Abstract
The benzylic positions in drugs are sites that readily react with cytochrome P450 oxidases via single-electron oxidation. New synthetic methodologies to incorporate a fluoroalkyl group at the benzylic site are continually being developed, and in this paper, we report a metal-free and site-selective organophotoredox-catalyzed trifluoromethylthiolation of benzylic C-H bonds for a wide variety of alkyl arenes and heteroarenes. The precise and predictive regioselectivity among various C(sp3)-H bonds originates from an inner-sphere benzylic radical initiation mechanism, and avoids the use of external oxidants or hydrogen atom abstractors. Its practicality stems from the trifluoromethylthiolation of a series of drugs and complex organic molecules, which is overwhelmingly selective for benzyl groups. This operationally simple protocol can provide a general and practical access to structurally diverse benzylic trifluoromethyl sulfides produced from ubiquitous benzylic C-H bonds. Large scale trifluoromethylthiolation can be achieved with continuous flow photoredox technology.
Similar content being viewed by others
Introduction
Challenges in modern drug discovery have played an important role in the evolution of direct synthetic methodology1,2,3,4,5,6. The cytochrome P450 enzymatic metabolism of therapeutics in vivo is a common route of drug metabolism7. Due to the unique electron-negativity and high lipophilicity, the introduction of a trifluoromethylthio group (SCF3) into pharmaceutical candidates can significantly protect against in vivo enzymatic metabolism and increase the cell membrane permeability8. As a consequence, the development of organic synthetic strategies accessing trifluoromethylthiolated compounds has gained considerable momentum in recent years9,10,11,12,13,14,15. Although several trifluoromethylthiolated drugs (e.g., tiflorex, toltrazuril and tiflorex in Fig. 1a) have been approved by FDA, the future development of such compounds depends on the evolution of synthetic strategies entailing versatility, diversity and availability.
The state-of-the-art strategies of precise C–H trifluoromethylthiolation. a The importance of trifluoromethylthiolated drugs in the market. b Previous strategies for C(sp3)–H bond trifluoromethylthiolation via generation of key alkyl radicals by an outer-sphere radical initiation process. c The prevalence of the benzylic moiety in biologically important compounds. d Our work via inner-sphere radical initiation for precise benzylic C–H bond trifluoromethylthiolation
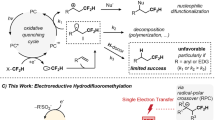
Direct radical trifluoromethylthiolation of C(sp3)–H bonds can provide a powerful platform with which to construct organofluorine compounds16,17,18,19. Seminal work from Qing and co-workers16, Chen and co-workers17, Tang and co-workers18, and Glorius and co-workers19 has significantly stressed the potential synthetic value of such reactions. Currently, the regioselectivity of the reaction relies mainly on the physicochemical properties (e.g., exchange constants and polarity) of intermolecular hydrogen-atom-transfer (HAT) reagents or oxidants in terms of C–H bond dissociation energy and electronic properties (Fig. 1b)20. However, based on this outer-sphere radical initiation mechanism, it is still rather difficult to predict the regioselectivity precisely, especially for the complex organic molecular architectures bearing nearly resembling C(sp3)–H bonds shown in Fig. 1b.
Benzylic C–H bonds are common in biologically important compounds and about 25% of top-selling 200 pharmaceuticals contain this structural motif 21. In general, the benzylic positions in small-molecule drugs are sites easily metabolized by cytochrome P450 oxidases. If a metal-free and unified benzylic C–H bond trifluoromethylthiolation strategy is available, drug discovery across of a wide range of structurally diversity compounds could be expedited (Fig. 1c). Although benzylic radical C–H bond functionalization has been well studied22,23,24,25,26, the late-stage benzylic C–H trifluoromethylthiolation strategy remains a significant obstacle. The use of strong external oxidants will result in rapid oxidation of benzylic C–H bonds into a carbonyl unit27. The only successful benzylic C–H trifluoromethylthiolation strategy is copper-catalyzed oxidative trifluoromethylthiolation with AgSCF3 but the reaction needs a large excess of simple toluene analogs (60 equivalents compared to AgSCF3), and thus this compromises its practicability and late-stage potential16. To achieve an exclusive benzylic C–H regioselectivity, the best approach may be the generation of a benzylic radical without involving an intermolecular HAT process. This leads to consideration of single-electron oxidation of phenyl rings. As shown in Fig. 1d, the resultant aryl radical cation species28,29,30,31,32,33,34,35,36 will lead to inner-sphere HAT with benzylic C–H bonds giving rise to benzylic radicals. Herein, we report the development of a metal-free, photoredox inner-sphere HAT process which predictably generates, from natural products or drug derivatives, a benzylic radical which can be trifluoromethylthiolated, avoiding the use of oxidants and HAT reagents. The practicality of the method is further illustrated by late-stage trifluoromethylthiolation of benzylic C–H bonds under flow photochemical conditions.
Results
Reaction optimization
To test our hypothesis, 2-isopentylbenzo[b]thiophene with Phth-SCF3 (2-((trifluoromethyl)thio)isoindoline-1,3-dione) was chosen as the model reaction since the electron-rich thiophene ring can usually support electrophilic trifluoromethylthiolation37,38. As shown in Table 1, both solvent and photocatalyst are crucial factors for successful benzylic C–H trifluoromethylthiolation. Among the solvents examined, only MeCN could produce a 75% yield of 3a. Other solvents gave only trace amounts of the desired product with commercially available 4CzIPN (2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile) as the photocatalyst (Table 1, entries 1–5). Notably, the reaction is highly regioselective (benzylic vs methine C–H, see Supplementary Fig. 9)19. Replacement of 4CzIPN with other photocatalysts delivered a much lower yield (Table 1, entries 5–10). The amount of Phth-SCF3 was further decreased from 1.5 equiv to 1.3 equiv by careful evaluation of reaction and base concentrations, affording the desired product (3a) in 81% yield with 98:2 regioselectivity ratio (Table 1, entry 11). Compared with previous work39, the use of a slight excess (1.3 equiv) of a trifluoromethylthiolated reagent enhances its synthetic value. In the absence of K2CO3, a 32% yield of 3a along with a decreased ratio of 88:12 was obtained (Table 1, entry 12). The role of inorganic base may benefit the deprotonation, generating a benzylic radical species. Control experiments suggested that both photocatalyst and light were crucial for benzylic C(sp3)–H trifluoromethylthiolation (Table 1, entries 13–14).
Substrate scope
With the optimal reaction conditions in hand, we investigated the scope of aromatic hydrocarbons (Fig. 2). Several electron-rich five-membered ring heteroaromatic compounds, including thiophene-, furan-, and indole-based substrates (3a–3d) are compatible with the reaction. In general, it is challenging to realize site-selective benzylic C–H trifluoromethylthiolation of indole substrates (3c) due to the competing trifluoromethylthiolation at C3 position40,41. This further indicates the advantages of this inner-sphere radical initiation mechanism. When substrates contain more than one different benzylic C–H bond, the secondary (3e), tertiary (3f), and less acidic C–H bond (3g, 3h) were preferentially trifluoromethylthioylated. Interestingly, with 4-(3-phenylpropyl)-pyridine, excellent regioselectivity was observed, focusing on the C–H bond in the proximity of the more electron-rich phenyl ring rather than the pyridinyl ring (3i). Distinguishing between nearly identical C–H bonds with traditional outer-sphere HAT strategy remains a significant challenge. It has been reported that even the outer-sphere HAT mechanism can robustly achieve trifluoromethylthiolation of unactivated methine and methylene C(sp3)–H bonds while failing with benzylic C–H bonds19. In contrast, our strategy addresses this unresolved problem (3j–3l). Also, the predicted regioselectivity is obtained with substrates bearing competitive methine C–H and the α-C–H of a heteroatom (3l).
Subsequently, we examined the generality of this protocol with substrates containing primary, secondary, or tertiary benzylic C–H bonds. Substrates bearing electron-withdrawing functional groups on the phenyl rings tolerated the reaction conditions, furnishing the desired products (3m, 3o–3q) in satisfactory yields. A wide variety of alkyl arenes are good coupling partners and uniformly afford the desired products (3r–3cc) in moderate to good yields with exclusive regioselectivity. The length of an alkyl chain on the aromatic ring has no influence on the regioselectivity and reaction efficiency. Another advantage of this redox-neutral strategy is that it can successfully trifluoromethylthiolate easily oxidized diphenylmethanes and benzylic ethers (3v–x). Importantly, PinB-substituted alkyl arenes are tolerated, albeit with moderate isolated yields (3z, 3dd), and can participate in a variety of downstream diversification reactions. With 2.5 equivalents of Phth-SCF3 reagents, double trifluoromethylthiolation can be realized (3ee, 3ff). With fluorenes, this affords direct access to the gem-trifluoromethylthiolation product (3ee) in moderate yield.
Late-stage and flow chemistry
Distinct from previous outer-sphere radical initiation C–H trifluoromethylthiolation reactions, an inner-sphere mechanism can exclusively generate a benzylic radical without depending on external strong oxidants. To demonstrate the generality and practice of the reaction, we applied this redox-neutral strategy to achieve late-stage benzylic trifluoromethylthiolation of biologically important natural products and drugs, with an aim of modularly constructing trifluoromethylthioylated drug candidates (Fig. 3a). A variety of complex molecules can be trifluoromethylthioylated at the benzylic position in satisfactory yield with excellent functional group compatibility. The inner-sphere benzylic radical generation process allows competing C–H bonds little influence in complex molecules. For example, one of the top selling drugs, pirfenidone can undergo benzylic C–H trifluoromethylthiolation exclusively to give the desired product (4) in 63% yield. The methyl ester of ibuprofen, an anti-inflammatory drug can incur this transformation at the more electron-rich benzylic C–H bond in 81% yield (11). The less sterically hindered benzylic C–H bond in gemfibrozil and d-phenylalanine derivatives can be preferentially trifluoromethylthiolated (13, 14). Complex alkyl arenes bearing an amide NH moiety are also good substrates for highly site-selective benzylic C–H trifluoromethylthiolation (14–16), and late-stage trifluoromethylthiolation can be scaled up to 1 mmol with shorter reaction times by the use of continuous micro-tubing reactors42,43, thus enhancing its utility in synthetic applications (Fig. 3b).
Synthetic application
A large number of benzyl sulfides were synthesized during research of pesticides44 and the benzophenone benzyl trifluoromethyl sulfide is a core substructure in such compounds. With our protocol, a series of benzyl trifluoromethyl sulfide analogs were successfully obtained from aryl (4-ethylphenyl)methanones in 51–73% yield under mild reaction conditions (Fig. 3c).
Mechanistic studies
To elucidate the possible reaction mechanism, electron paramagnetic resonance (EPR) experiments with N-tert-butyl-α-phenylnitrone (PBN) as the electron-spin trapping reagent were carried out. A significant EPR signal was observed for the model reaction, indicating a possible radical pathway (Fig. 4a). To further validate this, a radical clock experiment with cyclopropylbenzene as a substrate was performed and the results (Fig. 4b) clearly demonstrated the involvement of a benzylic radical. In addition, the KIE result (kH/kD = 6.9) suggests that C–H cleavage is involved in the rate-determining step. Luminescence quenching experiments suggest that 1a may be the quencher in the reductive quenching cycle (Fig. 4d). The quantum yield of a model reaction was determined to be 0.33 and thus a radical chain pathway is less likely.
Accordingly, a plausible mechanism is proposed and is shown in Fig. 4e. 4CzIPN is a commercially available organic photocatalyst, and it is reported that photoexcited 4CzIPN is a strong oxidant (1/2E = +1.35 V). Although alkyl arenes hold higher oxidative potential45, the potential overlap between the excited 4CzIPN and alkyl arenes could promote the single-electron oxidation for irreversible inner-sphere HAT as the driving force (see Supplementary Figs. 31 and 32)46. The aryl radical cation species that is formed (25 or 25′) will lead to an intramolecular 1,2-HAT with benzylic C–H bonds and subsequent deprotonation47 of cyclohexadienyl cation (26) with base gives rise to a benzylic radical (27). This benzylic radical can couple with a Phth-SCF3 anion radical (28) to deliver the desired benzylic trifluoromethylthiolation products (3) and a Phth anion (29). Generation of the Phth anion can further abstract one proton from the cyclohexadienyl cation (26) to yield phthalimide and this can explain why a catalytic amount of K2CO3 can initiate the reaction. Alternatively, the radical addition of benzylic radical (27) to electrophilic Phth-SCF3 reagent is also a likely candidate19 for the generation of trifluoromethylthiolation products (3).
Discussion
In conclusion, we have developed an organophotoredox-catalyzed reaction for site-selective benzylic C–H bond trifluoromethylthiolation of a wide variety of alkyl arenes and heteroarenes through an inner-sphere radical initiation mechanism, affording structurally diverse benzylic trifluoromethyl sulfides with moderate to good yields. The broad scope, excellent functional group compatibility, and predictable regioselectivity allow for efficient late-stage benzylic C–H trifluoromethylthiolation of a variety of drug candidates and complex molecules. We believe that this strategy will expedite precise benzylic C–H functionalization in complex molecules and that it will promote the construction of a library of benzylic trifluoromethyl sulfide leads for drug discovery.
Methods
General procedure for benzylic trifluoromethylthiolation
Substrate 1 (0.2 mmol), Phth-SCF3 (64.3 mg, 0.26 mmol), 4CzIPN (3.2 mg, 0.004 mmol), and K2CO3 (5.52 mg, 0.04 mmol) were placed in a transparent Schlenk tube equipped with a stirring bar. The anhydrous MeCN (4.0 mL) was added under argon atmosphere. If the substrate 1 is liquid, anhydrous MeCN and 1 were added in turn. The reaction mixture was stirred under the irradiation of two 45 W blue LEDs (distance app. 4.0 cm from the bulb) at room temperature for 12–24 h. When the reaction finished, the mixture was quenched with water and extracted with ethyl acetate (3 × 10 mL). The organic layers were combined and concentrated under vacuo. The product was purified by flash column chromatography on silica gel (petroleum ether:ethyl acetate).
General procedure for flow chemistry
Complex molecule (1.0 mmol), Phth-SCF3 (321 mg, 1.3 mmol), 4CzIPN (16 mg, 0.02 mmol), and K2CO3 powder (120 mesh, 27.6 mg, 0.2 mmol) were placed in a sample bottle (20 mL). After placing in the glove box, anhydrous MeCN (20.0 mL) was added and the yellow mixture was then transferred into the syringe (20 mL) in the glove box. Next, the reaction mixture was subjected to the irradiation of three 45 W blue LEDs (distance app. 4.0 cm) with a small fan at room temperature in the mode of perfusion/extraction at the speed of 2.0 mL/h. When the reaction finished, the mixture was quenched with water and extracted with ethyl acetate (3 × 10 mL). The organic layers were combined and concentrated under vacuo. The product was purified by flash column chromatography on silica gel (petroleum ether:ethyl acetate).
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
References
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Voreakos, K., Devel, L. & Georgiadis, D. Late-stage diversification of phosphinic dehydroalanine pseudopeptides based on a giese-type radical C-alkylation strategy. Org. Lett. 21, 4397–4401 (2019).
Lapuh, M. I. et al. Late-stage C-H amination of abietane diterpenoids. Org. Biomol. Chem. 17, 4736–4746 (2019).
White, M. C. & Zhao, J. Aliphatic C-H oxidations for late-stage functionalization. J. Am. Chem. Soc. 140, 13988–14009 (2018).
Prakash, G. K. & Hu, J. Selective fluoroalkylations with fluorinated sulfones, sulfoxides, and sulfides. Acc. Chem. Res. 40, 921–930 (2007).
Prakash, G. K., Jog, P. V., Batamack, P. T. & Olah, G. A. Taming of fluoroform: direct nucleophilic trifluoromethylation of Si, B, S, and C centers. Science 338, 1324–1327 (2012).
Meunier, B., de Visser, S. P. & Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem. Rev. 104, 3947–3980 (2004).
Hansch, C. et al. Aromatic substituent constants for structure-activity correlations. J. Med. Chem. 16, 1207–1216 (1973).
Shao, X., Xu, C., Lu, L. & Shen, Q. Shelf-stable electrophilic reagents for trifluoromethylthiolation. Acc. Chem. Res. 48, 1227–1236 (2015).
Tlili, A. & Billard, T. Formation of C-SCF3 bonds through direct trifluoromethylthiolation. Angew. Chem. Int. Ed. 52, 6818–6819 (2013).
Xu, X. H., Matsuzaki, K. & Shibata, N. Synthetic methods for compounds having CF3-S units on carbon by trifluoromethylation, trifluoromethylthiolation, triflylation, and related reactions. Chem. Rev. 115, 731–764 (2015).
Barata-Vallejo, S., Bonesi, S. & Postigo, A. Late stage trifluoromethylthiolation strategies for organic compounds. Org. Biomol. Chem. 14, 7150–7182 (2016).
Zhang, P., Lu, L. & Shen, Q. Recent progress on direct trifluoromethylthiolating reagents and methods. Acta Chim. Sin. 75, 744 (2017).
Chu, L. & Qing, F. L. Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source. Acc. Chem. Res. 47, 1513–1522 (2014).
Hamzehloo, M., Hosseinian, A., Ebrahimiasl, S., Monfared, A. & Vessally, E. Direct C-H trifluoromethylthiolation of (hetero)arenes: a review. J. Fluor. Chem. 224, 52–60 (2019).
Chen, C., Xu, X.-H., Yang, B. & Qing, F.-L. Copper-catalyzed direct trifluoromethylthiolation of benzylic C–H bonds via nondirected oxidative C(sp3)–H activation. Org. Lett. 16, 3372–3375 (2014).
Wu, H. et al. Direct trifluoromethylthiolation of unactivated C(sp3)–H using silver(I) trifluoromethanethiolate and potassium persulfate. Angew. Chem. Int. Ed. 54, 4070–4074 (2015).
Guo, S., Zhang, X. & Tang, P. Silver-mediated oxidative aliphatic C-H trifluoromethylthiolation. Angew. Chem. Int. Ed. 54, 4065–4069 (2015).
Mukherjee, S., Maji, B., Tlahuext-Aca, A. & Glorius, F. Visible-light-promoted activation of unactivated C(sp3)–H bonds and their selective trifluoromethylthiolation. J. Am. Chem. Soc. 138, 16200–16203 (2016).
Luo, Y.-R. in Comprehensive Handbook of Chemical Bond Energies 255–369 (Taylor & Francis, 2007).
McGrath, N. A., Brichacek, M. & Njardarson, J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010).
Paeth, M. et al. Copper-mediated trifluoromethylation of benzylic C(sp3)–H bonds. Chem. Eur. J. 24, 11559–11563 (2018).
Xiao, H. et al. Copper-catalyzed late-stage benzylic C(sp3)–H trifluoromethylation. Chem 5, 940–949 (2019).
Guo, S., AbuSalim, D. I. & Cook, S. P. Aqueous benzylic C-H trifluoromethylation for late-stage functionalization. J. Am. Chem. Soc. 140, 12378–12382 (2018).
Yang, H. D. et al. Silver-promoted oxidative benzylic C-H trifluoromethoxylation. Angew. Chem. Int. Ed. 57, 13266–13270 (2018).
Qin, Y., Zhu, L. & Luo, S. Organocatalysis in inert C-H bond functionalization. Chem. Rev. 117, 9433–9520 (2017).
Recupero, F. & Punta, C. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Chem. Rev. 107, 3800–3842 (2007).
Nicholas, A. Md. P. & Arnold, D. R. Thermochemical parameters for organic radicals and radical ions. Part 1. The estimation of the pKa of radical cations based on thermochemical calculations. Can. J. Chem. 60, 2165–2179 (1982).
Bordwell, F. G. & Cheng, J. P. Radical-cation acidities in solution and in the gas phase. J. Am. Chem. Soc. 111, 1792–1795 (1989).
Wayner, D. D. M. & Parker, V. D. Bond energies in solution from electrode potentials and thermochemical cycles. A simplified and general approach. Acc. Chem. Res. 26, 287–294 (2002).
Ohkubo, K. et al. Simultaneous production of p-tolualdehyde and hydrogen peroxide in photocatalytic oxygenation of p-xylene and reduction of oxygen with 9-mesityl-10-methylacridinium ion derivatives. Chem. Commun. 46, 601–603 (2010).
Pandey, G., Pal, S. & Laha, R. Direct benzylic C-H activation for C-O bond formation by photoredox catalysis. Angew. Chem. Int. Ed. 52, 5146–5149 (2013).
Bloom, S., McCann, M. & Lectka, T. Photocatalyzed benzylic fluorination: shedding “light” on the involvement of electron transfer. Org. Lett. 16, 6338–6341 (2014).
Yi, H., Bian, C. L., Hu, X., Niu, L. B. & Lei, A. W. Visible light mediated efficient oxidative benzylic sp3 C-H to ketone derivatives obtained under mild conditions using O2. Chem. Commun. 51, 14046–14049 (2015).
Liu, H. et al. One-pot photomediated giese reaction/Friedel-Crafts hydroxyalkylation/oxidative aromatization to access naphthalene derivatives from toluenes and enones. ACS Catal. 8, 6224–6229 (2018).
Pandey, G., Laha, R. & Mondal, P. K. Heterocyclization involving benzylic C(sp3)-H functionalization enabled by visible light photoredox catalysis. Chem. Commun. 55, 9689–9692 (2019).
Zhang, P. et al. N-trifluoromethylthio-dibenzenesulfonimide: a shelf-stable, broadly applicable electrophilic trifluoromethylthiolating reagent. J. Org. Chem. 81, 7486–7509 (2016).
Wang, Q., Qi, Z., Xie, F. & Li, X. Lewis acid-catalyzed electrophilic trifluoromethylthiolation of (hetero)arenes. Adv. Synth. Catal. 357, 355–360 (2015).
Xu, W. et al. Synergistic catalysis for the umpolung trifluoromethylthiolation of tertiary ethers. Angew. Chem. Int. Ed. 57, 10357–10361 (2018).
Jiang, L. et al. Direct trifluoromethylthiolation and perfluoroalkylthiolation of C(sp2)–H bonds with CF3SO2Na and RFSO2Na. Angew. Chem. Int. Ed. 54, 14965–14969 (2015).
Honeker, R., Ernst, J. B. & Glorius, F. Transition-metal-free trifluoromethylthiolation of N-heteroarenes. Chem. Eur. J. 21, 8047–8051 (2015).
Fan, X. et al. Neutral-eosin-Y-photocatalyzed silane chlorination using dichloromethane. Angew. Chem. Int. Ed. 58, 12580–12584 (2019).
Deng, H. P., Zhou, Q. & Wu, J. Microtubing-reactor-assisted aliphatic C-H functionalization with HCl as a hydrogen-atom-transfer catalyst precursor in conjunction with an organic photoredox catalyst. Angew. Chem. Int. Ed. 57, 12661–12665 (2018).
Toriyabe, K. et al. Preparation of benzyl sulfides, sulfoxides and sulfones as pesticides. WO 9633168 A1 (1996).
Nicewicz, D., Roth, H. & Romero, N. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2015).
Primer, D. N., Karakaya, I., Tellis, J. C. & Molander, G. A. Single-electron transmetalation: an enabling technology for secondary alkylboron cross-coupling. J. Am. Chem. Soc. 137, 2195–2198 (2015).
Zhou, R., Liu, H., Tao, H., Yu, X. & Wu, J. Metal-free direct alkylation of unfunctionalized allylic/benzylic sp3 C–H bonds via photoredox induced radical cation deprotonation. Chem. Sci. 8, 4654–4659 (2017).
Acknowledgements
We thank the National Natural Science Foundation of China (21971108, 21971111, 21702098, 21732003, and 21672099), the Natural Science Foundation of Jiangsu Province (Grant No. BK20190006), Fundamental Research Funds for the Central Universities (020514380176), “Jiangsu Six Peak Talent Project”, “1000−Youth Talents Plan”, and start-up funds from Nanjing University for financial support. W.X. was supported by the Scientific Research Foundation of Graduate School of Nanjing University (2018CL05). L.T. was supported by the innovation experiment program for undergraduates of Jiangsu province.
Author information
Authors and Affiliations
Contributions
J.X. conceived and supervised the whole project and wrote the manuscript with input from all authors. W.X., C.Z., and J.X. designed and discussed the experiments; W.X., W.W., and T.L. performed and analyzed the experiments.
Corresponding authors
Ethics declarations
Competing interests
J.X., C.Z., and W.X. declare the following competing interests that one patent has been registered (201910828905.0).
Additional information
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, W., Wang, W., Liu, T. et al. Late-stage trifluoromethylthiolation of benzylic C-H bonds. Nat Commun 10, 4867 (2019). https://doi.org/10.1038/s41467-019-12844-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-12844-9
This article is cited by
-
Highly selective single and multiple deuteration of unactivated C(sp3)-H bonds
Nature Communications (2022)
-
Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)-H functionalization
Nature Communications (2021)
-
Benzylic C−H acylation by cooperative NHC and photoredox catalysis
Nature Communications (2021)
-
Efficient access to aliphatic esters by photocatalyzed alkoxycarbonylation of alkenes with alkyloxalyl chlorides
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.