vector pTrcHis2B, pfabA or pfabB, after acidic challenge at pH 4.2 for 1 h. The membrane fluidity is determined by the anisotropy of fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH). F0F1-ATPase activity is assayed in terms of the release of inorganic phosphate using permeabilized cells in ATP-containing buffer. The intracellular pH was monitored using ratiometric pH-sensitive GFP pHluorin2. d Recovery of E. coli BW25113 wild-type strain and mutant with substitution in fabA CpxR site from feces following oral administration to BALB/c mice. Three independent trials with six mice in each trail were performed for each strain. e 3-Hydroxypropionate production, OD600, and final pH of fermentation broth of recombinant strains with and without fabA overexpression. For experiments with pH adjustment, the medium was adjusted to pH 7.0 every 12 h. Data are presented as mean ± SEM of three independent experiments. Two-tailed Student’s t tests were performed to determine the statistical significance for two group comparisons. The source data are provided as a Source Data file.
As E. coli has to combat a moderate acidic environment (pH 4.0–6.0) and proliferate in host’s small intestine, we carried out mouse gastrointestinal passage experiment to test the in vivo effect of the UFAs-CpxRA system. BALB/c mice were administered BW25113 wild-type strain or mutant with substituted fabA CpxR binding site, and three independent trials with six mice in each trial were performed for each strain. After 24 h, fecal samples were collected to test the presence of each strain. Out of 18 inoculated mice, the wild-type BW25113 strain was recovered from a total of 8 mice, while the fabA CpxR box mutant was detected in only 2 fecal samples (Fig. 5d). The results between the parental and mutant strains were statistically different (p < 0.05), demonstrating that this ATR system significantly promotes E. coli survival in mouse intestinal lumens.
In bio-production of organic acids, product accumulation acidifies the fermentation broth and inhibits the growth of producing strain. Previously, we had constructed a 3-hydroxypropionate (3HP)-producing E. coli recombinant strain. In shaking flask cultivation, the pH of culture decreased to pH 5.2–5.5 along with the production of 3HP, repressing the further production and cell growth (Fig. 5e). So, the pH of fermentation broth had to be adjusted to 7.0 periodically. Then fabA gene was overexpressed to enhance E. coli tolerance to acidic environments, leading to similar 3HP production and cell growth with and without pH adjustment (Fig. 5e). This result demonstrates the great prospect of UFAs-CpxRA-dependent ATR system in organic acids bio-production.
The UFAs-CpxRA system functions in diverse bacteria species
Salmonella Typhimurium LT2 and Shigella flexneri 2a str. 2457T are both common enteric pathogens, and their FabA proteins share more than 98% identity with E. coli FabA (Supplementary Fig. 7). To test whether UFAs are required for growth under acidic pH in these bacteria, the empty vector and recombinant plasmid carrying E. coli fabA gene were transformed into Salmonella Typhimurium LT2 and S. flexneri 2a str. 2457T. In acidic challenge tested at pH 5.0, the transformation of empty vector did not affect the acid tolerance of those two strains, but the overexpression of fabA gene elevated those CFU ratios by 2.32- and 1.99-fold, respectively (Fig. 6a). Moreover, mRNA level of fabA and fabB was upregulated by acid challenge in Salmonella, Klebsiella and Pseudomonas strains (Fig. 6b), and the consensus sequence of CpxR site was also found upstream of the fabA and fabB genes in additional bacteria species (Table 1). Additionally, Cronobacter sakazakii clone carrying transposon insertion in cpxR gene was identified as acid-sensitive mutant49. All these facts suggest this ATR system functioning in exponential phase is highly conserved across bacteria species.
a Acid tolerance of Salmonella Typhimurium LT2 and Shigella flexneri 2a str. 2457T wild-type strain and strains carrying empty vector or pfabA after acidic challenge at pH 5.0 for 1 h (n = 3 biologically independent samples). b Relative mRNA level of fabA and fabB in Salmonella Typhimurium LT2, Klebsiella pneumoniae ATCC25955 and Pseudomonas aeruginosa PAO1 grown at pH 7.0 and pH 5.0 (n = 2 biologically independent samples with three technical repeats). Error bars, mean ± SEM. Two-tailed Student’s t tests were performed to determine the statistical significance for two group comparisons. The source data are provided as a Source Data file.
Discussion
According to the results shown above, we present a previously uncharacterized ATR system as well as its regulatory pathway in exponentially growing E. coli. We demonstrated that the two-component system CpxRA can directly sense the acidic environments, and activate transcription of UFAs synthetic genes, resulting in increased UFAs contents in cell membrane lipid and normal growth of E. coli at pH 4.2. Our findings greatly enriched our understanding of the networks contributing to bacterial acid resistance, and the mechanisms for stress response governed by the CpxRA system.
As reported, the acid limit for E. coli growth is pH 4.0–4.5, and all known AR and ATR systems only prolong survival of E. coli cells under acidic conditions, but cannot support growth at pH 4.0–4.518,21,22. In our study, overexpression of fabA and fabB genes for UFAs biosynthesis, or activation of the two-component regulatory system CpxRA both restore the growth capability of E. coli at pH 4.2. Compared with those previously known AR systems, this growth-conferring ATR system is expected to have more important physiological significance. Firstly, bacteria can grow normally under acidic pH with activation of the CpxRA- and UFAs-dependent system, achieving higher biomass, which may be required for successful pathogenesis and efficient bio-production. Secondly, de novo mutations during DNA duplication play a critical role in bacterial stress resistance development50, and the rapid proliferation at low pH provides an opportunity for the evolution of novel AR and ATR systems. Thirdly, functioning of previously known AR systems is subject to more external limitations, such as AR1 is repressed by glucose and AR2−AR5 are dependent on exogenous amino acids, whereas the UFAs-CpxRA system can be activated by acidic pH alone because CpxA is capable of phosphorylating CpxR upon exposure to pH 4.2 in a reconstituted proteoliposome system.
We proved that the transcription of fabA stimulated by CpxRA is required for multiplication of E. coli in mouse intestine, indicating that UFAs-CpxRA system is also related to pathogenesis of E. coli pathovars, as well as those previously known AR systems. While various pathotypes of E. coli colonize and infect different organs, they all have to combat acidic environments during invading the host’s digestive tract. With pH values as low as 1.5–2.5, the stomach has been recognized as a natural antibiotic barrier1. Benefited from AR1−AR5 systems, E. coli can survive in the gastric acid for hours22,51. With their passage into the small intestine, E. coli cells will encounter a less acidic environment (pH 4.0–6.0) with the presence of organic acids produced by the normal intestinal flora2. As pathogenic E. coli strains must reproduce rapidly to cause disease ultimately52, the UFAs-CpxRA system is likely to play a key role. In summary, the successful enteric pathogen must possess two abilities, survival in extreme acidic condition and quick growth in moderate acidic environment. Consequently, this ATR system may be a new target for the development of antimicrobials.
UFAs-CpxRA-dependent ATR system also has potential application in bio-production of organic acids, which are valuable platform chemicals and have been successfully produced by recombinant E. coli strains53,54. However organic acids cause acidification of fermentation broth and inhibit E. coli growth at concentrations far below what is required for economical production. Now large quantity of base titrant are required to raise pH of the media in organic acids production process, and large amounts of acid must be consumed to recover the organic acids in the protonated form after production. If we could construct acid-tolerant strains growing at a pH less than the pKa of the produced acid, the additional consumption of acid and base titrants will be circumvented and the overall production cost will be lowered remarkably. As the UFAs-CpxRA system functions in exponential phase, is not repressed by glucose (the carbon source in most fermentation), and does not need exogenous amino acids, it is believed that this ATR system could be effectively applied in the field of organic acids bio-production.
Our data suggested that UFAs played an important role in protection of exponential phase E. coli from acid shock. The increase of UFAs content in membrane lipid not only affected the fluidity of lipid bilayer but also changed the activity of the F0F1-ATPase, conducing to reduced membrane proton permeability and improved internal pH homeostasis. This phenomenon is consistent with that S. mutants cells grown at pH 5.0 had higher UFAs composition and lower proton permeability than those grown at pH 7.027,55. Moreover, changes in fatty acid composition probably also affect the PTS system and enzyme secretion56,57. Overall, changing membrane fatty acid composition may improve the bacterial ability to adapt to acidic environment and be an important factor in bacterial acid response. In this study, the cyclopropane fatty acid (CFA) could not be detected in exponential phase E. coli cells, although it was regarded as a major factor in acid resistance of stationary phase E. coli24,58. However, UFA can be converted into CFA by CFA synthase59, and increased UFAs content will potentially enable the synthesis of CFA in stationary phase.
We demonstrate that the E. coli kinase CpxA is a direct sensor for acidic pH. Our data provide strong evidence that a decrease in pH protonates the histidine residues at positions 52 and 117 in CpxA periplasmic domain, leads to events catalyzed by its cytoplasmic domain, including phosphorylation of CpxA, transfer of phosphoryl group to regulator CpxR, as well as activation of CpxR-dependent gene transcription. As titratable by pH, histidine has been regarded as sensor detecting mild acidic pH, and also plays an essential role in the activation of sensor kinase PhoQ by acidic pH60. But the molecular details of how histidine senses the low pH signal in PhoQ and CpxA are different. Protonation of residues at positions 52 and 117 is effective to activate CpxA at acidic pH, whereas the imidazole ring of histidine is important in maintaining the response of PhoQ to acidity60.
Our results evidenced that CpxRA is a key system in the acid stress response of exponentially growing E. coli (Fig. 7), consistent with a previous proteomic analysis highlighting the importance of CpxRA in acid stress61. Upon exposure to acidic environments, CpxRA system stimulates the transcription of UFAs synthetic genes, resulting in improved intracellular pH homeostasis. Additionally, CpxRA upregulates some genes involved in cell wall modification, including peptidoglycan (PG) cross-linking proteins YcfS, YcbB and DacC; PG cleaving proteins AmiA, AmiC and Slt61,62,63. The induction of those genes led to an increase of cross-linking between PG and outer membrane proteins, and an increase of cell wall stability, which may help protecting E. coli cells from acidic challenge. That proteomic study also indicated the repression of AR2 system by CpxRA61. Because AR2 system is responsible for survival below pH 3.0 and log phase cells with overexpression of AR2 genes are not more acid resistant4, the CpxRA-mediated repression of AR2 in exponentially growing cells above pH 3.0 will guarantee that AR2 system is not induced in an inappropriate situation to avoid the metabolic burden. Furthermore, the sensor kinase CpxA was proved to cross-talk with noncognate response regulator OmpR64, which itself is involved in the acid stress response of E. coli65,66. All of these data indicate that E. coli has evolved multiple acid response mechanisms as well as precise regulatory circuits, to target specific stress conditions.
In exponentially growing E. coli, CpxRA system is activated by moderate acidic pH through protonation of periplasmic histidine residues, and upregulates the transcription of UFAs biosynthesis genes fabA and fabB, and cell wall modification genes including ycfS, ycbB, dacC, slt, amiA and amiC, and represses the expression of AR2 system genes. The sensor kinase CpxA also interacts with noncognate response regulator OmpR, which itself plays an important role in E. coli acid resistance.
In many Gram-negative pathogens, the CpxRA system plays an important role in the regulation of virulence factors, including pilus, secreted virulence effectors and type III secretion system36. For CpxRA to upregulate expression of these virulence genes, there must be an activating signal for CpxA under in vivo conditions. The identity of this signal still remains a topic of much debate. According to the results in this study, we propose that moderate acidic pH (such as the intestine and macrophage) is the activating signal of CpxA in vivo. As the previous study showed that CpxRA also contributed to bacterial resistance to antimicrobial peptides, a component of the host antimicrobial response62, acidic pH and antimicrobial peptide may have synergistic effect on CpxRA activation. Further study is ongoing to address this hypothesis.
Methods
Bacterial strains and growth conditions
All strains and plasmids used in this study are listed in Supplementary Data 1, and all primers used are listed in Supplementary Data 2. Phage P1 was used for generalized transductions in E. coli. Bacteria were grown at 37 °C in Luria-Bertani broth (Oxoid) or in E minimal medium (0.8 mM MgSO4, 10 mM citric acid, 57.5 mM K2HPO4, 16.7 mM NaNH3HPO4, 0.5% glucose). When necessary, antibiotics were added at final concentrations of 100 μg mL−1 for ampicillin, 20 μg mL−1 for chloramphenicol or 50 μg mL−1 for kanamycin. E. coli DH5α was used as host for the preparation of plasmid DNA, and E. coli χ7213 was used for preparation of suicide vectors. Diaminopimelic acid (50 μg mL−1) was used for the growth of χ7213 strain. LB agar containing 10% sucrose was used for sacB gene-based counter selection in allelic exchange experiments. The software ImageJ (version 1.52a) was used to analyze western blot results, and the online version of Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo) was used for sequence alignment.
Plasmids were constructed by digesting PCR fragments containing target gene and cloning into corresponding vectors as normal. Derivatives of pTrc-cpxA with nucleotide substitutions were constructed using Q5 Site-Directed Mutagenesis Kit (New England Biolabs) according to the manufacturer’s specifications. All plasmids were confirmed by DNA sequencing. Strains harboring chromosomal epitope-tagged proteins were generated using λ Red recombinase system67,68. DNA fragments encoding PhoQ-CpxA fusion or carrying substituted CpxR site were generated by joint PCR using primers shown in Supplementary Data 2, and cloned into the suicide vector pRE11269. The resulting plasmids were used to mediate the allelic exchange to generate strains with chromosomal PhoQ-CpxA fusion and CpxR site mutation.
In acidic challenge experiments, the strains were grown in E medium (pH 7.0) to an OD600 of 0.6. For strains with plasmid, IPTG was added to final concentration of 0.5 mM at an OD600 of 0.4, and the strain was further grown to an OD600 of 0.6. Then, the cells were harvested and washed twice with fresh E medium (pH 7.0), and inoculated into E medium with various pH as indicated, and strains were grown for another 30–60 min before the cells were collected to determine the CFU, membrane lipid composition, mRNA and protein levels.
Determination of fatty acid compositions
Phospholipids were extracted as described by Wang and Cronan23. Briefly, E. coli cells were harvested and resuspended in 1 mL sterile water, and 5 mL of chloroform-methanol (2:1 vol/vol) was added and vortexed for 3 min then stewing overnight. The solution was centrifuged and the upper phase was removed, an equal volume of 2 M KCl was added, followed by mixing and centrifugation. The top KCl phase was removed, an equal volume of water was added, followed by mixing and centrifugation. The resulting bottom organic phase was dried under a stream of nitrogen. Two milliliters of methyesterification reagent (BF3:CH3OH = 1:4) was added and incubated at 60 °C for 30 min. The resulting solution was extracted with n-hexane for three times and then detected with Agilent GC-MS (7890A–5975C; Column: Agilent-HP-INNOWax). The fatty acids were identified by comparing the retention times and mass fragmentation patterns with authentic standards. Content of each fatty acid is given as the relative peak area [(peak area of one fatty acid/total peak area) × 100%].
Immunoblot analysis of His6-tagged proteins
The E. coli cells were disrupted by sonication, the cell lysates were centrifuged, and the supernatants were used for western blot. Protein concentration was determined using BCA protein assay kit (Pierce), and the same amount of protein sample was separated in 12% SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad), and incubated with monoclonal HRP-conjugated anti-6×His antibodies (1:10,000 diluted) (Abcam). Protein signals were detected using Immobilon Western HRP substrate (Millipore) and X-ray film. To separate the phosphorylated proteins, 20–50 μM Phos-tag Acrylamide (WAKO) and 0.1 mM Mn2+ were added into the SDS-PAGE. Quantification was conducted using ImageJ software (NIH).
Quantitative RT-PCR and RACE
Total RNA was isolated from bacterial culture using EASYSpin Plus bacterial RNA quick extract kit (Aidlab Biotechnologies, China) according to the manufacturer’s instructions. RNA concentration was determined by spectrophotometry at 260 nm. Removal of genomic DNA and synthesis of cDNA were carried out using PrimeScript RT reagent Kit with gDNA Eraser (Takara). qRT-PCR was conducted using TB Green Premix Ex Taq (Takara) with the QuantStudio 1 system (Applied Biosystems). Constitutively transcribed gene rpoD was used as a reference control to normalize total RNA quantity of different samples. The relative difference of mRNA level was calculated using the ΔΔCt method70. Two independent biological samples with three technical repeats for each sample were performed for each qRT-PCR analysis.
The RACE experiment was performed using SMARTer RACE cDNA Amplification Kit (Clontech), according to the manufacturer’s instructions. The primers 1128 + 1129 and 1130 + 1131 were used to determine the transcription start sites of fabA and fabB under the NlpE overexpression conditions, respectively.
Purification of His6-CpxR and CpxA-His6
Purification of His6-CpxR and CpxA-His6 was conducted according to Fleischer et al. 37. Briefly, E. coli strain BW25113 with pTrc-cpxR or pTrc-cpxA was grown at 37 °C with aeration in LB medium. Gene expression was induced with 0.5 mM IPTG for 3–4 h. Membrane fractions and cytosolic fraction were separated by ultracentrifugation37. His6-CpxR was solubilized in cytosolic fraction and purified by Ni-affinity chromatography. Membrane proteins were solubilized with 1% dodecyl-β-d-maltoside (DM), and then CpxA-His6 was also purified by Ni-affinity chromatography.
Preparation of proteoliposomes
Proteoliposome was reconstructed as previously described with small modification37. Briefly, E. coli phospholipids (Avanti) were dried under a stream of nitrogen, and slowly dissolved in sodium citrate-hydrochloric acid buffer (pH 7.0, 5.0, 4.2) with 10% glycerol (vol/vol) and 0.47% Triton X-100 (vol/vol), respectively. Purified CpxA-His6 was added to the mixture and stirred at room temperature for 20 min. Bio-Beads SM-2 (Bio-Rad) were added in a bead/detergent ratio of 10:1(w/w), and the mixture was gently stirred at 4 °C overnight. After 16 h, fresh Bio-Beads were added, and the mixture was stirred for another 6 h. The proteoliposomes were collected by ultracentrifugation. To test autophosphorylation, proteoliposomes were incubated with 300 μmol ATP in phosphorylation buffer (50 mM Tris-HCl, pH 7.5, 10% glycerol (vol/vol), 2 mM dithiothreitol (DTT), 50 mM KCl, 5 mM MgCl2) at room temperature for 30 min. 5× SDS sample buffer was loaded to termination reaction. To analyze phosphortransfer, purified His6-CpxR was added to this mixture and incubated at room temperature for 20 min. Then samples were ultracentrifugated and the upper phase was collected. 5× SDS sample buffer was loaded to stop the reaction. To detect the phosphorylation level of CpxA and CpxR, all the samples were subjected to 8% SDS-PAGE with 20–50 μM Phos-tag Acrylamide (WAKO) and 0.1 mM Mn2+, which can retard the mobility of phosphoproteins to show the phosphorylated and nonphosphorylated forms in two separated bands.
Electrophoretic mobility shift assay (EMSA)
Primers 683 and 685 were labeled using T4 polynucleotide kinase (New England Biolabs) and γ-32P ATP (PerkinElmer Life Sciences). The promoter regions of fabA and fabB genes were amplified with primers 682 + 683 and 684 + 685, respectively. Ten nmol of 32P-labeled DNA was incubated at room temperature for 30 min with 0 or 50 pmol of His6 -CpxR protein in 20 μL of an EMSA buffer consisting of 10 mM Tris-HCl, pH 7.5, 1 mM ethylene diamine tetraacetic acid, 5 mM DTT, 10 mM NaCl, 1 mM MgCl2, and 5% glycerol. The mixture was subjected directly to 4% TAE-PAGE. Signals were detected by autoradiography.
DNase I footprinting assay
DNase I footprinting assays were carried out using the fabA promoter region amplified from BW25113 chromosome with primers 682 and 32P-683 and using the fabB promoter region amplified with primers 684 and 32P-685 for the noncoding strand. Approximately 25 pmol of 32P-labeled DNA and 0, 25, 50, or 100 pmol of His6-CpxR protein were mixed in a 100-μL reaction containing 20 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid pH 8.0, 10 mM KCl, 1 mM DTT, and 0.1 mg mL−1 bovine serum albumin. The reaction mixture was incubated at room temperature for 20 min. Then 1 μL of 100 mM CaCl2, 1 μL of 100 mM MgCl2, and 0.005 units of DNase I (Fermentas) were added, and the mixture was incubated at room temperature for 2 min. The DNase I digestion was stopped by phenol treatment, and the DNA was precipitated. Samples were analyzed by 6% polyacrylamide electrophoresis by comparison with a DNA sequence ladder generated with the same primers using a Maxam and Gilbert A + G reaction.
Membrane property assays
The membrane fluidity was measured by applying DPH as a fluorescence probe44. Briefly, the washed cells were incubated in DPH (Sigma) at a final concentration of 2 μM, then shaken in the dark at 30 °C for 40 min. After incubation, the unincorporated DPH was removed by washing with phosphate-buffered saline (PBS) twice. The cells were resuspended in PBS to get a final density of OD600 = 0.2 for the measurement of fluorescence anisotropy on a FluoroMax-4 spectrofluorometer (HORIBA Jobin Yvon). The wavelengths were 342 and 432 nm and slit widths were 5 and 10 nm for excitation and emission light, respectively. The fluorescence anisotropy, which was negatively correlated with membrane fluidity, was calculated44. For the permeability assay, the washed cells were resuspended in PBS for 30 min. The release of nucleotides was then measured at an optical density of 260 nm.
Intracellular pH measurement
The gene encoding ratiometric pH-sensitive green florescent protein pHluorin2 was synthesized and cloned into vector pBAD-18kan. Excitation assays were performed at wavelengths 395 and 475 nm with the emission at 510 nm using FluoroMax-4 Spectrofluorometer (HORIBA Jobin Yvon), and the ratio of fluorescence at 395 to 475 nm was used to calculate the intracellular pH according to a standard curve46.
F0F1-ATPase activity assay
The cells were washed once and resuspended in 1.8 mL membrane buffer (75 mM Tris pH 7.0, 10 mM MgSO4). Toluene was added to a final concentration of 10% (vol/vol), and the suspension was vortexed for 30 s and subjected to two rounds of freeze-thawing. Cells were collected, resuspended in membrane buffer. ATPase activity was assayed in terms of the release of inorganic phosphate in 50 mM Tris-maleate buffer pH 7.0, containing 10 mM MgSO4 and 50 μM ATP. Phosphate was assayed by the malachite green method, using Malachite Green Phosphate Assay Kit (Cayman Chemical).
Mouse survival passage experiments
Female BALB/c mice were housed individually in pathogen-free facility and given food and water ad libitum until 6-week-old. BW25113 strain and mutant with substitution in fabA CpxR site were grown in LB at 37 °C to log phase, and then 104 CFUs of bacteria were orally administrated to mice. To allow for inoculum clearance through the stomach, the mice were not provided feed for 4 h after oral administration. Fecal samples were collected 24 h after inoculation and suspended in PBS (0.5 g feces/4.5 mL PBS) and subsequently diluted. The diluted samples were then plated on LB agar containing nalidixic acid (20 μg mL−1) to test for the presence or absence of corresponding strains. Three independent trials were performed for each strain, and in each trial six mice were inoculated. This experiment was performed following the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1985).
3-Hydroxypropionate production
The strains were grown overnight in LB broth and 1:100 diluted into 250 mL Erlenmeyer flasks with 50 mL of minimal medium containing 14 g L−1 K2HPO4•3H2O, 5.2 g L−1 KH2PO4, 1 g L−1 NaCl, 1 g L−1 NH4Cl, 0.5 g L−1 MgSO4, 0.2 g L−1 yeast extract, and 20 g L−1 glucose. All shake flask experiments were carried out in triplicates. After incubation at 37 °C, 0.05 mM IPTG was added for induction at OD600 0.8, and 3 h later, biotin (40 mg L−1) and NaHCO3 (20 mM) were added. The antibiotics were supplied periodically after induction of IPTG every 12 h until 48 h. 10 g L−1 glucose was added once again after 24 h induction. 3HP concentration in medium was determined using Agilent 1200 Infinity HPLC system with an Aminex HPX-87H column (300 × 7.8 mm, Bio-Rad)54.
Ethics declarations
The animal experiments were performed according to the standards set forth in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1985). Experimental protocols were approved by the Institutional Animal Care Committee at Qingdao Institute of Bioenergy and Bioprocess Technology.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The source data underlying Figs. 1a, b, d−g, 2c−f, 3c−g, 4a, c, e−g, 5a−e, and 6a, b and Supplementary Figs. 1, 2, 3b, c, 4, 5, and 6 are provided as a Source Data file. The FabA sequences used in this study are under the accession numbers AIN31422, NP_460041, and EFS15200. The genome sequences used in this study are under the accession numbers CP009273, NC_003197, NC_002516, NC_011283, NC_013716, CP001235, NC_004741, CP001589, and NC_009778. Other data supporting the findings of this study are available from the corresponding authors upon request.
References
Foster, J. W. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2, 898–907 (2004).
Lin, J. et al. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62, 3094–3100 (1996).
Lund, P., Tramonti, A. & De Biase, D. Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 38, 1091–1125 (2014).
Foster, J. W. Acid stress responses of Salmonella and E. coli: survival mechanisms, regulation, and implications for pathogenesis. J. Microbiol. 39, 89–94 (2001).
Castanie-Cornet, M. P., Penfound, T. A., Smith, D., Elliott, J. F. & Foster, J. W. Control of acid resistance in Escherichia coli. J. Bacteriol. 181, 3525–3535 (1999).
Lin, J., Lee, I. S., Frey, J., Slonczewski, J. L. & Foster, J. W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177, 4097–4104 (1995).
Zhao, B. & Houry, W. A. Acid stress response in enteropathogenic gammaproteobacteria: an aptitude for survival. Biochem. Cell Biol. 88, 301–314 (2010).
Sen, H. et al. Structural and functional analysis of the Escherichia coli acid-sensing histidine kinase EvgS. J. Bacteriol. 199, e00310–e00317 (2017).
Hersh, B. M., Farooq, F. T., Barstad, D. N., Blankenhorn, D. L. & Slonczewski, J. L. A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 178, 3978–3981 (1996).
De Biase, D., Tramonti, A., Bossa, F. & Visca, P. The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol. Microbiol. 32, 1198–1211 (1999).
Iyer, R., Williams, C. & Miller, C. Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J. Bacteriol. 185, 6556–6561 (2003).
Soksawatmaekhin, W., Kuraishi, A., Sakata, K., Kashiwagi, K. & Igarashi, K. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51, 1401–1412 (2004).
Richard, H. & Foster, J. W. Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J. Bacteriol. 186, 6032–6041 (2004).
Zhang, M. et al. A genetically incorporated crosslinker reveals chaperone cooperation in acid resistance. Nat. Chem. Biol. 7, 671–677 (2011).
Mujacic, M. & Baneyx, F. Chaperone Hsp31 contributes to acid resistance in stationary-phase Escherichia coli. Appl. Environ. Microbiol. 73, 1014–1018 (2007).
Tramonti, A., De Canio, M. & De Biase, D. GadX/GadW-dependent regulation of the Escherichia coli acid fitness island: transcriptional control at the gadY-gadW divergent promoters and identification of four novel 42 bp GadX/GadW-specific binding sites. Mol. Microbiol. 70, 965–982 (2008).
Seo, S. W., Kim, D., O'Brien, E. J., Szubin, R. & Palsson, B. O. Decoding genome-wide GadEWX-transcriptional regulatory networks reveals multifaceted cellular responses to acid stress in Escherichia coli. Nat. Commun. 6, 7970 (2015).
Harden, M. M. et al. Acid-adapted strains of Escherichia coli K-12 obtained by experimental evolution. Appl. Environ. Microbiol. 81, 1932–1941 (2015).
Krulwich, T. A., Sachs, G. & Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9, 330–343 (2011).
Pienaar, J. A., Singh, A. & Barnard, T. G. Acid-happy: survival and recovery of enteropathogenic Escherichia coli (EPEC) in simulated gastric fluid. Micro. Pathog. 128, 396–404 (2019).
Kaur, P. & Asea, A. Loss of biofilm formation in an emerging foodborne pathogen Enteroaggregative Escherichia coli (EAEC) under acid stress. J. Cell Sci. Ther. 8, 260 (2017).
Small, P., Blankenhorn, D., Welty, D., Zinser, E. & Slonczewski, J. L. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J. Bacteriol. 176, 1729–1737 (1994).
Wang, H. & Cronan, J. E. Only one of the two annotated Lactococcus lactis fabG genes encodes a functional β-ketoacyl-acyl carrier protein reductase. Biochemistry 43, 11782–11789 (2004).
Brown, J. L., Ross, T., McMeekin, T. A. & Nichols, P. D. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37, 163–173 (1997).
Keweloh, H., Diefenbach, R. & Rehm, H. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch. Microbiol. 157, 49–53 (1991).
Fozo, E. M. & Quivey, R. G. Jr Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl. Environ. Microbiol. 70, 929–936 (2004).
Quivey, R. G. Jr, Faustoferri, R., Monahan, K. & Marquis, R. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol. Lett. 189, 89–92 (2000).
Cao, Y. J., Yang, J. M., Xu, X., Liu, W. & Xian, M. Increasing unsaturated fatty acid contents in Escherichia coli by coexpression of three different genes. Appl. Microbiol. Biotechnol. 87, 271–280 (2010).
De Wulf, P., McGuire, A. M., Liu, X. & Lin, E. C. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277, 26652–26661 (2002).
Snyder, W. B. & Silhavy, T. J. Beta-galactosidase is inactivated by intermolecular disulfide bonds and is toxic when secreted to the periplasm of Escherichia coli. J. Bacteriol. 177, 953–963 (1995).
DiGiuseppe, P. A. & Silhavy, T. J. Signal detection and target gene induction by the CpxRA two-component system. J. Bacteriol. 185, 2432–2440 (2003).
Henry, M. F. & Cronan, J. E. Jr. Escherichia coli transcription factor that both activates fatty acid synthesis and represses fatty acid degradation. J. Mol. Biol. 222, 843–849 (1991).
Feng, Y. & Cronan, J. E. Escherichia coli unsaturated fatty acid synthesis: complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J. Biol. Chem. 284, 29526–29535 (2009).
Campbell, J. W. & Cronan, J. E. Jr. Escherichia coli FadR positively regulates transcription of the fabB fatty acid biosynthetic gene. J. Bacteriol. 183, 5982–5990 (2001).
Vogt, S. L. & Raivio, T. L. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol. Lett. 326, 2–11 (2012).
Raivio, T. L. Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim. Biophys. Acta 1843, 1529–1541 (2013).
Fleischer, R., Heermann, R., Jung, K. & Hunke, S. Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J. Biol. Chem. 282, 8583–8593 (2007).
Perez, J. C. & Groisman, E. A. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol. Microbiol. 63, 283–293 (2007).
Perez, J. C. et al. Evolution of a bacterial regulon controlling virulence and Mg2+ homeostasis. PLoS Genet 5, e1000428 (2009).
Lioliou, E. E. et al. Phosphorylation activity of the response regulator of the two-component signal transduction system AtoS-AtoC in E. coli. Biochim. Biophys. Acta 1725, 257–268 (2005).
Fritz, R., Stiasny, K. & Heinz, F. X. Identification of specific histidines as pH sensors in flavivirus membrane fusion. J. Cell Biol. 183, 353–361 (2008).
Lee, D. et al. RAP uses a histidine switch to regulate its interaction with LRP in the ER and Golgi. Mol. Cell 22, 423–430 (2006).
Thompson, A. N., Posson, D. J., Parsa, P. V. & Nimigean, C. M. Molecular mechanism of pH sensing in KcsA potassium channels. Proc. Natl Acad. Sci. USA 105, 6900–6905 (2008).
Laroche, C., Beney, L., Marechal, P. A. & Gervais, P. The effect of osmotic pressure on the membrane fluidity of Saccharomyces cerevisiae at different physiological temperatures. Appl. Microbiol. Biotechnol. 56, 249–254 (2001).
Sturr, M. G. & Marquis, R. E. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl. Environ. Microbiol. 58, 2287–2291 (1992).
Mahon, M. J. pHluorin2: an enhanced, ratiometric, pH-sensitive green florescent protein. Adv Biosci. Adv. Biosci. Biotechnol. 2, 132–137 (2011).
Hickey, E. W. & Hirshfield, I. N. Low-pH-induced effects on patterns of protein synthesis and on internal pH in Escherichia coli and Salmonella typhimurium. Appl. Environ. Microbiol. 56, 1038–1045 (1990).
Slonczewski, J. L., Rosen, B. P., Alger, J. R. & Macnab, R. M. pH homeostasis in Escherichia coli: measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc. Natl Acad. Sci. USA 78, 6271–6275 (1981).
Alvarez-Ordonez, A. et al. Acid stress management by Cronobacter sakazakii. Int. J. Food Microbiol. 178, 21–28 (2014).
Ragheb, M. N. et al. Inhibiting the evolution of antibiotic resistance. Mol. Cell 73, 157–165 e155 (2019).
Gorden, J. & Small, P. L. Acid resistance in enteric bacteria. Infect. Immun. 61, 364–367 (1993).
Gilbert, R. J. & Roberts, D. Food hygiene aspects and laboratory methods. PHLS Micorbiol. Dig. 3, 32–34 (1986).
Tong, W. et al. Biosynthetic pathway for acrylic acid from glycerol in recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 100, 4901–4907 (2016).
Liu, C. et al. Functional balance between enzymes in malonyl-CoA pathway for 3-hydroxypropionate biosynthesis. Metab. Eng. 34, 104–111 (2016).
Ma, Y. & Marquis, R. E. Thermophysiology of Streptococcus mutans and related lactic-acid bacteria. Antonie van. Leeuwenhoek 72, 91–100 (1997).
Aboulwafa, M. & Saier, M. H. Jr Lipid dependencies, biogenesis and cytoplasmic micellar forms of integral membrane sugar transport proteins of the bacterial phosphotransferase system. Microbiology 159, 2213–2224 (2013).
Markevics, L. J. & Jacques, N. A. Enhanced secretion of glucosyltransferase by changes in potassium ion concentrations is accompanied by an altered pattern of membrane fatty acids in Streptococcus salivarius. J. Bacteriol. 161, 989–994 (1985).
Chang, Y. Y. & Cronan, J. E. Jr Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33, 249–259 (1999).
Wang, A. Y., Grogan, D. W. & Cronan, J. E. Jr Cyclopropane fatty acid synthase of Escherichia coli: deduced amino acid sequence, purification, and studies of the enzyme active site. Biochemistry 31, 11020–11028 (1992).
Prost, L. R. et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol. Cell 26, 165–174 (2007).
Surmann, K., Cudic, E., Hammer, E. & Hunke, S. Molecular and proteome analyses highlight the importance of the Cpx envelope stress system for acid stress and cell wall stability in Escherichia coli. Microbiologyopen 5, 582–596 (2016).
Weatherspoon-Griffin, N. et al. The CpxR/CpxA two-component system up-regulates two Tat-dependent peptidoglycan amidases to confer bacterial resistance to antimicrobial peptide. J. Biol. Chem. 286, 5529–5539 (2011).
Bernal-Cabas, M., Ayala, J. A. & Raivio, T. L. The Cpx envelope stress response modifies peptidoglycan cross-linking via the L,D-transpeptidase LdtD and the novel protein YgaU. J. Bacteriol. 197, 603–614 (2015).
Siryaporn, A. & Goulian, M. Cross-talk suppression between the CpxA-CpxR and EnvZ-OmpR two-component systems in E. coli. Mol. Microbiol. 70, 494–506 (2008).
Stincone, A. et al. A systems biology approach sheds new light on Escherichia coli acid resistance. Nucleic Acids Res. 39, 7512–7528 (2011).
Chakraborty, S. & Kenney, L. J. A new role of OmpR in acid and osmotic stress in Salmonella and E. coli. Front. Microbiol. 9, 2656 (2018).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Zhao, G., Weatherspoon, N., Kong, W., Curtiss, R. 3rd & Shi, Y. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella typhimurium. Proc. Natl Acad. Sci. USA 105, 20924–20929 (2008).
Edwards, R. A., Keller, L. H. & Schifferli, D. M. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207, 149–157 (1998).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 25, 402–408 (2001).
Acknowledgements
We thank Dr. Roy Curtiss III (University of Florida) for pRE112 and E. coli χ7213, Dr. Lixue Zhang (Qingdao University) for help on proteoliposome reconstruction, Dr. Qinggang Wang (Qingdao Institute of Bioenergy and Bioprocess Technology) for helpful discussion, and NBRP-E.coli at NIG for BW25113 strain. This study was financially supported by the NSFC (31722001 and 31670089), and Natural Science Foundation of Shandong Province (JQ201707).
Author information
Authors and Affiliations
Contributions
G.Z. designed the experiments. Y.X., Z.Z., W.T., Y.S., Y.D., B.L., J.W., M.L., Y.W. and S.S. performed the experiments. G.Z., M.X., Y.X. and Q.Q. analyzed the results. G.Z., M.X., Y.X. and Q.Q. wrote the manuscript. All authors edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Daniela De Biase, Peter Lund and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source Data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Y., Zhao, Z., Tong, W. et al. An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat Commun 11, 1496 (2020). https://doi.org/10.1038/s41467-020-15350-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-15350-5
This article is cited by
-
Divergent responses of growth rate and antioxidative system of ten Bacillus strains to acid stresses
Soil Ecology Letters (2024)
-
Strategies to increase the robustness of microbial cell factories
Advanced Biotechnology (2024)
-
Characterization of the Rhizosphere Bacterial Microbiome and Coffee Bean Fermentation in the Castillo-Tambo and Bourbon Varieties in the Popayán-Colombia Plateau
BMC Plant Biology (2023)
-
Integration of (S)-2,3-oxidosqualene enables E. coli to become Iron Man E. coli with improved overall tolerance
Biotechnology for Biofuels and Bioproducts (2023)
-
Discovery and remodeling of Vibrio natriegens as a microbial platform for efficient formic acid biorefinery
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.