Abstract
Thiacalix[4]arenes as a family of promising ligands have been widely used to construct polynuclear metal clusters, but scarcely employed in silver nanoclusters. Herein, an anion-templated Ag88 nanocluster (SD/Ag88a) built from p-tert-butylthiacalix[4]arene (H4TC4A) is reported. Single-crystal X-ray diffraction reveals that C4-symmetric SD/Ag88a resembles a metal-organic super calix comprised of eight TC4A4− as walls and 88 silver atoms as base, which can be deconstructed to eight [CrO4@Ag11(TC4A)(EtS)4(OAc)] secondary building units arranged in an annulus encircling a CrO42− in the center. Local and global anion template effects from chromates are individually manifested in SD/Ag88a. The solution stability and hierarchical assembly mechanism of SD/Ag88a are studied by using electrospray mass spectrometry. The Ag88 nanocluster represents the highest nuclearity metal cluster capped by TC4A4−. This work not only exemplify the specific macrocyclic effects of TC4A4− in the construction of silver nanocluster but also realize the shape heredity of TC4A4− to overall silver super calix.
Similar content being viewed by others
Introduction
Due to the esthetic structures and a plethora of promising properties, silver nanoclusters have emerged as a hot topic garnering great interests over the last decade.1,2,3,4,5,6,7,8,9,10,11,12 However, their synthetic chemistry is still in the embryo, and trial and error is now the most popular synthetic routine. Regarding their assembly, protecting ligand is one of the most important prerequisites we must consider, and the widely recognized candidates are thiols, alkynes, and phosphines, or their combinations.13,14,15 Later, the advancements of anion template strategy16,17,18,19 and geometric polyhedral principle20,21,22 promote the rational design and synthesis of silver nanoclusters to a higher level. Compared with the above-mentioned organic ligands bearing single-coordination site, macrocyclic ligands with multiple preorganized coordination sites are much more desired in the construction of silver nanoclusters because of their reinforcement effect originating from the cooperative coordination of multiple binding groups. These considerations are reminiscent of “third generation” calixarenes–thiacalix[4]arenes, which are macrocyclic tetramers of phenols joined by sulfur atoms23 and have been recognized as a family of good ligands in the assembly of polynuclear metal clusters and cages.24,25,26,27 Based on the multiple coordination sites of –OH and –S– groups on them, a series of large metal nanoclusters or nanocages, including Co16, Co24, Mn24, Co32, Ni32, and Ni40, have been reported by Liao and Hong groups.28,29,30,31,32,33 However, thiacalix[4]arene-protected silver nanocluster is still rudimentary, and only two closely related p-tert-butylthiacalix[4]arene (H4TC4A)-capped reductive Ag34 and Ag35 nanoclusters have been reported.34,35 Although the coordination sites of H4TC4A favor to support silver nanoclusters, the bulky skeleton of H4TC4A also brings a big challenge in growth of single crystals, which is very crucial to understand the structural details of both metal core and metal–ligand interface.
Except for the ligand selection, oxoanion template plays another dominant role in constructing silver nanoclusters due to the strong directional effect arising from Ag–O interaction.36,37,38,39,40,41 In most cases of anion-templated assembly, oxoanions commonly exert the global effect that means the metal ions aggregate around them in a roughly chaotic fashion without any precedence. As we know, shuttlecock-like {M4(TC4A)} (M = Mn, Fe, Co, and Ni) is a very common secondary building unit (SBU) in TC4A4−-capped metal nanoclusters;42 however, we do not know what is the SBU if metal is switched to silver in the presence of the anion template. More importantly, we are also unclear that whether the as-formed {(template)@Agx(TC4A)} SBUs can be further reorganized around the oxoanion template again to form the hierarchical motif. Thus, correlating the SBUs and the final structure of TC4A4−-capped silver nanocluster is very important for understanding their syntheses and assembly mechanism.
Considering the rich advantages of H4TC4A in coordination chemistry and the powerful anion template effect in silver nanoclusters, we are extending our researches to combine them together in the synthesis of silver nanoclusters. Herein, we present a C4-symmetric silver nanocluster (K2[(CrO4)9@Ag88(TC4A)8(EtS)32(OAc)8]·8CH3CN·4DMF; SD/Ag88a) with a super calix shape containing the [CrO4@Ag11(TC4A)(EtS)4(OAc)] as SBU. Eight SBUs are further cyclized into an Ag88 cluster around a central CrO42−. This silver nanocluster is the highest-nuclearity metal cluster capped by TC4A4−. The structural features including the special ligand effect, local and global anion template effects, as well as the hierarchical assembly in SD/Ag88a will be discussed in detail.
Results
Structures of SD/Ag88a and SD/Ag88b
Briefly, SD/Ag88a was facilely prepared by the reaction of (EtSAg)n, H4TC4A, AgOAc, and K2Cr2O7 in the mixed solvent system containing acetonitrile, dichloromethane, and DMF at room temperature (Fig. 1). The red prism crystals can be crystallized after 2 weeks and collected together by filtration as bulk samples (~10%). The higher-yield synthesis of SD/Ag88a can be achieved using solvothermal reaction at 65 °C (~40%). The AgOAc in the synthesis of SD/Ag88a is very crucial because we have tried the other eight different silver salts available in our laboratory, including AgBF4, CF3COOAg, CH3SO3Ag, CF3SO3Ag, AgNO3, AgSbF6, PhCOOAg, and p-TOSAg, but none of them can produce SD/Ag88a. Auxiliary EtS− ligand also shows steric hindrance-related influence on the formation of SD/Ag88a because other larger alkylthiols such as tBuSH or iPrSH cannot produce SD/Ag88a under the same assembly condition. Of note, by mixing AgOAc with AgSbF6 in this system, we can isolate a similar Ag88 cluster (K2[(CrO4)9@Ag88(TC4A)8(EtS)32(OAc)8(CH3CN)]·8CH3CN; SD/Ag88b), but crystallized in monoclinic P21/c space group. The detailed structure diagrams for SD/Ag88b are shown in Supplementary Fig. 1. A series of characterization techniques such as single-crystal X-ray diffraction (SCXRD), powder X-ray diffraction (PXRD), Fourier transform-infrared spectroscopy (FTIR), UV–Vis spectroscopy, thermogravimetric analysis (TGA), dynamic light scattering (DLS), energy-dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM) were used in this system (Supplementary Figs. 7–17).
X-ray diffraction analyses on single crystals (Supplementary Fig. 18) of SD/Ag88a and SD/Ag88b revealed that they crystallize in tetragonal P4/n and monoclinic P21/c space groups, respectively (Supplementary Table 1). More structural diagrams and crystallographic data plots for them are shown in Supplementary Figs. 19–29. The composition of SD/Ag88a was determined as {K2[(CrO4)9@Ag88(TC4A)8(EtS)32(OAc)8]·8CH3CN·4DMF}. The composition of SD/Ag88b has one more coordinated CH3CN on the surface of the cluster compared with SD/Ag88a. The asymmetric unit of SD/Ag88a contains a quarter of Ag88 cluster and a crystallographic fourfold axis passes through Cr atom of the central CrO42−, whereas no crystallographic symmetry element coincides with the Ag88 cluster of SD/Ag88b, so a complete molecule was observed in the asymmetric unit. As a result, the overall 88-silver metallic framework of SD/Ag88b is more distorted than that of SD/Ag88a.
Due to the structural similarity between SD/Ag88a and SD/Ag88b, we just describe and discuss their structures below by taking SD/Ag88a as a representative. As shown in Fig. 2, SD/Ag88a looks like a super calix composed of 88 silver atoms, 32 EtS−, 8 TC4A4−, 8 OAc−, and 9 CrO42− anions. Among them, 88 silver atoms and 8 TC4A4− ligands roughly constitute the base and wall of the super calix, respectively. The equator diameter and the height of SD/Ag88a are 2.2 and 1.1 nm, respectively, by removing the organic shell.
a and b Total structures of Ag88 super calix viewed along two orthogonal directions. The inset in Fig. 2a is the photograph of crystals of SD/Ag88a taken by using a digital camera under the microscope. c and d The skeletal structure of SD/Ag88a by removing all organic ligands and anion templates viewed along two orthogonal directions. Color labels: purple, Ag; yellow, S; gray, C; red, O; green polyhedra, CrO42−.
The metallic skeleton of 88 silver atoms can be divided into eight CrO42−-templated Ag11 SBUs and each of them is capped by one TC4A4− with a cone-shaped conformation to form an irregular SBU with a composition of [CrO4@Ag11(TC4A)(EtS)4(OAc)] (Fig. 3a). The CrO42− plays the local templating effect in such SBU using a μ10-κ3:κ3:κ2:κ2 mode. In the asymmetric unit, there are two Ag11 SBUs fused together with two TC4A4− ligands locating in a nearly perpendicular orientation (Supplementary Fig. 2). In each cavity of TC4A4−, one CH3CN molecule is encapsulated and its N atom points out of the bigger opening of TC4A4− (Supplementary Fig. 3). The sunken voids formed after removing CH3CN molecule from TC4A4− are clearly shown in Supplementary Fig. 4. Two crystallographic unique TC4A4− ligands show different coordination modes using both phenolic hydroxyl and bridging sulfur atoms, μ6-κo2:κo3:κo3:κo3:κs1:κs1:κs1:κs2 (Fig. 3b) and μ7-κo3:κo3:κo3:κo3:κs1:κs1:κs1:κs2 (Fig. 3c). The Ag–O and Ag–S bond lengths related to TC4A4− fall in the ranges of 2.255(5)−2.70(1) Å and 2.511(5)−2.753(5) Å, respectively (Supplementary Table 2). The OAc− uses bidentate bridging (μ2-κ1:κ1) mode to coordinate on the Ag11 SBU (Ag–O: 2.14(2)−2.41(2) Å). Two of four EtS− ligands in each SBU adopt μ4 mode to cap on Ag11 SBU (Ag–S: 2.397(5)−2.641(7) Å), whereas the other two (one in μ3 and another in μ4 mode) combine with TC4A4− bridges to consolidate the joints between SBUs (Supplementary Fig. 5). One remaining central CrO42− anion (μ4-κ2:κ2:κ0:κ0) uses the global templating effect to organize eight Ag11 SBUs into an annulus finally (Fig. 3d); thus the best description for SD/Ag88a is {CrO4@[CrO4@Ag11(TC4A)(EtS)4(OAc)]8}. Although the TC4A4− ligands effectively cover on SD/Ag88a, we should not neglect the importance of auxiliary small EtS− and OAc− ligands that fill into the coordination unsaturation regions left after TC4A4− coverage. The argentophilic interactions, featured as the Ag···Ag distances shorter than 3.44 Å falling in the range of 2.825(2)−3.418(2) Å, reinforce the overall Ag88 skeleton.43,44,45 Although linking cationic shuttlecock-like {M4(TC4A)} (M = Mn, Fe, Co, and Ni) SBUs by carboxylates can form a very large nanocage with total metal counts more than 30,29 there are no TC4A4−-protected metal clusters with nuclearity higher than 80; thus SD/Ag88a is the highest-nuclearity metal cluster capped by TC4A4−. Compared with the known biggest silver cluster, [Ag490S188(StC5H11)114],15 the 88-nuclei silver super calix represents a brand-new structure model in the silver cluster family.
a The ball-and-stick mode of the structure of [CrO4@Ag11(TC4A)(EtS)4(OAc)] SBU. b, c Two different coordination modes of TC4A4− ligands. Color labels: purple, Ag; yellow, S; gray, C; red, O; cyan, Cr. d The Ag88 annulus built from eight Ag11 SBUs around the central CrO42− anion. Eight Ag11 SBUs are individually colored.
The packing of SD/Ag88a is also quite interesting and shown in Fig. 4a, c. The Ag88 super calix is lined in a face-to-face fashion to form a 1D nanotube running along [001] direction. Such packing is mainly dictated by intercluster van der Waals interaction between t-butyl groups on the upper rims and generates some voids as shown in Supplementary Fig. 6. The distance between adjacent two SD/Ag88a nanoclusters is 23.14 Å based on the separation between two Cr1 atoms. The similar packing was also observed in a chiral lead metal–organic nanotube based on β-cyclodextrin.46 Interestingly, the packing fashion of SD/Ag88b is completely different from that of SD/Ag88a. The intercluster van der Waals interactions that dominated face-to-side arrangement were found in the packing of SD/Ag88b (Fig. 4b, d). The centroid separation between two Ag88 clusters of SD/Ag88b is 26.27 Å. The larger separations between clusters indicated their loose packing that may be sensitive to co-crystallized solvents, although no satisfactory structural model for all solvent positions could be determined from SCXRD analysis. The different weight losses in the first step upon heating in N2 stream observed in the TGA curves suggested the different solvent-filling in the crystals (Supplementary Fig. 7a) and the residues after TGA are primarily metallic silver (Supplementary Fig. 7b).
Solution behaviors of SD/Ag88a
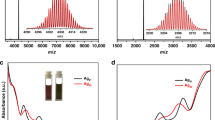
Mass spectrometry, DLS, and TEM were utilized to check the solution behavior of SD/Ag88a dissolved in CH2Cl2. The ESI-MS of SD/Ag88a contains five major isotope-distribution envelops (1a–1e) in the m/z range lower than 4000 (Fig. 5a). They are trivalent species deduced from the difference (Δm/z = 0.33) between adjacent isotopic peaks in each envelop. The most dominant envelop centered at m/z = 3038.487 (1d) can be assigned to [(CrO4)4@Ag44(TC4A)4(EtS)16(OAc)]3+ (Calcd. m/z = 3038.513), which is roughly equal to a half of SD/Ag88a but losing one central CrO42−, three OAc− anions, and some guest solvent molecules. In other words, the species 1d is equivalent to four fused Ag11 SBUs after losing three OAc− anions. Based on the isotope distributions, the envelop 1c centered at m/z = 3009.850 can be assigned to [(CrO4)4@Ag43(TC4A)4(EtS)14(OAc)2(CH2Cl2)]3+ (Calcd. m/z = 3009.859). Interestingly, the m/z spacing between 1a and 1c, 1b and 1d, and 1d and 1e is 55.30 or 55.96, which can be attributed to the mass of one AgOAc divided by the charge state of +3, indicating the coordination–dissociation equilibrium between them involving losing or gaining one AgOAc unit. In the m/z range higher than 4000, we also observed two weak but recognizable peaks centered at 4503.247 (1f) and 4587.207 (1g). After checking the spacing of adjacent isotopic peaks, we found that 1f and 1g are divalent species and can be assigned to [(CrO4)4@Ag43(TC4A)4(EtS)16(OAc)]2+ (1f, Calcd. m/z = 4503.318) and [(CrO4)4@Ag44(TC4A)4(EtS)16(OAc)2]2+ (1g, Calcd. m/z = 4587.277), respectively. The detailed formulae of 1a–1g are listed in Supplementary Table 3.
To rule out the possible fragmentation pathway of Ag88 cluster occurred in ESI-MS measurement, we also used DLS and TEM to examine the original CH2Cl2 solution of SD/Ag88a. Both Supplementary Figs. 8, 9a show some particles with diameters smaller than SD/Ag88a, which clearly suggest that the fragmentation happens in the course of dissolution instead of the ESI-MS process. From the above-combined results, we can conclude that (i) partial SD/Ag88a can keep intact but mainly coexists with a half of it in the CH2Cl2; (ii) the coordination–dissociation equilibria involving few ligands and AgOAc exist in this system; (iii) the encapsulation of the central CrO42− may not happen until the final stage enclosing the overall Ag88 super calix from two halves of Ag44 fragments. Based on the above structural analysis and the cluster fragmentation path revealed by mass spectrometry, we can retrodict a growth route for SD/Ag88a from bowl-like Ag11 SBU, a tetrameric semicircular Ag44 fragment to the final octameric circular Ag88 super calix (Fig. 5b).
UV–Vis spectra and photocurrent response properties
As shown in Fig. 6a, the solid-state UV–Vis spectra of SD/Ag88a, SD/Ag88b, and (EtSAg)n precursor were measured at 250–1000 nm at room temperature. The (EtSAg)n precursor looks yellow to the naked eye, whereas the SD/Ag88a and SD/Ag88b appear to be dark red. Both SD/Ag88a and SD/Ag88b show similar double-hump absorption profile, one narrow peak at ca. 340 nm, and one broad peak starting from ca. 370 to 800 nm. The absorption peak at 340 nm can be attributed to the n → π* transition of EtS−, as similarly observed in the absorption spectrum of the (EtSAg)n precursor. The broad absorption band can be attributed to the charge transfer transition from S 3p to Ag 5 s orbitals, which thus cause 240 and 190 nm redshifts of the absorption edges for SD/Ag88a and SD/Ag88b, respectively, compared with (EtSAg)n. The bandgaps of SD/Ag88a, SD/Ag88b, and (EtSAg)n precursor were determined as 1.37, 1.48, and 2.19 eV, respectively, according to the Kubelka–Munk function (Supplementary Fig. 10), which indicates that the aggregation of silver atoms into the cluster can influence the bandgap structures that include broadening of the absorption edge and narrowing of the bandgap. In addition, both SD/Ag88a and SD/Ag88b are almost emission silent at both room temperature and liquid nitrogen temperature.
a The normalized UV–Vis spectra of SD/Ag88a, SD/Ag88b, and (EtSAg)n precursor in the solid state. Insets are the digital photographs of SD/Ag88a, SD/Ag88b, and (EtSAg)n taken under the ambient environment. b Compared photocurrent responses of blank, (EtSAg)n, SD/Ag88a, and SD/Ag88b ITO electrodes in a 0.2 M Na2SO4 aqueous solution under repetitive irradiation.
Considering the wide visible light absorption, we performed photocurrent measurements for (EtSAg)n, SD/Ag88a, and SD/Ag88b in a typical three-electrode system by coating them on indium-doped SnO2 (ITO) as working electrodes (platinum wire as the assisting electrode and Ag/AgCl as the reference electrode) and keeping the bias voltage at 0.6 V. The photocurrent experiments were carried out in a 0.2 M Na2SO4 aqueous solution under illumination upon on/off cycling irradiation with LED light (λ = 420 nm; 50 W; intervals of 10 s). Upon irradiation, photocurrent density increases at 0.16, 0.12, and 0.20 μA cm−2 for (EtSAg)n, SD/Ag88a, and SD/Ag88b, respectively (Fig. 6b), which indicates that the SD/Ag88b possesses the best efficiency in the generation and separation of photoinduced electron/hole pairs in ITO electrodes.47 The photocurrent density can be still kept after ten on/off cycles, suggesting the response reproducibility. The generation of photocurrent may involve photoinduced charge migration from S 3p to the Ag 5 s orbits.
The stability of the electrode was further proved by the compared IR spectra and PXRD patterns.48 After the photocurrent tests, both IR spectra (Supplementary Figs. 11, 12) and PXRD patterns (Supplementary Figs. 13, 14) of samples were basically identical to those of original samples, which indicates that these samples did not undergo decomposition in the process of electrode preparation and during the photocurrent measurements.
Discussion
In summary, we have assembled and characterized a silver-organic super calix comprising 88 silver atoms and 8 TC4A4− ligands. SD/Ag88a is the highest-nuclearity metal cluster based on TC4A4−. Structural analysis revealed important chromate-templated Ag11 SBUs, which are further fused into a super calix of SD/Ag88a with a remaining CrO42− sitting on the center. Both local and global anion-templating effects from chromates are clearly manifested in the hierarchical structure of SD/Ag88a. The hierarchical assembly mechanism from bowl-like Ag11 SBU, a semicircular Ag44 fragment to the final circular Ag88 super calix was also revealed by using electrospray mass spectrometry (ESI-MS). The successful installation of TC4A4− ligand on silver nanocluster exemplifies its powerful chelating ability and macrocyclic effects, which surely open a bright road to assembly of silver nanoclusters using such kind of macrocyclic ligands.
Methods
Synthesis of SD/Ag88a
Method A: the mixture of (EtSAg)n (0.05 mmol, 8.5 mg), H4TC4A (0.015 mmol, 10.8 mg), and K2Cr2O7 (0.025 mmol, 7.3 mg) were dissolved in mixed solvent of acetonitrile, dichloromethane, and N,N’-dimethylformamide (6.5 mL, v:v:v = 10:2:1). The mixed solution was stirred for 1 h at room temperature, then AgOAc (0.1 mmol, 16.7 mg) was added to the above mixture for another 3 h of stirring. The red solution was filtrated and evaporated in the dark for 2 weeks. The red prism crystals of SD/Ag88a were obtained in a yield of 10%.
Synthesis of SD/Ag88a
Method B: the mixture of (EtSAg)n (0.05 mmol, 8.5 mg), H4TC4A (0.015 mmol, 10.8 mg), and K2Cr2O7 (0.025 mmol, 7.3 mg) were dissolved in mixed solvent of acetonitrile, dichloromethane, and N,N’-dimethylformamide (6.5 mL, v:v:v = 10:2:1). The mixed solution was stirred for 1 h at room temperature. To this solution AgOAc (0.1 mmol, 16.7 mg) was added. The reaction continued for further 3 h of stirring, then the red mixture was sealed in a 25-mL Teflon-lined reaction vessel and kept at 65 °C for 2000 min. After cooling to room temperature, the red solution was filtrated and evaporated in the dark for 1 week. The red prism crystals of SD/Ag88a were isolated in a yield of 40%. Elemental analyses calc. (found) for SD/Ag88a (C428H588Ag88Cr9K2N12O88S64): C, 26.50 (26.51); H, 3.06 (3.08); N 0.87 (0.85)%. Selected IR peaks (cm−1): 3382 (w), 2949 (w), 1658 (w), 1550 (w), 1434 (s), 1301 (w), 1245 (m), 1209 (w), 848 (m), 828 (s), 760 (m), 724 (m), 650 (w), 612 (w), 540 (w), 520 (w).
Synthesis of SD/Ag88b
The synthesis conditions were similar to those described for Method B above, but using AgOAc (0.1 mmol, 16.7 mg) and AgSbF6 (0.05 mmol, 17.2 mg) instead. Red prism crystals of SD/Ag88b were isolated in a yield of 37%. Elemental analyses calc. (found) for SD/Ag88b (C418H563Ag88Cr9K2N9O84S64): C, 26.22 (26.14); H, 2.96 (3.00); N 0.66 (0.59)%. Selected IR peaks (cm−1): 2949 (w), 1549 (w), 1472 (w), 1433 (s), 1356 (m), 1304 (m), 1239 (m), 1051 (w), 966 (w), 855 (m), 830 (s), 764 (m), 720 (m), 648 (w), 528 (w).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC: 1920453 and 1920454 for SD/Ag88a and SD/Ag88b. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Liu, Y., Najafabadi, B. K., Fard, M. A. & Corrigan, J. F. A functionalized Ag2S molecular architecture: facile assembly of the atomically precise ferrocene-decorated nanocluster [Ag74S19(dppp)6(fc(C{O}OCH2CH2S)2)18]. Angew. Chem. Int. Ed. 54, 4832–4835 (2015).
Dhayal, R. S. et al. Ag21{S2P(OiPr)2}12 +: an eight-electron superatom. Angew. Chem. Int. Ed. 54, 3702–3706 (2015).
Hau, S. C. K., Cheng, P.-S. & Mak, T. C. W. Enlargement of globular silver alkynide cluster via core transformation. J. Am. Chem. Soc. 134, 2922–2925 (2012).
Chakraborty, I. & Pradeep, T. Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem. Rev. 117, 8208–8271 (2017).
Kurasawa, M., Arisaka, F. & Ozeki, T. Asymmetrically fused polyoxometalate-silver alkynide composite cluster. Inorg. Chem. 54, 1650–1654 (2015).
Shen, H. & Mizuta, T. An alkynyl-stabilized Pt5Ag22 cluster featuring a two-dimensional alkynyl-platinum “crucifix motif”. Chem.-Eur. J. 23, 17885–17888 (2017).
Li, G., Lei, Z. & Wang, Q.-M. Luminescent molecular Ag-S nanocluster [Ag62S13(SBut)32](BF4)4. J. Am. Chem. Soc. 132, 17678–17679 (2010).
Xie, Y.-P., Jin, J.-L., Lu, X. & Mak, T. C. W. High-nuclearity silver thiolate clusters constructed with phosphonates. Angew. Chem. Int. Ed. 54, 15176–15180 (2015).
Wang, Z.-Y. et al. Atomically precise site-specific tailoring and directional assembly of superatomic silver nanoclusters. J. Am. Chem. Soc. 140, 1069–1076 (2018).
Yang, H. et al. Plasmonic twinned silver nanoparticles with molecular precision. Nat. Commun. 7, 12809 (2016).
Jin, S. et al. Crystal structure and optical properties of the [Ag62S12(SBut)32]2+ nanocluster with a complete face-centered cubic kernel. J. Am. Chem. Soc. 136, 15559–15565 (2014).
Gao, G.-G., Cheng, P.-S. & Mak, T. C. W. Acid-induced surface functionalization of polyoxometalate by enclosure in a polyhedral silver-alkynyl cage. J. Am. Chem. Soc. 131, 18257–18259 (2009).
Corrigan, J. F., Fuhr, O. & Fenske, D. Metal chalcogenide clusters on the border between molecules and materials. Adv. Mater. 21, 1867–1871 (2009).
Anson, C. E. et al. Synthesis and crystal structures of the ligand-stabilized silver chalcogenide clusters [Ag154Se77(dppxy)18], [Ag320(StBu)60S130(dppp)12], [Ag352S128(StC5H11)96], and [Ag490S188(StC5H11)114]. Angew. Chem. Int Ed. 47, 1326–1331 (2008).
Vilar, R. Anion-templated synthesis. Angew. Chem. Int. Ed. 42, 1460–1477 (2003).
Campos-Fernandez, C. S. et al. Anion template effect on the self-assembly and interconversion of metallacyclophanes. J. Am. Chem. Soc. 127, 12909–12923 (2005).
Wang, Q.-M., Lin, Y.-M. & Liu, K.-G. Role of anions associated with the formation and properties of silver clusters. Acc. Chem. Res. 48, 1570–1579 (2015).
Rais, D. et al. Anion-templated syntheses of rhombohedral silver-alkynyl cage compounds. Angew. Chem. Int Ed. 40, 3464–3467 (2001).
Wang, Z. et al. Johnson solids: anion-templated silver thiolate clusters capped by sulfonate. Chem.-Eur. J. 24, 1640–1650 (2018).
Li, X.-Y. et al. A platonic solid templating Archimedean solid: an unprecedented nanometre-sized Ag37 cluster. Nanoscale 7, 8284–8288 (2015).
Wang, Z. et al. Assembly of silver trigons into a buckyball-like Ag180 nanocage. Proc. Natl Acad. Sci. USA 114, 12132–12137 (2017).
Kumar, R., Lee, Y. O., Bhalla, V., Kumar, M. & Kim, J. S. Recent developments of thiacalixarene based molecular motifs. Chem. Soc. Rev. 43, 4824–4870 (2014).
Liu, M., Liao, W., Hu, C., Du, S. & Zhang, H. Calixarene-based nanoscale coordination cages. Angew. Chem. Int. Ed. 51, 1585–1588 (2012).
Bi, Y., Du, S. & Liao, W. p-tert-Butylthiacalix[4]arene-supported high-nuclearity {Co24M8} (M = Mo or W) nanospheres and the hybrids with Keggin polyoxometalates. Chem. Commun. 47, 4724–4726 (2011).
Bi, Y., Wang, S., Liu, M., Du, S. & Liao, W. A tetragonal prismatic {Co32} nanocage based on thiacalixarene. Chem. Commun. 49, 6785–6787 (2013).
Geng, D. et al. Merohedral icosahedral M48 (M = CoII, NiII) cage clusters supported by thiacalix[4]arene. Chem. Sci. 9, 8535–8541 (2018).
Bi, Y., Du, S. & Liao, W. Thiacalixarene-based nanoscale polyhedral coordination cages. Coord. Chem. Rev. 276, 61–72 (2014).
Bi, Y. et al. A {Co32} nanosphere supported by p-tert-butylthiacalix[4]arene. J. Am. Chem. Soc. 131, 11650–11651 (2009).
Hang, X. et al. Discrete {Ni40} coordination cage: a calixarene-based Johnson-type (J 17) hexadecahedron. J. Am. Chem. Soc. 138, 2969–2972 (2016).
Wang, S. et al. Calixarene-based {Ni18} coordination wheel: highly efficient electrocatalyst for the glucose oxidation and template for the homogenous cluster fabrication. J. Am. Chem. Soc. 140, 6271–6277 (2018).
Su, K. et al. Open pentameric calixarene nanocage. Inorg. Chem. 53, 18–20 (2014).
Xiong, K. C. et al. Chlorine-induced assembly of a cationic coordination cage with a μ5-carbonato-bridged MnII 24 core. Chem. Eur. J. 18, 5536–5540 (2012).
Guan, Z.-J. et al. Thiacalix 4 arene: new protection for metal nanoclusters. Sci. Adv. 2, e1600323 (2016).
Guan, Z.-J. et al. The stability enhancement factor beyond eight-electron shell closure in thiacalix 4 arene-protected silver clusters. Chem. Sci. 10, 3360–3365 (2019).
Liu, J.-W. et al. Anisotropic assembly of Ag52 and Ag76 nanoclusters. J. Am. Chem. Soc. 140, 1600–1603 (2018).
Wang, Z. et al. Trapping an octahedral Ag6 kernel in a seven-fold symmetric Ag56 nanowheel. Nat. Commun. 9, 2094 (2018).
Liu, H. et al. Acid–base-triggered structural transformation of a polyoxometalate core inside a dodecahedrane-like silver thiolate shell. Angew. Chem. Int. Ed. 55, 3699–3703 (2016).
Liu, J.-W. et al. A giant 90-nucleus silver cluster templated by hetero-anions. Chem. Commun. 54, 4461–4464 (2018).
Zhang, S.-S. et al. Anion-templated nanosized silver alkynyl clusters: cluster engineering and solution behavior. Chem.-Eur. J. 23, 3432–3437 (2017).
Wang, Z., Su, H.-F., Tung, C.-H., Sun, D. & Zheng, L.-S. Deciphering synergetic core-shell transformation from [Mo6O22@Ag44] to [Mo8O28@Ag50]. Nat. Commun. 9, 4407 (2018).
Xiong, K. et al. Truncated octahedral coordination cage incorporating six tetranuclear-metal building blocks and twelve linear edges. Chem. Sci. 3, 2321–2325 (2012).
Huang, R.-W. et al. Tandem silver cluster isomerism and mixed linkers to modulate the photoluminescence of cluster-assembled materials. Angew. Chem. Int. Ed. 57, 8560–8566 (2018).
Li, S. et al. Atom-precise modification of silver(I) thiolate cluster by shell ligand substitution: a new approach to generation of cluster functionality and chirality. J. Am. Chem. Soc. 140, 594–597 (2018).
Schmidbaur, H. & Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 54, 746–784 (2015).
Wei, Y. et al. Pb(II) metal-organic nanotubes based on cyclodextrins: biphasic synthesis, structures and properties. Chem. Sci. 3, 2282–2287 (2012).
Wu, T. et al. Monocopper doping in Cd-In-S supertetrahedral nanocluster via two-wtep strategy and enhanced photoelectric response. J. Am. Chem. Soc. 135, 10250–10253 (2013).
Wang, C., Liu, C., Li, L.-J. & Sun, Z.-M. Synthesis, crystal structures, and photochemical properties of a family of heterometallic titanium oxo clusters. Inorg. Chem. 58, 6312–6319 (2019).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21822107, 91961105, 21571115, and 21827801), the Natural Science Foundation of Shandong Province (Nos. JQ201803, ZR2019ZD45, and ZR2017MB061), the Taishan Scholar Project of Shandong Province of China (Nos. tsqn201812003 and ts20190908), the Qilu Youth Scholar Funding of Shandong University, and the Fundamental Research Funds of Shandong University (104.205.2.5).
Author information
Authors and Affiliations
Contributions
The original idea was conceived by D.S., experiments and data analyses were performed by Z.W., Y.-W.G., Q.-P.Q., and D.S., ESI-MS data were collected by H.-F.S., structure characterization was performed by Z.W., Y.F.B., and D.S., and the paper was drafted by D.S., Z.W., C.-H.T., and L.-S.Z. All authors have given approval to the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Aude Demessence and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Su, HF., Gong, YW. et al. A hierarchically assembled 88-nuclei silver-thiacalix[4]arene nanocluster. Nat Commun 11, 308 (2020). https://doi.org/10.1038/s41467-019-13682-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-13682-5
This article is cited by
-
A reasonable approach for the generation of hollow icosahedral kernels in metal nanoclusters
Nature Communications (2021)
-
4.8 nm Concave {M72} (M=Co, Ni, Fe) metal-organic polyhedra capped by 18 calixarenes
Science China Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.