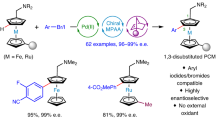
Abstract
Planar chiral ferrocenes have received great attention in both academia and industry. Although remarkable progresses have been made over the past decade, the development of efficient and straightforward methods for the synthesis of enantiopure planar chiral ferrocenes remains highly challenging. Herein, we report a rhodium(I)/phosphonite catalyzed thioketone-directed enantioselective C-H bond arylation of ferrocenes. Readily available aryl iodides are used as the coupling partners in this transformation, leading to a series of planar chiral ferrocenes in good yields and excellent enantioselectivities (up to 86% yield, 99% ee). Of particular note, heteroaryl coupled ferrocenes, which are difficult to access with previous approaches, can be obtained in satisfactory results.
Similar content being viewed by others
Introduction
Transition-metal-catalyzed inert C–H bond direct functionalization has become a powerful tool in modern synthetic chemistry1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17. One of the remained challenges for the widespread application of C–H bond functionalization is how to control the regioselectivity and stereoselectivity. Up to now, extensive attention has been paid on this field of research and significant results have been achieved18,19,20,21,22. Recently, we developed a Pd(II)-catalyzed C–H direct arylation of ferrocenes with thioketones as effective directing groups23. The importance of planar chiral ferrocenes24,25,26,27 prompted us to explore a corresponding enantioselective arylation reaction. In this regard, Pd(II)/monoprotected amino acid (MPAA) catalytic system28,29,30,31,32,33,34,35,36, established by Yu and coworkers, was firstly examined as our initial attempts for this asymmetric C–H bond arylation. The desired product could be obtained but without any enantioselectivity. Chiral phosphate anion as a counteranion with palladium has also been demonstrated as an effective catalytic system in a number of asymmetric C–H bond functionalization reactions37,38,39,40,41. Unfortunately, no positive results on enantioselective control were obtained when various chiral phosphoric acids (CPAs) were introduced (Fig. 1a). Therefore, the development of enantioselective C–H arylation reaction to provide structurally diverse planar chiral ferrocenes is highly desirable42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61.
Recently, Rh(I)-catalyzed enantioselective C–H functionalizations progressed rapidly. In 1997, Murai and coworkers reported Rh(I)-catalyzed asymmetric C–H/olefin coupling reaction with modest enantioselectivity62. Later, Ellman, Bergman and coworkers achieved a remarkable progress on Rh(I)-catalyzed enantioselective intramolecular C–H alkylation reaction, which gave cyclic products with up to 96% ee63. Several elegant Rh(I)-catalyzed asymmetric intramolecular dehydrogenative silylations have been demonstrated by the groups of Takai64,65, He66, and Shibata67, respectively. Notably, Glorius and coworkers have made breakthroughs that the combination of a rhodium(I) precatalyst with chiral N-heterocyclic carbene (NHC) or monodentate phosphonite ligand enabled highly enantioselective Csp3–H arylations68,69.
Inspired by these pioneering results, we explore Rh(I) catalyzed enantioselective arylation of thiocarbonylferrocenes (Fig. 1b) after achieving the synthesis of axially chiral heterobiaryls via isoquinoline directed C-H functionalization70. In the presence of Rh(I) catalyst derived from a chiral phosphonite, direct arylation of thioketone substituted ferrocenes with readily available aryl iodides proceeds in excellent enantioselectivity. Herein, we report the results of this study.
Results
Optimization of reaction conditions
We began our initial attempts by utilizing chiral diphosphines L1-2 (BINAP, DIOP) as the ligand. However, no product was observed when thiocarbonylferrocene 1a was treated with 1.1 equiv of iodobenzene 2a in the presence of 5 mol% [Rh(C2H4)2Cl]2 and 3.0 equiv of LiOtBu in THF at 80 °C (Table 1, entries 1–2). When Feringa ligand L3 was used, to our delight, planar chiral product (3a) was obtained in 19% yield with 88% ee (Table 1, entry 3). Stereochemical induction is predominantly determined by the BINOL backbone of the phosphoramidite ligand by comparing the results of that of diastereomeric ligand L4 (Table 1, entry 4, 15% yield, −71% ee). The utilization of TADDOL-derived phosphoramidite L5 could further increase the yield to 29% (Table 1, entry 5). Notably, 3a was isolated in 46% yield and 90% ee when TADDOL-derived phosphonite L7, introduced by the Glorius group69, was used (Table 1, entry 7). Promoted by these encouraging results, we further investigated other TADDOL-derived phosphonite ligands (Table 1, entries 8–13). It was found that the chiral backbone assembled with 2-naphthyl groups delivered 3a in 26% yield with 91% ee (Table 1, entry 8). Further evaluation of substituents [Ar = 3,5-(CF3)2C6H3 and 3,5-(tBu)2C6H3] on the TADDOL backbone revealed that both ligands could promote the reaction (33% and 44% yield, respectively) albeit with decrease of enantioselectivity (Table 1, entries 9–10). Ligand L11 bearing a tbutyl group could not promote this reaction (Table 1, entry 11). On the other hand, varying the aromatic moiety on the P atom afforded product 3a with comparative results (45–46% yields, 90% ee) as those of L7 (Table 1, entries 12–13). Thus, the easily accessible ligand L7 was chosen as the optimal one. The addition of molecular sieves was found to increase the yield of 3a (Table 1, entry 14, 59% yield with 92% ee). Increasing the amount of iodobenzene 2a to 1.3 equivalents also has a positive influence on the reaction outcome (Table 1, entry 15, 78% yield, 94% ee). Other molecular sieves did not give a noticeable improvement of the yield. Various solvents were next screened, and it was found that the reaction in dioxane afforded 3a in 76% yield with 97% ee (Table 1, entry 18, see the Supplementary Table 1 for complete optimization). The ratio between Rh(I) and ligand had a profound effect on the results. The use of 20 mol% of L7 ([Rh]/L = 1/2) provided 3a without erosion in term of yield and enantioselectivity (Table 1, entry 17, 75% yield, 97% ee). The loading of L7 could be further reduced to 15 mol % (Table 1, entry 18, [Rh]/L = 1/1.5), 76% 97% ee). However, both yield and enantioselectivity were decreased when 10 mol% of L7 ([Rh]/L = 1/1) was used (Table 1, entry 19).
Substrate scope
Under the above optimized reaction conditions [1a (0.2 mmol), 2a (0.26 mmol), [Rh(C2H4)2Cl]2 (5 mol%), L7 (15 mol%), LiOtBu (0.6 mmol) and 3 Å MS (100 mg) in dioxane at 80 °C], C–H arylation reactions of thiocarbonylferrocene 1a with various aryl iodides were investigated (Table 2). Notably, the absolute configuration of product 3a (99% ee after recrystallization) was determined by X-ray crystallographic diffraction as Sp (see the Supplementary Table 2 for details). Both electron-donating groups (such as methyl, methoxy), halogen groups (F, Cl, and Br) and electron-withdrawing groups (cyano and trifluoromethyl) on the phenyl ring were all well tolerated, and the desired products were delivered in good yields with good to excellent enantioselectivity (3b–h, 58–82% yields, 89–95% ee). Delightedly, the reaction of 1-bromo-4-iodobenzene 2f afforded product 3f in 68% yield with 90% ee, leaving the Br atom intact. Meta- and ortho-substituted aryl iodides 1i, 1j, and 1k also proceeded smoothly to give the arylated products 3i–k in 70–80% yields with 91–97% ee. 2-Iodonaphthalene was found to be a suitable substrate, and 3l was obtained in 62% yield with 97% ee. Notably, heteroaryl iodides such as benzofuranyl, thienyl and pyridyl iodides were found to be effective coupling partners, providing the desired products 3m–q in 53–75% yields with 74–94% ee. However, 30 mol% of L7 was required to obtain reasonable yields for thienyl and pyridyl iodides. This is likely due to their relatively strong coordination with rhodium. This asymmetric C–H arylation reaction is also compatible with other thiocarbonylferrocenes such as 1r and 1s, leading to the formation of 3r and 3s in 65–76% yields with 99% ee.
To demonstrate the potential utility of such an asymmetric C–H bond arylation reaction, a gram-scale reaction was performed. Thioketone 1a (5 mmol) could be efficiently converted into 3a (1.56 grams) with 96% ee in the presence of 2.5 mol% [Rh(C2H4)2Cl]2 and 7.5 mol% L7 (Fig. 2a). Furthermore, planar chiral ferrocenyl ketone 4 was obtained in 93% yield without erosion of enantioselectivity by the treatment with Ag2CO3 under mild conditions (Fig. 2b).
Mechanistic studies
To gain insights into this enantioselective C–H arylation reaction of ferrocenes, several control and deuteration experiments were carried out. No desired arylation product was observed when ketone substituted ferrocene 1a’ was subjected to the standard conditions, which indicated the unique role of thioketone group in the C–H bond activation process (Fig. 3a). 3a was obtained in 25% yield with 95% ee when bromobenzene was used as a coupling partner (Fig. 3b). The competition experiment between 4-iodoanisole 2c and 4-iodobenzonitrile 2g revealed that electron-deficient substrate was more reactive than the electron-rich one (Fig. 3c). Significant deuteration was observed for the recovered starting material 1a when the C–H arylation reaction was performed in the presence of 20 equiv of CD3OD (Fig. 3d). More importantly, we attempted this H/D exchange experiment in the absence of iodobenzene, which led to the recovered 1a with 70% deuterium (Fig. 3e). These results indicate that the C–H cleavage of thioketone substituted ferrocene is reversible and may be not the rate-determining step.
Discussion
In conclusion, we have developed an efficient thioketone-directed Rh(I)-catalyzed enantioselective C–H bond arylation of ferrocenes. In the presence of Rh(I) catalyst derived from [Rh(C2H4)2Cl]2 and a TADDOL-based chiral phosphonite ligand, various aryl iodides reacted with ferrocenyl thioketones smoothly in good yields. The reactions displayed a broad substrate scope, and the planar chiral ferrocenes were obtained in excellent enantioselectivity. Heteroaryl iodides also work well in this catalytic system, affording heteroaryl substituted ferrocenes, which are difficult to be obtained by the previously known methods. Further mechanistic studies and applications of the products are ongoing in our laboratory.
Methods
Representative procedure
3 Å MS (100 mg) was added to a dry Schlenk tube (25 mL). The flask was evacuated and backfilled with argon for 3 times. Then, LiOtBu (0.6 mmol, 48.1 mg), L7 (0.03 mmol, 17.2 mg), [Rh(C2H4)2Cl]2 (0.01 mmol, 3.9 mg), and 1 (0.2 mmol) were added to the Schlenk tube. The flask was evacuated and backfilled with argon for 3 times again, and followed by addition of dioxane (1.5 mL). Aryl iodide 2 (0.26 mmol, 1.3 equiv.) was added at last. The mixture was stirred at 80 °C. After the reaction was complete (monitored by TLC), the mixture was cooled to room temperature. The mixture was diluted with petroleum ether (~10 mL). Then, silica gel was added and the solvent was evaporated under reduced pressure. The product was isolated by sicila gel column chromatography (petroleum ether or petroleum ether/ethyl acetate = 20/1).
Data availability
The X-ray crystallographic coordinates for product 3a have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code 1894751. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this work, including experimental procedures and compound characterization data, are available within the article and its Supplementary Information files.
References
Yu, J.-Q. & Shi, Z. (eds.). C–H activation. In Topics in Current Chem istry, Vol. 292 (Springer, 2010).
Ding, K. & Dai, L.-X. (eds.). Transition metal-catalyzed C-H functionalization: synthetically enabling reactions for building molecular complexity. In Organic Chemistry-Breakthroughs and Perspectives (Wiley-VCH, 2012).
Herrerias, C. I., Yao, X., Li, Z. & Li, C.-J. Reactions of C−H bonds in water. Chem. Rev. 107, 2546–2562 (2007).
Díaz-Requejo, M. M. & Perez, P. J. Coinage metal catalyzed C−H bond functionalization of hydrocarbons. Chem. Rev. 108, 3379–3394 (2008).
Bellina, F. & Rossi, R. Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C−H bonds and some of their synthetic equivalents with aryl halides and pseudohalides. Chem. Rev. 110, 1082–1146 (2009).
Mkhalid, I. A., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C−H activation for the construction of C−B bonds. Chem. Rev. 110, 890–931 (2009).
Balcells, D., Clot, E. & Eisenstein, O. C-H bond activation in transition metal species from a computational perspective. Chem. Rev. 110, 749–823 (2010).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C−C bond formation via heteroatom-directed C−H bond activation. Chem. Rev. 110, 624–655 (2009).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Yeung, C. S. & Dong, V. M. Catalytic dehydrogenative cross-coupling: forming carbon−carbon bonds by oxidizing two carbon− hydrogen bonds. Chem. Rev. 111, 1215–1292 (2011).
Sun, C.-L., Li, B.-J. & Shi, Z.-J. Direct C−H transformation via iron catalysis. Chem. Rev. 111, 1293–1314 (2010).
Ackermann, L. Carboxylate-assisted ruthenium-catalyzed alkyne annulations by C–H/Het–H bond functionalizations. Acc. Chem. Res. 47, 281–295 (2013).
Liu, C. et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 115, 12138–12204 (2015).
Daugulis, O., Roane, J. & Tran, L. D. Bidentate, monoanionic auxiliary-directed functionalization of carbon–hydrogen bonds. Acc. Chem. Res. 48, 1053–1064 (2015).
Song, G. & Li, X. Substrate activation strategies in rhodium (III)-catalyzed selective functionalization of arenes. Acc. Chem. Res. 48, 1007–1020 (2015).
He, G., Wang, B., Nack, W. A. & Chen, G. Syntheses and transformations of α-amino acids via palladium-catalyzed auxiliary-directed sp3 C–H functionalization. Acc. Chem. Res. 49, 635–645 (2016).
Baudoin, O. Ring construction by palladium (0)-catalyzed C (sp3)–H activation. Acc. Chem. Res. 50, 1114–1123 (2017).
You, S.-L. (ed.). Asymmetric functionalization of C-H bonds (RSC, Cambridge, UK, 2015).
Zheng, C. & You, S.-L. Recent development of direct asymmetric functionalization of inert C–H bonds. RSC. Adv. 4, 6173–6214 (2014).
Newton, C. G., Wang, S.-G., Oliveira, C. C. & Cramer, N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem. Rev. 117, 8908–8976 (2017).
He, J., Wasa, M., Chan, K. S., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2016).
Saint-Denis, T. G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C (sp3)‒H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Cai, Z.-J., Liu, C.-X., Gu, Q. & You, S.-L. Thioketone‐directed palladium (II)-catalyzed C−H arylation of ferrocenes with aryl boronic acids. Angew. Chem. Int. Ed. 57, 1296–1299 (2018).
Hayashi, T. & Togni, A. (eds.). Ferrocenes (VCH, Weinheim, Germany 1995).
Togni, A. & Haltermann, R. L. (eds.). Metallocenes (VCH, Weinheim, Germany 1998).
Štěpnička, P. (ed.) Ferrocenes (Wiley, Chichester 2008).
Dai, L.-X. & Hou, X.-L. (eds.). Chiral Ferrocenes in Asymmetric Catalysis (Wiley, 2010).
Schaarschmidt, D. & Lang, H. Selective syntheses of planar-chiral ferrocenes. Organometallics 32, 5668–5704 (2013).
Shi, B.-F., Maugel, N., Zhang, Y.-H. & Yu, J.-Q. Pd (II)-catalyzed enantioselective activation of C(sp2)-H and C(sp3)-H bonds using monoprotected amino acids as chiral ligands. Angew. Chem. Int. Ed. 47, 4882–4886 (2008).
Shi, B.-F., Zhang, Y.-H., Lam, J. K., Wang, D.-H. & Yu, J.-Q. Pd (II)-catalyzed enantioselective C−H olefination of diphenylacetic acids. J. Am. Chem. Soc. 132, 460–461 (2009).
Wasa, M., Engle, K. M., Lin, D. W., Yoo, E. J. & Yu, J.-Q. Pd (II)-catalyzed enantioselective C–H activation of cyclopropanes. J. Am. Chem. Soc. 133, 19598–19601 (2011).
Musaev, D. G., Kaledin, A., Shi, B. F. & Yu, J.-Q. Key mechanistic features of enantioselective C–H bond activation reactions catalyzed by [(chiral mono-N-protected amino acid)–Pd (II)] complexes. J. Am. Chem. Soc. 134, 1690–1698 (2012).
Chu, L., Wang, X.-C., Moore, C. E., Rheingold, A. L. & Yu, J.-Q. Pd-catalyzed enantioselective C–H iodination: asymmetric synthesis of chiral diarylmethylamines. J. Am. Chem. Soc. 135, 16344–16347 (2013).
Cheng, X.-F. et al. Pd (II)-catalyzed enantioselective C–H activation/C–O bond formation: synthesis of chiral benzofuranones. J. Am. Chem. Soc. 135, 1236–1239 (2013).
Chu, L., Xiao, K.-J. & Yu, J.-Q. Room-temperature enantioselective C–H iodination via kinetic resolution. Science 346, 451–455 (2014).
Chan, K. S., Fu, H.-Y. & Yu, J.-Q. Palladium (II)-catalyzed highly enantioselective C–H arylation of cyclopropylmethylamines. J. Am. Chem. Soc. 137, 2042–2046 (2015).
Yan, S. B., Zhang, S. & Duan, W.-L. Palladium-catalyzed asymmetric arylation of C (sp3)–H bonds of aliphatic amides: Controlling enantioselectivity using chiral phosphoric amides/acids. Org. Lett. 17, 2458–2461 (2015).
Wang, H., Tong, H. R., He, G. & Chen, G. An enantioselective bidentate auxiliary directed palladium‐catalyzed benzylic C−H arylation of amines using a BINOL phosphate ligand. Angew. Chem. Int. Ed. 55, 15387–15391 (2016).
Jain, P., Verma, P., Xia, G. & Yu, J.-Q. Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nat. Chem. 9, 140–144 (2017).
Fukagawa, S. et al. Enantioselective C(sp3)-H amidation of thioamides catalyzed by a cobaltIII/chiral carboxylic acid hybrid system. Angew. Chem. Int. Ed. 58, 1153–1157 (2019).
Yan, S.-Y. et al. Palladium (II)‐catalyzed enantioselective arylation of unbiased methylene C(sp3)−H bonds enabled by a 2-pyridinylisopropyl auxiliary and chiral phosphoric acids. Angew. Chem. Int. Ed. 57, 9093–9097 (2018).
Zhu, D.-Y., Chen, P. & Xia, J.-B. Synthesis of planar chiral ferrocenes by transition-metal-catalyzed enantioselective C−H activation. ChemCatChem 8, 68–73 (2016).
Gao, D.-W., Gu, Q., Zheng, C. & You, S.-L. Synthesis of planar chiral ferrocenes via transition-metal-catalyzed direct C–H bond functionalization. Acc. Chem. Res. 50, 351–365 (2017).
Huang, J.-P., Gu, Q. & You, S.-L. Synthesis of planar chiral ferrocenes via transition-metal-catalyzed direct C-H bond functionalization. Chin. J. Org. Chem. 38, 51–61 (2018).
Gao, D.-W., Shi, Y.-C., Gu, Q., Zhao, Z. L. & You, S.-L. Enantioselective synthesis of planar chiral ferrocenes via palladium-catalyzed direct coupling with arylboronic acids. J. Am. Chem. Soc. 135, 86–89 (2012).
Pi, C. et al. Redox of ferrocene controlled asymmetric dehydrogenative heck reaction via palladium-catalyzed dual C–H bond activation. Chem. Sci. 4, 2675–2679 (2013).
Gao, D.-W., Yin, Q., Gu, Q. & You, S.-L. Enantioselective synthesis of planar chiral ferrocenes via Pd(0)-catalyzed intramolecular direct C-H bond arylation. J. Am. Chem. Soc. 136, 4841–4844 (2014).
Deng, R. et al. Palladium-catalyzed intramolecular asymmetric C–H functionalization/cyclization reaction of metallocenes: an efficient approach toward the synthesis of planar chiral metallocene compounds. J. Am. Chem. Soc. 136, 4472–4475 (2014).
Pi, C. et al. Synthesis of ferrocene derivatives with planar chirality via palladium-catalyzed enantioselective C–H bond activation. Org. Lett. 16, 5164–5167 (2014).
Liu, L. et al. Asymmetric synthesis of planar chiral ferrocenes by enantioselective intramolecular C–H arylation of N-(2-Haloaryl) ferrocenecarboxamides. Org. Lett. 16, 5336–5338 (2014).
Shibata, T. & Shizuno, T. Iridium‐catalyzed enantioselective C-H alkylation of ferrocenes with alkenes using chiral diene ligands. Angew. Chem. Int. Ed. 53, 5410–5413 (2014).
Gao, D.-W., Gu, Q. & You, S.-L. An enantioselective oxidative C–H/C–H cross-coupling reaction: highly efficient method to prepare planar chiral ferrocenes. J. Am. Chem. Soc. 138, 2544–2547 (2016).
Zhang, S., Lu, J., Ye, J. & Duan, W.-L. Asymmetric C-H arylation for the synthesis of planar chiral ferrocenes: controlling enantioselectivity using chiral phosphoric acids. Chin. J. Org. Chem. 36, 752–759 (2016).
Schmiel, D. & Butenschön, H. Directed iron-catalyzed ortho-alkylation and arylation: toward the stereoselective catalytic synthesis of 1, 2-disubstituted planar-chiral ferrocene derivatives. Organometallics 36, 4979–4989 (2017).
Luo, S., Xiong, Z., Lu, Y. & Zhu, Q. Enantioselective synthesis of planar chiral pyridoferrocenes via palladium-catalyzed imidoylative cyclization reactions. Org. Lett. 20, 1837–1840 (2018).
Xu, J., Liu, Y., Zhang, J., Xu, X. & Jin, Z. Palladium-catalyzed enantioselective C(sp2)–H arylation of ferrocenyl ketones enabled by a chiral transient directing group. Chem. Commun. 54, 689–692 (2018).
Zhao, W.-T., Lu, Z.-Q., Zheng, H., Xue, X.-S. & Zhao, D. Rhodium-catalyzed 2-arylphenol-derived six-membered silacyclization: straightforward access toward dibenzooxasilines and silicon-containing planar chiral metallocenes. ACS Catal. 8, 7997–8005 (2018).
Xu, B.-B., Ye, J., Yuan, Y. & Duan, W.-L. Palladium-catalyzed asymmetric C–H arylation for the synthesis of planar chiral benzothiophene-fused ferrocenes. ACS Catal. 8, 11735–11740 (2018).
Kong, W.-J. et al. Copper-mediated diastereoselective C–H thiolation of ferrocenes. Organometallics 37, 2832–2836 (2018).
Cai, Z.-J., Liu, C.-X., Gu, Q., Zheng, C. & You, S.-L. PdII‐catalyzed regio‐and enantioselective oxidative C−H/C−H cross‐coupling reaction between ferrocenes and azoles. Angew. Chem. Int. Ed. 131, 2171–2175 (2019).
Yetra, S. R. et al. Micellar catalysis for ruthenium(II)-catalyzed C−H arylation: weak-coordination-enabled C−H activation in H2O. Angew. Chem. Int. Ed. 58, 7490–7494 (2019).
Fujii, N., Kakiuchi, F., Yamada, A., Chatani, N. & Murai, S. Asymmetric intramolecular C-H/olefin coupling: Asymmetric cyclization reactions of 1, 5-dienes catalyzed by rhodium complexes. Chem. Lett. 26, 425–426 (1997).
Thalji, R. K., Ellman, J. A. & Bergman, R. G. Highly efficient and enantioselective cyclization of aromatic imines via directed C− H bond activation. J. Am. Chem. Soc. 126, 7192–7193 (2004).
Kuninobu, Y., Yamauchi, K., Tamura, N., Seiki, T. & Takai, K. Rhodium‐catalyzed asymmetric synthesis of spirosilabifluorene derivatives. Angew. Chem. Int. Ed. 52, 1520–1522 (2013).
Murai, M., Matsumoto, K., Takeuchi, Y. & Takai, K. Rhodium-catalyzed synthesis of benzosilolometallocenes via the dehydrogenative silylation of C(sp2)–H bonds. Org. Lett. 17, 3102–3105 (2015).
Zhang, Q.-W., An, K., Liu, L.-C., Yue, Y. & He, W. Rhodium‐catalyzed enantioselective intramolecular C-H silylation for the syntheses of planar‐chiral metallocene siloles. Angew. Chem. Int. Ed. 54, 6918–6921 (2015).
Shibata, T., Shizuno, T. & Sasaki, T. Enantioselective synthesis of planar-chiral benzosiloloferrocenes by Rh-catalyzed intramolecular C–H silylation. Chem. Commun. 51, 7802–7804 (2015).
Kim, J.-H., Greßies, S., Boultadakis-Arapinis, M., Daniliuc, C. & Glorius, F. Rh(I)/NHC*-catalyzed site-and enantioselective functionalization of C(sp3)–H bonds toward chiral triarylmethanes. ACS Catal. 6, 7652–7656 (2016).
Greßies, S., Klauck, F. J. R., Kim, J. H., Daniliuc, C. G. & Glorius, F. Ligand‐enabled enantioselective C–H activation of tetrahydroquinolines and saturated Aza‐heterocycles by RhI. Angew. Chem. Int. Ed. 57, 9950–9954 (2018).
Wang, Q., Cai, Z.-J., Liu, C.-X., Gu, Q. & You, S.-L. Rhodium-catalyzed atroposelective C–H arylation: efficient synthesis of axially chiral heterobiaryls. J. Am. Chem. Soc. 141, 9504–9510 (2019).
Acknowledgements
We thank the National Key R&D Program of China (2016YFA0202900), National Natural Science Foundation of China (21821002, 91856201, and 21572250), the CAS (XDB20000000 and QYZDY-SSW-SLH012) and Science and Technology Commission of Shanghai Municipality (18JC1411302) for generous financial support.
Author information
Authors and Affiliations
Contributions
S.L.Y. came up with the original idea. Z.J.C., C.X.L., and Q.W. performed the experiments and analyzed the data. Z.J.C., Q.G., and S.L.Y. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Takanori Shibata and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cai, ZJ., Liu, CX., Wang, Q. et al. Thioketone-directed rhodium(I) catalyzed enantioselective C-H bond arylation of ferrocenes. Nat Commun 10, 4168 (2019). https://doi.org/10.1038/s41467-019-12181-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-12181-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.