Abstract
Reducing or eliminating persistent disparities in lung cancer incidence and survival has been challenging because our current understanding of lung cancer biology is derived primarily from populations of European descent. Here we show results from a targeted sequencing panel using NCI-MD Case Control Study patient samples and reveal a significantly higher prevalence of PTPRT and JAK2 mutations in lung adenocarcinomas among African Americans compared with European Americans. This increase in mutation frequency was validated with independent WES data from the NCI-MD Case Control Study and TCGA. We find that patients carrying these mutations have a concomitant increase in IL-6/STAT3 signaling and miR-21 expression. Together, these findings suggest the identification of these potentially actionable mutations could have clinical significance for targeted therapy and the enrollment of minority populations in clinical trials.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer-related death in the United States (U.S.) and the second most common form of cancer diagnosed in both men and women1. Since public health records began tracking differences in lung cancer incidence and mortality by racial and ethnic groups in the U.S., disparities between European Americans (EAs) and African Americans (AAs) have been identified2,3. Specifically, lung cancer incidence is higher in AAs, especially among men1. AAs also have the highest mortality rate and the lowest 5-year survival rate compared with other racial and ethnic groups1. The factors contributing to this health disparity are multifactorial4. For example, access to high quality health care is an important factor in lung cancer outcomes. In terms of incidence, it is likely that tobacco plays a role in the observed differences given that it is the leading etiological exposure associated with the lung cancer development4. However, AAs have a lower tobacco consumption overall compared with EAs5 and data show that the difference in lung cancer incidence persists at equal categories of cigarettes smoked per day6. This suggests a divergence in the etiology of lung cancer in the U.S. between racial and ethnic groups. As exposures are tightly linked with tumor biology7, it is possible that such differences in disease etiology could be reflected at the genomic level.
Our current understanding of lung cancer biology is primarily derived from populations of European descent. Given the persistent disparities that exist in lung cancer incidence and survival between AAs and EAs, it is important to characterize tumor biology across racial and ethnic groups. Large-scale genomic studies have highlighted genetic heterogeneity in lung cancer8,9,10. By identifying driver mutations, these studies have greatly contributed to the development of targeted pharmacological drugs for the treatment of cancer, and, through the ability to detect circulating tumor DNA, are also being leveraged for early diagnostics11. To date, few studies have investigated the somatic mutation landscape of lung cancer in AAs, and of those that have, the studies often included a small panel of genes or focused on hotspot mutations; others have focused on tumor tissue only12,13,14,15. Here, we report two genes, PTPRT and JAK2, that are recurrently mutated in lung adenocarcinoma (LUAD) among AAs.
Results
AAs have a complex lung cancer mutational landscape
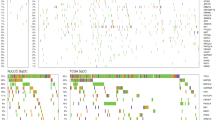
We conducted targeted exome sequencing of 129 tumor/adjacent non-involved pairs of fresh-frozen tissue from self-reported AAs (Supplementary Table 1) in the NCI-MD Case Control Study. Admixture analysis was consistent with self-reported race for 98% of the samples, comparable with The Cancer Genome Atlas (TCGA16; Supplementary Data 1). Of the 564 genes examined (Supplementary Data 2), 67 were not mutated in any of the patients (Supplementary Data 3) and 13 patients did not have mutations in the genes sequenced. We identified 4,136 somatic single-nucleotide variants (SNVs) and indel events (Supplementary Data 4; median/patient = 24, range = 0–426; Fig. 1a; Supplementary Data 4), reflecting the genetic heterogeneity of the population. As expected, tumors from smokers had more mutations than never smokers (average = 38, 37, and 5 for current, former and never smokers, respectively). The median number of mutations that passed the second filter, i.e., likely to alter protein function, was 14 (range = 0–132; Supplementary Data 5). Roughly a quarter (24%) of tumors did not harbor a mutation in the Oncovar gene panel, which is consistent with the previous observations8,10,17. It is possible that other somatic copy number-based genomic events, rare driver mutations, or epigenomic changes drive carcinogenesis in these tumors. Using a recent definition for hypermutation (>10 somatic SNVs/megabase (Mb))18, 59 samples were classified as hypermutated. The patient with the highest mutation burden was a current smoker with 64 pack-years of tobacco smoke consumption, who presented with adenocarcinoma. Known DNA repair genes—XRCC1, FANCA, BRCA1, PARP1, and ERCC4—were mutated and a somatic mutation signature consistent with defects in mismatch repair (signature 20) were observed in hypermutated patients (Supplementary Fig. 1). Mutations in mismatch repair genes have been associated with a hypermutated phenotype. MSH2, MSH6, MLH1, and PMS2 were included in the gene panel and only one of the patients with a hypermutated tumor had a mutation, which was a missense R638S mutation in MSH2.
a Distribution of somatic mutation number across patient samples. b Summary of mutational signatures in LUAD and LUSC samples from the NCI-MD Case Control Study. c Oncoprint outlining the co-occurrence of somatic mutations in all samples in the NCI-MD Case Control Study (histo, histology; AD, adenocarcinoma; BAC, bronchioalveolar carcinoma; SCC, squamous cell carcinoma; adenosq, adenosquamous cell carcinoma; pack, pack-years of smoking; smk, smoking status). Dashed line indicates the median.
As expected19, the most common nucleotide change was a C > A transversion (Supplementary Fig. 2a). Each tumor somatic profile was further contextualized in terms of known mutational signatures20. Eleven dominant mutational signatures were observed across AA lung cancers (Fig. 1b; Supplementary Data 6). Consistent with previous work21, signature 4 was the main signature observed in both LUAD and lung squamous cell carcinoma (LUSC) tumors from AAs and associated with smoking exposure (Fig. 1b). The APOBEC signatures 2 and 13 were also observed. Mutational signatures 3 (homologous recombination deficiency), 18 (potentially due to reactive oxygen-species-induced DNA damage)22, and 24 (which, like 4, has a C > A bias, is associated with aflatoxin exposure; Fig. 1b; Supplementary Data 6) were also observed in many of the AA tumor samples. However, as this was a targeted gene panel, future studies should conduct a more thorough study with whole-exome sequencing (WES).
Consistent with published studies, we observed a complex mutational landscape of lung cancer in AAs with considerable heterogeneity in the somatic landscape between individuals and little evidence for dominant driver genes (Supplementary Fig. 1c). We confirmed genomic alterations previously identified in lung cancer including TP53, which was the most mutated gene (Fig. 1c).
AAs have high PTPRT and JAK2 mutation frequencies
As the frequency of somatic mutations varies by histological subtype, we report mutation frequencies for LUAD and LUSC separately. Fifteen genes were significantly mutated in LUSC (Supplementary Fig. 2b; FDR P < 0.1). For most of these genes, the mutation frequency was comparable among AAs and EAs (Supplementary Data 7). In LUAD, 18/54 samples (33%) did not have a significantly recurrent mutation compared with 14/52 (27%) in LUSC, suggesting that, as in EAs, a large proportion of genomic drivers of lung cancer remain to be identified.
Eleven genes were significantly mutated in LUAD (Supplementary Fig. 2c, d). A comparison of driver genes between AAs and EAs (TCGA) shows that the global pattern of driver gene mutation frequencies is generally similar between EAs and AAs (Supplementary Data 7). However, STK11 and RB1 mutations occurred in 19% and 11% of LUAD tumors among AAs, respectively, which is higher than the frequency reported for EA patients in TCGA9 (Supplementary Fig. 2c). TP53 mutations were slightly higher among AAs compared with EAs, consistent with previous observations16.
We further found that the frequency of mutations in PTPRT and JAK2 are higher in AAs compared with EAs (Fig. 2a). Our data indicate that 13/54 (24%) of LUAD patients have mutations in PTPRT and that 4/54 (7.4%) have mutations in JAK2, compared with 8% and 2% in EAs, respectively (Fig. 2a). These mutations do not tend to co-occur in the same patient. Specifically, of the 15 patient samples (all histology combined) that carried a mutation in PTPRT and the 11 that carried a mutation in JAK2, only 1 sample had a mutation in both genes (two-sided Fisher’s exact test P < 0.001). In LUAD, no sample carried a mutation in both genes suggesting that these mutations are mutually exclusive (two-sided Fisher’s exact test P = 0.001). PTPRT was not mutually exclusive of other known key oncogenes and tumor suppressors (Supplementary Data 8).
a PTPRT and JAK2 mutations in the NCI-MD Case Control Study using targeted sequencing and WES, and in TCGA using WES and b combined. c Graphical distribution of individual mutations in PTPRT in EAs and AAs. d GSEA of gene expression changes in PTPRT and JAK2 mutant samples compared with wild type. e Levels of miR-21 in PTPRT and JAK2 mutant samples compared with wild type. Error bars indicate the s.d. *P < 0.05, **P < 0.01, ***P < 0.001, two-sided Student’s t-test. Source data are provided as a Source Data file. WES, whole-exome sequencing; AA, African American, EA, European American; TCGA, The Cancer Genome Atlas; GSEA, Gene set enrichment analysis.
Combined, PTPRT and JAK2 are mutated in >30% of tumors from AAs and ~10% of tumors from EAs (Fig. 2b). To validate these observations, we first used data from TCGA (Supplementary Data 1) and replicated the statistically higher frequency of PTPRT (AA 20%, EA 8%, two sample test of proportions P = 0.0004) and JAK2 (AA 6%, EA 2%, P = 0.025) mutations in LUAD from AAs (Fig. 2a; Supplementary Data 7). Secondly, we conducted WES on an additional independent set of 50 tumor and normal pairs from AAs and EAs in the NCI-MD Case Control Study (Supplementary Data 1, 2 and 9). Again, we observed a higher frequency of PTPRT (AAs 21%, EAs 9.6%, two sample test of proportions P = 0.014) and JAK2 (AAs 10%, EAs 0%, two sample test of proportions P = 0.08) mutations in tumors from AAs (Fig. 2a). Similar to data in lung cancer among EAs and other cancer types23, there were no clear hotspot mutations and the mutated codons were spread throughout PTPRT, including the phosphatase and extracellular domains (Fig. 2c). To our knowledge, this is the first time this observation has been reported in AAs. Previous studies based on targeted sequencing panels did not include PTPRT12,13,14, which likely explains why this observation was not reported before. Also, our inclusion of matched normal samples indicates that the events are somatic and not germline, which is an important observation given to the recent description that 10% of the pan-African genome is not represented in the current reference genome24.
PTPRT and JAK2 function downstream of cytokine and interferon signaling to regulate STAT323, which is an oncogenic driver and hallmark of cancer25. Integrating total RNAseq data for 23 samples for which we had both targeted exome sequencing and RNAseq data (n = 6 mutant and 17 wild type), we observed an enrichment of IL6/JAK2/STAT3 and interferon signaling among lung tumors carrying either PTPRT or JAK2 mutations (Fig. 2d). We also observed an enrichment of PI3K signaling, consistent with the literature26. We then analyzed microRNA (miRNA) transcriptional targets of STAT327, and observed increased miR-21 (Fig. 2e) and miR-181b (Supplementary Fig. 2) in tumor samples carrying mutations in PTPRT or JAK2, while non STAT3 targets, such as miR-126, were similar (Supplementary Fig. 3). These data suggest that an increased frequency of loss of function PTPRT and JAK2 mutations may drive STAT3 activity in subsets of non-small cell lung cancer (NSCLC) that are enriched among AAs.
Discussion
We report the somatic mutation profiles of 129 matched lung cancers from AAs across the coding regions of 564 pan-cancer genes (and six whole gene regions) and confirm key findings with data from (1) TCGA and (2) WES of 50 EAs and AAs. Roughly, a quarter (24%) of the tumors in our analysis did not harbor a mutation in the Oncovar gene panel, which is consistent with the previous observations8,10,17. It is possible that other somatic copy number-based genomic events, rare driver mutations, or epigenomic changes drive carcinogenesis in these tumors. We did not observe substantial differences in the mutation frequency of known driver genes according to ancestry in either LUAD or LUSC. However, we identified an increased prevalence of PTPRT and JAK2 mutations in LUAD from AAs. We validated this observation using whole-exome data from both TCGA and an independent set of samples from NCI-MD. Combined, ~30% of tumors from AAs carried mutations in PTPRT and/or JAK2 genes compared with 10% of EAs. To our knowledge, this is the first time this observation has been reported in AAs. Other protein phosphatases mutated in cancer, e.g., PTPRD, also negatively regulate STAT3 activation. A comprehensive study on the mutation frequency of these phosphatases and other STAT3 pathway regulators in LUAD from AAs is also warranted28.
TCGA has reported a fusion partner of PTPRT in lung cancer, EXD2. Therefore, although calling fusion genes from WES data can be problematic and error prone29, if searching for a specific gene the likelihood of false positive findings can be reduced. We detected putative PTPRT fusion genes in nine samples, though none had a similar partner gene or the same partner as previous reported fusions in TCGA. Further, as the minor allele fraction for either split reads or spanning pairs is very low in our study (Supplementary Data 10), it suggests that these subclonal fusions are not pathogenic or biologically relevant. Because fusion events that result in a well-expressed transcript are more easily and more reliably detectable from RNAseq data, future studies with RNAseq data should explore whether these putative fusion genes manifest as transcribed variants.
Interestingly, our recent work demonstrated that while IL-6 is associated with lung cancer diagnosis in both EAs30 and AAs31, the effect size was considerably larger among AAs, which is further evidence that this IL-6/JAK2/STAT3 pathway is important among AAs. We hypothesize that patients with PTPRT and JAK2 mutations could be candidates for targeted therapy and as such, our findings have implications for the recruitment of patients into clinical trials. For example, the initial conception to use JAKs as therapeutic targets was based on the identification of an activating mutation in JAK2 linked to myeloproliferative neoplasms32. The rationale for their use in these disorders has also been linked with perturbed JAK/STAT signaling, either due to somatic mutations or transcriptomic changes33. Recent work by Pitroda and colleagues found that a selective JAK2 inhibitor is cytotoxic to NSCLC cells in the context of constitutive IFN-stimulated JAK/STAT gene expression and that tumor cell-intrinsic expression of IFN-inducible PD-L1 was abrogated by the selective inhibitor34. In fact, somatic JAK1/2 mutations were shown to mediate primary resistance to PD-1 blockade because of an inability to signal through the interferon gamma receptor pathway, making it possible that patients harboring such mutations would be unlikely to respond to PD-1 blockade therapy35. Taken together, these findings suggest a potential role for JAK2 inhibitors in lung cancer in the context of a specific genomic background that could also possibly work in tandem with immune checkpoint inhibition.
Current JAK inhibitors are not always selective and most do not target specific mutations, though newer generations of JAK inhibitors demonstrate selective inhibition. JAK2 inhibitors might not work in PTPRT mutant tumors because other JAKs can, in theory, activate STAT3. As such, STAT3 inhibitors are good candidates for the tumors, we describe in our study. Interestingly, we conducted an agnostic analysis of differential drug sensitivity among cell lines mutant for JAK2 or PTPRT using the depmap database36 [https://depmap.org] and identified a STAT inhibitor with selective growth inhibition in PTPRT mutant cells (Supplementary Data 11). Our findings therefore raise the hypothesis that patients carrying these mutations may be more likely to respond to drugs that target this pathway than patients without these mutations. However, detailed mechanistic experiments will be needed to determine whether these are indeed actionable mutations, especially given a recent report that up to half of JAK2 mutations in nonsmall cell lung cancer can be inactivating37.
Our study has several strengths. It uses fresh-frozen tissues and matched tumor and non involved adjacent tissues. This study design gives us the ability to call true somatic mutations and is especially important in light of recent findings showing that up to 10% of the genome in individuals of African ancestry are not captured, using the current reference genome24. Most of these differences map to intergenic and noncoding regions, as such, their impact on a targeted exome-sequencing panel would be expected to be limited in nature. However, future work should address these novel genomic sequences and assess them for potential health-associated variants. Second, we used two additional datasets to confirm our results. As TCGA includes participants from across the U.S. and our samples were from the Baltimore region of Maryland, leveraging the TCGA database allowed us to compare our results to AAs from across the U.S. Whether or not population differences in PTPRT/JAK2 mutations extend to populations of Asian descent, or indeed other minority and under-represented populations, remains to be determined. TCGA has eight LUAD patients classified as Asian, one (12.5%) of which carries a PTPRT mutation, suggesting that the frequency in Asian populations is more closely aligned with EAs.
In summary, we show that the global frequency of somatic mutations is similar in tumors from EAs and AAs. However, we present evidence that somatic mutations in PTPRT and JAK2 are enriched in AAs and hypothesize that these mutations may be actionable. As this is a putatively targetable pathway, preclinical studies are needed to determine whether tumors carrying these mutations affect outcome or response to therapy directed against IL-6/JAK2/STAT3 signaling.
Methods
Patient samples and DNA extraction
Patients were selected from an ongoing case control study conducted by the NCI and the University of Maryland (Supplementary Data 1). This NCI-MD Case Control Study was conducted in accordance with the Declaration of Helsinki. Institutional review board approval was granted from NCI and participating hospitals and registered on clinicaltrials.gov [https://clinicaltrials.gov/ct2/show/NCT00339859]. Written informed consent was obtained from all patients. Patients for this study were recruited between 1984 and 2013. At the time of surgery, a portion of the tumor specimen and non involved adjacent lung tissue was flash frozen and stored at −80 °C until needed. Clinical and pathological information was obtained from medical records, tumor boards, and pathology reports.
Total genomic DNA was extracted using DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA). DNA quality and yield were determined using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). The initial study population included 141 tumor–normal pairs. One sample failed QC and was not suitable for sequencing. Four samples had poor quality normal tissue and were excluded due to the inability to match with tumor tissue. After sequencing was complete, seven samples were excluded due to quality of sequencing data. Thus, in total, 12 samples were excluded and the final study cohort consisted of 129 tumor–normal pairs.
The validation study population included an independent sample set (n = 50 samples) from the same ongoing case-control study. DNA was extracted from 15 µm sections of FFPE tissue using the Qiagen DNA FFPE Tissue Kit. Input for library prep was 500 ng.
Targeted exome sequencing and data processing
Simultaneous fragmentation and adaptor ligation was performed on input gDNA (50 ng) by tagmentation, using the Nextera DNA Library Preparation kit, according to the manufacturer’s protocol (Illumina). Products with a mean size of 350 bp +/−20% were purified using the Agencourt AmpureXP Purification System (Beckman Coulter). Amplification and dual indexing of purified samples was performed using Illumina PCR primers InPE1.0 and InPE2.0, and primer indices (8 bp). Hybridization capture of pooled indexed libraries was performed according to the manufacturer’s protocol using NCI Oncovar V4, an Agilent SureSelect Custom DNA kit (Agilent Technologies) targeting 2.93 Mb of exonic sequence in 564 genes found to be mutated in diverse solid tumors (Supplementary Data 2) with full coverage of six genes (CDKN2A, PTEN, SDHA, SDHC, TP53, and VHL)38. In addition, xGen Blocking Oligos (Integrated DNA Technologies Inc., Coralville, IA) specific to Nextera library adaptor sequences were used during hybridization according to manufacturer’s recommendations. The libraries were sequenced on an Illumina NextSeq 500 or HiSeq 2500 instrument by paired-end 2 × 75 bp to an average target region depth of ~140×. Alignments to the hg19 human reference genome assembly were performed with BWA-MEM (release 0.7.10, July 13 2014, r789)39, indel realignment by GATK IndelRealigner (version 3.4-0- g7e26428)40, and duplicates were marked with picard MarkDuplicates (version 1.129)41. Somatic SNVs and small insertions and deletions were called with Strelka 2.0.1742. All variants are reported as filter 1, while those mutations likely to alter protein function, i.e., nonsynonymous, frame shift, splice site, start/stop site SNVs, and codon insertion or deletions, are reported as filter 2. Sequencing statistics are reported in Supplementary Data 12. A two-sample test of proportions was used to analyze statistical differences in the frequency of somatic mutations between populations.
WES and data processing
WES was performed at Personal Genome Diagnostics (Baltimore, MD)43. In brief, DNA was extracted from FFPE tissue and matched blood or saliva samples, using the Qiagen DNA FFPE Tissue Kit or Qiagen DNA Blood Mini Kit (Qiagen). Genomic DNA from tumor and normal samples was fragmented and used for Illumina TruSeq library construction (Illumina), according to the manufacturer’s instructions. Briefly, 500 ng of genomic DNA in 100 ml of TE (tris-EDTA) was fragmented and purified using Agencourt AMPure XP beads (Beckman Coulter). Exonic regions were captured in solution using the Agilent SureSelect kit (Agilent). PE sequencing, resulting in 100 bases from each end of the fragments for exome libraries, was performed using Illumina instrumentation (Illumina). Sequence reads were aligned against the human reference genome (version hg19). Somatic mutations were identified using VariantDx and Cerebro custom software43,44. Fusion genes were called with the Manta program45.
Mutation calls in TCGA
Somatic mutations calls for LUAD and LUSC were downloaded from Firehose for EAs and AAs separately (Supplementary Data 7).
Mutation significance analysis
Mutation significance was performed using the MutSig2CV algorithm [https://software.broadinstitute.org/cancer/cga/mutsig_run]. The current version improves the background mutation rate estimation by pooling data from neighbor genes in covariate space, and substantially reduces the number of false–positive findings. Tables with mutation data, per-sample coverage, gene covariables, and mutation type were imported to the software. Genes with a Bonferroni-corrected P < 0.05 are considered significant46.
Mutational signature analysis
Mutational signatures in the targeted sequencing data were analyzed using R/Bioconductor package “MutationalPatterns”. The package covers a wide range of tools including: mutational signatures, transcriptional and replicative strand bias, genomic distribution, and association with genomic features. References mutation signature were obtained from the COSMIC website [https://cancer.sanger.ac.uk/cosmic/signatures] for 30 signatures. The current signatures were then determined by the contribution of 30 known mutational signatures on a single sample level by finding the optimal linear combination of mutational signatures that most closely reconstructs the mutation matrix47.
Measurement of miR-21
miRNA expression for miR-21 in lung adenocarcinoma tumor and normal tissues was extracted from our previous Nanostring analysis of miR-21, and compared between PTPRT- and JAK2-mutated samples (n = 4) and wild-type samples (n = 9). The miRNA microarray data discussed in this publication have been deposited in National Center for Biotechnology Information’s GEO and are accessible through GEO Series accession number GSE63805. Tests for statistical differences in miR-21 expression between mutated and nonmutated samples were conducted using two-sided Student’s t-test.
Gene set enrichment analysis
We integrated total RNAseq data for 23 samples, where we had both mutation and RNAseq data (n = 6 mutant and 17 wild type) in the NCI-MD study using the Palantir Foundry platform. Genes with fewer than 1 read per million in at least three members of each group were removed. Following quantile normalization and differential expression analysis using the R/Bioconductor package limma, gene set enrichment analysis (GSEA) was performed using the fgsea package and the MSigDB Hallmark Pathways.
Genetic ancestry
For admixture analysis, we utilized the 1000 Genomes Project phase III48 superpopulations as reference populations, where we removed rare variants (i.e., <5% across all of the phase III 1000 genomes), all INDELs and any SNPs that were not biallelic. We then used the tool Admixture v1.3.049 to estimate ancestry proportions for each of the 1000 Genomes Project superpopulations.
Data availability
The datasets generated during the current study have been uploaded to the dbGaP repository in compliance with the NIH Genomic Data Sharing Policy. Data can be accessed at [https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001895.v1.p1]. Raw data for Figs. 1 and 2, and Supplementary Figs. 1–3 are provided in the Source Data File.
Change history
30 January 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Schneiderman, M. A. & Levin, D. L. Trends in lung cancer. Mortality, incidence, diagnosis, treatment, smoking, and urbanization. Cancer 30, 1320–1325 (1972).
Burbank, F. & Fraumeni, J. F. U. S. cancer mortality: nonwhite predominance. J. Natl Cancer Inst. 49, 649–659 (1972).
Ryan, B. M. Lung cancer health disparities. Carcinogenesis 39, 741–751 (2018).
Holford, T. R., Levy, D. T. & Meza, R. Comparison of smoking history patterns among African American and white cohorts in the United States born 1890 to 1990. Nicotine Tob. Res. 18(Suppl 1), S16–S29 (2016).
Haiman, C. A. et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N. Engl. J. Med. 354, 333–342 (2006).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550 (2014).
Campbell, J. D. et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 48, 607–616 (2016).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Campbell, J. D. et al. Comparison of prevalence and types of mutations in lung cancers among black and white populations. JAMA Oncol. 3, 801–809 (2017).
Araujo, L. H. et al. Genomic characterization of non-small-cell lung cancer in African Americans by targeted massively parallel sequencing. J. Clin. Oncol. 33, 1966–1973 (2015).
Bollig-Fischer, A. et al. Racial diversity of actionable mutations in non-small cell lung cancer. J. Thorac. Oncol. 10, 250–255 (2015).
Kytola, V. et al. Mutational landscapes of smoking-related cancers in Caucasians and African Americans: precision oncology perspectives at Wake Forest Baptist Comprehensive Cancer Center. Theranostics 7, 2914–2923 (2017).
Yuan, J. et al. Integrated analysis of genetic ancestry and genomic alterations across cancers. Cancer Cell 34, 549–560 e549 (2018).
Lusk, C. M. et al. Profiling the mutational landscape in known driver genes and novel genes in African American non-small cell lung cancer patients. Clin. Cancer Res. 25, 4300–4308 (2019).
Campbell, B. B. et al. Comprehensive analysis of hypermutation in human cancer. Cell 171, 1042–1056 e1010 (2017).
Govindan, R. et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150, 1121–1134 (2012).
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Campbell, P. J. & Stratton, M. R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 3, 246–259 (2013).
Alexandrov, L. B. et al. Mutational signatures associated with tobacco smoking in human cancer. Science 354, 618–622 (2016).
Petljak, M. et al. Characterizing mutational signatures in human cancer cell lines reveals episodic APOBEC mutagenesis. Cell 176, 1282–1294.e1220 (2019).
Julien, S. G., Dube, N., Hardy, S. & Tremblay, M. L. Inside the human cancer tyrosine phosphatome. Nat. Rev. Cancer 11, 35–49 (2011).
Sherman, R. M. et al. Assembly of a pan-genome from deep sequencing of 910 humans of African descent. Nat. Genet. 51, 30–35 (2018).
Bromberg, J. F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999).
Hart, J. R., Liao, L. J., Yates, J. R. & Vogt, P. K. Essential role of Stat3 in PI3K-induced oncogenic transformation. Proc. Natl Acad. Sci. USA 108, 13247–13252 (2011).
Zhang, J. F., Luo, X., Li, H. M., Deng, L. & Wang, Y. Genome-wide uncovering of STAT3-mediated miRNA expression profiles in colorectal cancer cell lines. Biomed Res. Int. 2014, 11 (2014). https://doi.org/10.1155/2014/187105.
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Raphael, B. J. Chapter 6: Structural variation and medical genomics. PLoS Comput. Biol. 8, e1002821 (2012).
Brown D. et al. Relationship between circulating inflammation proteins and lung cancer diagnosis in the National Lung Screening Trial. Cancer Epidemiol. Biomarkers Prev. 28, 110–118 (2018).
Meaney C. L. et al. Circulating inflammation proteins associated with lung cancer in African Americans. J. Thorac. Oncol. 14, 1192–1203 (2019).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Disco. 17, 395–412 (2018).
Pitroda, S. P. et al. JAK2 inhibitor SAR302503 abrogates PD-L1 expression and targets therapy-resistant non-small cell lung cancers. Mol. Cancer Ther. 17, 732–739 (2018).
Shin, D. S. et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Disco. 7, 188–201 (2017).
Corsello S. M. et al. Non-oncology drugs are a source of previously unappreciated anti-cancer activity. bioRxiv, Preprint at https://www.biorxiv.org/content/10.1101/730119v1.full (2019).
Saigi, M. et al. MET-oncogenic and JAK2-inactivating alterations are independent factors that affect regulation of PD-L1 expression in lung cancer. Clin. Cancer Res. 24, 4579–4587 (2018).
Chaisaingmongkol, J. et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 32, 57–70 e53 (2017).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817 (2012).
Jones, S. et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci. Transl. Med. 7, 283ra253 (2015).
Wood, D. E. et al. A machine learning approach for somatic mutation discovery. Sci. Transl. Med. 10, eaar7939 (2018).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Blokzijl, F., Janssen, R., van Boxtel, R. & Cuppen, E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Genome Med. 10, 33 (2018).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Acknowledgements
This study was supported by the NIH Intramural Research Program at the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
These authors contributed equally: Khadijah A. Mitchell and Noah Nichols. Conception and design: K.A.M, N.N., and B.M.R. Development of methodology: D.C.E., J.W., P.M., J.S., and E.W. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): K.A.M, A.Z., B.M.R., E.B., D.C.E., P.M., J.W., H.S., M.P., J.S., and E.W. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis): K.A.M., N.N., W.T., R.S., A.T.G., S.S., D.C.E., Y.J.Z., J.L., J.W., S.P., J.S., P.M., and B.M.R. Writing, review, and/or revision of the manuscript: K.A.M., N.N., W.T., R.S., A.T.G., S.S., Y.J.Z., J.L., J.W., S.P., E.W., J.S., P.M., B.M.R., J.W., H.S., M.P., D.C.E., A.Z., E.B., E.R., and R.F.A. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K.A.M, N.N., and B.M.R. Study supervision: B.M.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Takashi Kohno and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source Data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitchell, K.A., Nichols, N., Tang, W. et al. Recurrent PTPRT/JAK2 mutations in lung adenocarcinoma among African Americans. Nat Commun 10, 5735 (2019). https://doi.org/10.1038/s41467-019-13732-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-13732-y
This article is cited by
-
Cancer health disparities in racial/ethnic minorities in the United States
British Journal of Cancer (2021)
-
Dysmenorrhea and Heavy Menstrual Bleeding in Elite Female Athletes: Quality of Life and Perceived Stress
Reproductive Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.