Abstract
Ocean acidification is expected to negatively impact calcifying organisms, yet we lack understanding of their acclimation potential in the natural environment. Here we measured geochemical proxies (δ11B and B/Ca) in Porites astreoides corals that have been growing for their entire life under low aragonite saturation (Ωsw: 0.77–1.85). This allowed us to assess the ability of these corals to manipulate the chemical conditions at the site of calcification (Ωcf), and hence their potential to acclimate to changing Ωsw. We show that lifelong exposure to low Ωsw did not enable the corals to acclimate and reach similar Ωcf as corals grown under ambient conditions. The lower Ωcf at the site of calcification can explain a large proportion of the decreasing P. astreoides calcification rates at low Ωsw. The naturally elevated seawater dissolved inorganic carbon concentration at this study site shed light on how different carbonate chemistry parameters affect calcification conditions in corals.
Similar content being viewed by others
Introduction
Ocean acidification is projected to lead to negative effects on calcifying organisms, particularly tropical corals1,2,3. Our understanding of the potential fate of corals in the face of changing pCO2 in the ocean is based primarily on controlled laboratory studies (e.g. refs. 4,5), mesocosm studies mimicking coral community composition6,7,8, alkalinisation versus carbon dioxide-enrichment studies in natural coral reef sites9,10, and a number of field studies with naturally reduced calcium carbonate saturation state (Ωarag)1,11,12,13,14. These efforts have provided strong evidence that the calcification rates of a large number of coral species investigated to date will decline in response to projected pCO215. However, some studies also report that certain coral species were able to maintain high calcification rates or even benefit from elevated pCO21,16,17,18, suggesting a high resilience potential of some coral species to changing carbonate chemistry19. Specifically, the ability of an organism to control the biomineralization process clearly determines its ecological and physiological success under reduced pH conditions14. The process of calcification in corals is linked to their ability to control the pH at the site of calcification (pHcf) by removing protons out of the calicoblastic space between the tissue and skeleton, where calcification takes place5. This enables corals to sustain pHcf well above seawater pH (pHSW)5,19,20. The physiological capacity of corals to control pHcf may alleviate the decline in coral growth and increase coral resilience to future climate change19. Knowledge about internal calcifying fluid pHcf in corals has been derived from a few direct measurements under the calcifying cell layer either using microsensors21,22 or pH-sensitive dyes5,23. These studies confirmed an elevated pHcf of between 0.4 and 2 pH units above ambient seawater in the calicoblastic space. Indirectly, boron isotopes (δ11B) of coral skeletons, which represent the pHcf of the calcifying solution, also suggest an elevated pHcf (e.g. refs. 19,24,25,26). Boron isotopes are more readily accessible compared to direct measurements and have the additional benefit that they integrate pHcf history over longer time periods19,20,24,27. Studies suggest that pHsw is an important driver affecting pHcf 25,28. However, it was recently demonstrated that changes in seawater dissolved inorganic carbon (DIC) or total alkalinity (TA) can also affect pHcf regulation23. Using B/Ca as a proxy for internal carbonate ion concentration (CO32−cf), provided geochemical evidence that corals can also modulate and adjust the internal DIC (DICcf) concentration. Together—the potential to upregulate DICcf and pHcf—allows for higher carbonate ion concentrations at the site of calcification and hence a higher Ωcf that facilitates calcification29,30.
Over the last decade, a growing body of literature has provided evidence that corals subjected to daily and seasonally fluctuating environmental conditions are able to exert a stronger control over their internal physiological attributes, potentially allowing them to better cope with future changes (reviewed in ref. 31). For instance, in situ flume experiments mimicking natural (daily, seasonal) fluctuating conditions coupled with future pCO2 conditions showed that corals from acidified treatments could maintain a constant calcification pHcf irrespective of changes in seawater pHsw27. The authors argued that the fluctuating conditions the corals were exposed to likely favour this strong control on internal conditions. That year long experiment, however, cannot tell whether corals can maintain such strong control when exposed to reduced mean seawater aragonite saturation state (Ωsw) for their entire life span. Corals living for their entire life under continuously low Ωsw and variable environmental conditions can be used to test whether corals can maintain pHcf homoeostasis over long time spans in their natural ecosystem with its complex biological interactions. Because many natural ocean acidification sites also show strongly fluctuating conditions11,32,33 these settings may be ideal for testing the relationship between environmental variability and acclimation potential of corals to low Ωsw34.
Here we measured geochemical proxies in the upper most recently formed skeletal parts of Porites astreoides corals that were collected along a natural aragonite saturation gradient at submarine springs (locally known as ojos) in Puerto Morelos, Mexico11. These geochemical proxies (δ11B-derived estimates of pHcf and B/Ca derived estimates of CO32−cf) allowed us to infer carbonate chemistry conditions at the site of calcification, which provide valuable new insights into the internal calcification regulation mechanisms in corals exposed to persistent low Ωsw, as well as fluctuating carbonate chemistry conditions33. Our results, combined with bio-inorganic calcification models19,30, identified critical regulation mechanisms and the inability of corals to fully acclimate to these conditions and sufficiently elevate their Ωcf to sustain growth rates similar to the same species of corals growing at ambient Ωsw.
Results
Natural conditions at the ojos
We used 12 cores from the coral P. astreoides: 5 cores collected from the centre of the low Ωsw ojos and 7 from control present-day Ωsw sites adjacent (within a few meters) to the ojos11. Porites astreoides, the species used in this study, represents one of only three calcifying coral species found growing within the discharge impacted area, while nine coral species are found nearby under ambient present-day Ωsw. Previous studies indicate that although the abundance of P. astreoides was not significantly reduced at the low Ωsw ojos, its growth rate (measured as net calcification) decreased significantly by 37% compared to the same species collected at control sites11. The control sites have a relatively consistent Ωsw (on average: 3.92 ± 0.03 sd) year round compared to the ojos where Ωsw is always <2 and ranges from 0.77 to 1.85 (on average: 1.49 ± 0.14 sd, Supplementary Table 1 11).
Skeletal δ11B and thus pHcf as a function of Ωsw
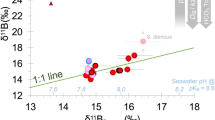
The δ11B in the 12 corals analysed ranged from 23.1‰ to 27.6‰, with slight but significantly lower values for corals affected by the ojo discharge where Ωsw was low (Fig. 1a, Supplementary Table 2; t-test: p = 0.022; r2 = 0.29, p = 0.04). These δ11B values translate into pHcf that are slightly lower (but not statistically significant) in the corals from sites with Ωsw < 2 with an average internal pHcf of 8.46 (±0.03 sem) compared to 8.54 (±0.01 sem) at the control sites (t-test: p = 0.085, Fig. 1b, Supplementary Table 2). The pHcf difference between the corals is relatively small (0.08 pH units) compared to the difference in environmental seawater pHsw of ~0.54 pH units between the sites. Hence, compared to pHsw in their surrounding environment, corals at the ojo centres maintained a higher pH gradient between seawater and the calcifying fluid (∆pH) in comparison to corals at control sites (Fig. 1c, Supplementary Table 2; t-test: p = 0.002, r2 = 0.89, p < 0.001).
Porites astreoides internal pHcf regulation based on skeletal proxies. Coral skeletal δ11B signature a from naturally different seawater aragonite saturation state (Ωsw) sites were translated into b internal calcifying fluid pH (pHcf) and c pH up-regulation intensity (∆pH). Circles represent values for each individual coral colony (mean ± confidence interval). Filled and non-filled symbols denotes the different locations: filled are the centres of the ojos with lower Ωsw and non-filled the control high Ωsw site. Dashed line represents regression line for site-specific significant different values in δ11B and ∆pH with Ωsw (radj2 = 0.29, p = 0.04, radj2 = 0.88, p < 0.001, respectively) and grey area denotes the 95% confidence band. Individual values are mean ± 95%-confidence interval
Skeletal B/Ca, thus DICcf and CO3 2− cf as a function of Ωsw
Changes in coral skeletal B/Ca were determined along with δ11B. This ratio varied between 442 and 721 µmol mol−1 and did not significantly correspond to Ωsw (Fig. 2a, p = 0.86). Using the δ11B and B/Ca skeletal proxies together to constrain the carbonate system at the site of calcification suggests an elevation of CO32−cf not only due to shifts in internal pHcf but also due to an increase in DICcf (Supplementary Table 2, Fig. 2b). The ratios of DICcf/DICsw —a measure of the upregulation of DICcf compared to seawater—were significantly higher at the control sites than at the low Ωsw sites (Fig. 2c, Supplementary Table 2; p = 0.036, r2 = 0.33, p = 0.029). This is mainly due to the higher than ambient DICsw (Supplementary Table 1) at the ojos because the DICcf did not vary significantly between the sites (Supplementary Fig. 1, Supplementary Table 2; p = 0.85).
Porites astreoides internal CO32− and DICcf based on skeletal proxies. Coral skeletal B/Ca ratio a from naturally different seawater aragonite saturation state (Ωsw) sites were translated into internal calcifying carbonate ion concentration (CO32-) values b and dissolved inorganic carbon (DICcf) concentration, as well as upregulation compared to seawater (DICcf/DICsw) (c, d respectively). Circles represent values for each individual coral colony (mean ± confidence interval). Filled and non-filled symbols denotes the different locations: filled are the centres of the ojos with lower Ωsw and non-filled the control high Ωsw site. Triangles in c represent seawater DICsw concentrations. Dashed line represents regression line for site-specific significant different values for DICcf/DICsw with Ωsw (radj2 = 0.33, p = 0.029) and grey area denotes the 95% confidence band. Individual values are mean ± 95%-confidence interval
Changes in calcification conditions and calcification rates
We applied a bio-inorganic model (IpHRAC model from McCulloch and colleagues19, see the “Methods” section) to calculate average Ωcf and associated relative calcification rates using our proxy data. We used the combined pHcf and the B/Ca-derived DICcf concentration at the site of calcification for corals from both ojo and control sites. With this bio-inorganic model both Ωcf (from 16.1 ± 0.3 sem to 13.1 ± 0.7 sem, Fig. 3a; p = 0.042) and calcification rates (from 1.0 ± 0.09 sem to 0.54 ± 0.09 sem; p = 0.036) decreased with decreasing Ωsw and correlated well with the observed calcification response previously reported for these corals11 (Fig. 3b). 41% of the variation in measured net calcification rates can be explained by internal changes in Ωcf derived from the geochemically determined calcifying conditions (Fig. 4a; r2adj = 0.41, p = 0.011). The internal calcifying fluid parameters are clearly distinct between corals from the different field sites, strongly indicating a combined effect of DICcf and pHcf modulation on P. astreoides calcification performance (Fig. 4b).
Growth response of Porites astreoides corals. The modelled growth response displays relative changes in calcification rate (relative calcification rate = mean control/individual colony). Calcification rates were calculated following the IpHRAC model19 (internal pH regulation and abiotic calcification: Calcification = k*(Ωcf−1)n) with calcifying fluid aragonite saturation state (Ωcf) was calculated from the average internal calcifying fluid pH (pHcf) of individual colonies and the dissolved inorganic carbon (DICcf): in a dependent variable Ωcf is based on DICcf and pHcf and b depicts the respective calculated calcification rates. Circles represent values for each individual coral colony (mean ± confidence interval). Filled and non-filled symbols denotes the different locations: filled are the centres of the ojos with lower seawater aragonite saturation state (Ωsw) and non-filled the control high Ωsw site. We compared calculated values with measured data11 (for better comparison also calculated as relative rate, open triangles). Individual values are mean ± 95%-confidence interval
Internal calcification conditions in Porites astreoides. a Calcifying conditions were used to calculate aragonite saturation state at the site of calcification (Ωcf) and related to the measured growth rate of the individual corals11. b Calcification conditions as internal calcifying fluid pH (pHcf) and dissolved inorganic carbon ratio of calcifying fluid to seawater (DICcf/DICsw) was determined for 12 Porites astroides coral colonies collected from sites with naturally different seawater aragonite saturation state (Ωsw). Circles represent values for each individual coral colony (mean ± confidence interval). Filled and non-filled symbols denotes the different locations: filled are the centres of the ojos with lower Ωsw and non-filled the control high Ωsw site. Dashed line represents regression line for relative calcification rate with Ωcf (radj2 = 0.41, p = 0.015) and grey area denotes the 95% confidence band. Individual values are mean ± 95%-confidence interval
Discussion
Coral calcification is one of the most fundamental processes in reef ecosystems and is essential for reef accretion and ecosystem diversity; however, calcification may be impacted by changes in seawater carbonate chemistry. Although corals are sensitive to changes in ocean carbonate chemistry15, the underlying physiological mechanisms that determine vulnerability are far from understood. Natural sites with low aragonite saturation that select for genotypes that can calcify under such conditions and permit decade-long developmental acclimation to changes in Ωsw are invaluable model systems for understanding the resilience of corals and coral calcification processes. Here we reveal that corals grown for their entire lifetime at low aragonite saturation conditions in their natural environment, at ojos in the Caribbean, exert strong control on both pHcf and DICcf, thereby modulating CO32−cf, Ωcf, and calcification rate. At the calcification site, both parameters that control Ωcf (pHcf and DICcf) decreased only slightly along the ambient Ωsw gradient in which the analysed corals live, highlighting the strong control of Porites asteroids corals over the biomineralization process. Yet the combined change in pHcf and DICcf corroborate the observed decline in calcification rate along the environmental gradient (Fig. 4b). Interestingly, at this field site ojos with low Ωsw had elevated DICsw, but this did not result in higher DICcf concentrations in the calcifying fluids, indicating a decoupling of internal and external DIC concentrations. This indicates that corals have significant control over the carbonate chemistry of the calcifying fluid, likely mediated by bicarbonate transporters (NBC, SLC4 family of ion transporters) that are localised in the calicoblastic epithelium, as well as other, not yet identified acid–base relevant transporters35. Carbonate chemistry at the calcification site clearly differs between coral growing at the control and ojo locations. The difference explains 41% of the observed difference in calcification rate; however, it still leaves 59% of the variation in calcification rate unexplained (Fig. 4b).
In our study, we took advantage of the inherent conditions of this submarine springs system, including the strong environmental fluctuations and the fact that carbonate chemistry is controlled by saline groundwater discharge, allowing us to provide new facets on drivers of coral calcification in natural settings affected by ocean acidification. In the subsequent discussion we will outline the novel insights we derive from the observed internal carbonate chemistry conditions at this natural low Ωsw site, discuss potential mechanisms that control calcification rates, add to the ongoing discussion on how seawater carbonate chemistry affects regulations of internal conditions at the site of calcification (e.g. ref. 23), and emphasise the importance of deciphering internal calcium regulation36,37,38.
The ability of organisms to modify pHcf reflects the strong effect of intracellular biological processes on coral calcification and is manifested in skeletal isotopic composition. The control of pHcf represents one mechanism to counter external seawater conditions39. The boron isotopic-derived pHcf values we report are similar to those reported in other studies for Porites25,26,27. The sensitivity of pHcf to changes in the environmental pHsw, however, differed between the different studies, as the environments the corals originated from were distinct27,40. All studies observed that pHcf stays within a narrower range (8.2–8.6) compared to large changes in seawater pHsw. They all highlight the generally strong control corals exert on pHcf. Despite this capacity for regulation, however, the observed pHcf was lower at lower Ωsw (e.g. refs. 20,25,28,41). Irrespective of whether corals maintained high pHcf, the corals exposed to low Ωsw maintained a higher proton gradient at lower pHsw (Fig. 2b). A potential driving force that fosters acclimation to various changes a coral may experience is the environmental history corals have been exposed to during their lifetime31. For example, pH homoeostasis—the maintenance of internal pHcf irrespective of the external seawater pHsw—was observed in corals that live in a highly dynamic naturally variable environment5,42. The underlying assumption is that these corals are better able to buffer external changes by exerting a stronger control over the calcifying fluids or by better exploiting times of favourable conditions27,40. Although the ojos represent a highly dynamic system33,43,44, coral performance measured in terms of net calcification was lower at these sites relative to the same species collected at control sites at the same location. Here, lifelong exposure to variable and persistently low Ωarag (<2) did not lead to full acclimation11. It is likely that there is a critical Ωarag threshold beyond which corals are no longer able to fully compensate for external acid–base changes. Such a critical threshold has been observed for corals grown at a Papua New Guinea CO2 seep site, where pHcf homoeostasis was only found for pHsw of >7.8 and Ωsw of >2.3. Beyond that, pHcf could not reach the same values as those under control conditions, and likely the coral’s physiological limit to compensate for changes was reached40. This lack of ability to fully compensate for the lower pHsw may be responsible for the slight differences in pHcf observed in this study.
The use of our dual geochemical proxy data to model coral growth (e.g. IpHRAC19,30) allowed us to further pinpoint potential mechanisms of how external seawater conditions affect internal calcifying conditions and ultimately skeletal growth. Calcification was once thought to be a passive diffusion process of seawater that brings external DIC to the site of calcification (potentially gaining DIC from metabolic CO2 by passing through the paracellular pathways)45 and by active ion transporters46 that result in an elevation of pHcf, thereby facilitating precipitation. At our study sites, DICsw is significantly higher at the low Ωsw sites (in average 2790 µmol kg−1 compared to control average DICsw of 2050 µmol kg−1) allowing us to decipher the role of external DICsw in modulating calcification regulation processes. If corals modify internal DICcf by simply up-regulating DICcf from the external concentrations baseline, we would expect higher DICcf values for the ojo corals where DICsw is higher. Under such assumption the elevated DICcf compensates for the slightly lower pHcf effect on Ωcf and calcification rates would essentially be similar between sites (Supplementary Fig. 1). However, our data clearly demonstrate that DICcf is not directly linked to external concentrations and can differ significantly from that of seawater22,30,47 (reported DICcf-upregulation values range from 1.6 to 3.230,38, with the ojos corals at our sites at the lower end), and this impacts Ωcf (more precisely CO32−) and calcification. A recent study under laboratory conditions with Stylophora pistillata23 observed that changes in DICsw concentration modulates internal pHcf regulation, with higher external DICcf facilitating higher internal pHcf, resulting in a clear correlation between seawater DICsw/H+sw and pHcf. Since DICsw at the ojos is significantly higher than at the control sites, one could expect this to compensate reduced pHcf up-regulation induced only due to changes in seawater pHsw. Yet we do not see a strong correlation between pHcf and seawater DICsw/H+sw, suggesting different drivers for Ωcf regulation in P. astreoides compared to those observed in S. pistillata23. Nevertheless, the change in pHcf and the limited ability to upregulate DICcf at the ojos corroborates the observed calcification rate decrease of corals at the ojos. However, these parameters may not be the only drivers for the decline in growth. Recent studies identified internal calcium (Ca2+cf) regulation as an additional player in coral calcification responses and emphasised that regulation of Ca2+cf can contribute to a corals’ resistance to future ocean changes36,37,38,48. In this sense, the good agreement of our model with the observed calcification response may imply that internal average steady-state calcium concentrations (Ca2+cf) at the ojos are lower by some proportion that is related to the pHcf changes, since our model based on pHcf and CO32−cf can explain only 41% of the observed calcification decline. This suggests a strong link between Ca2+cf and pHcf and supports the idea of a plasma-membrane Ca-ATPase (49, but see ref. 50) responsible for pHcf regulation. However, it is possible that pHcf and Ca2+cf were both regulated by additional and/or different ion transport mechanisms (e.g. potentially ion exchangers, Ca2+-channels)50,51.
The present study also indicates that the acclimation process in different corals encompass some degree of flexibility in terms of the relative role of pHcf and DICcf regulation in increasing the Ωcf, with some individuals compensating by adjusting their internal pHcf and others primarily by DICcf modulation. This may also be true of the role of Ca2+cf upregulation. The relative amount, source, and transportation pathways of DIC, H+ and Ca2+ to the site of calcification are still not fully understood52 and transport processes may differ between different coral species and even individual corals of the same species. Another potential driver for the observed differences among studies could be the number and type of symbionts the corals are hosting. Corals at the ojos harbour a higher density of symbionts53 that may potentially account for the higher energy demands for pHcf up-regulation resulting in the relatively small difference in the internal conditions (pHcf, DICcf) we see. Recent work provided the first evidence that coral symbionts (e.g. by modulating the chemical microenvironment within the diffusive boundary layer surrounding the coral that may buffer external changes54) and host genotypes can jointly affect coral calcification rates55. Similarly, possible interactions with the microbiome (e.g. restructuring of the corals microbiome56) or changes in energy acquisition and allocation processes to overcome environmental gradients57,58 can affect coral growth. Environmental factors may also affect pHcf and DICcf explaining some of the observed differences between the ojo and ambient corals at our study site. Studies have shown that a decrease in pHcf and DICcf is associated with increasing temperature38, yet at our sites the temperatures at the ojos is actually lower, on average, than at control sites. Salinity might also influence regulation processes, yet the measured average values (32.2 psu) as well as the salinity range measured (26–36 psu)44 at the springs can be tolerated by corals and the long-term exposure to such conditions may have allowed them to develop mechanisms to better cope and adapt to this variable environment59. Overall, these environmental and biological parameters may be responsible for the observed internal conditions in the calcifying fluid but likely also affect rates of processes that ultimately affect calcification, and thus contribute to the unexplained component in our relationship between calcification and the geochemically derived DICcf and pHcf. Our geochemical model approach assumes steady-state equilibrium conditions; however, the rates of the various transport processes involved in regulating the chemistry of the calcifying fluid will ultimately dictate the calcification response60: these rates may differ between individual coral genotypes, further contributing to the offsets between the model output and observations.
In this study, we utilised a dual geochemical proxy approach (δ11B and B/Ca) to constrain calcifying fluid carbonate chemistry in P. astreoides corals that spent their entire life (decades) under acidified low Ωsw conditions. We found that at the pHcf for corals at the low Ωsw was slightly lower than at the ambient conditions indicating inability to achieve optimal calcification conditions. We also determined that pHcf and DICcf are independently regulated and corroborated the calcification response in P. astreoides at this site. The study provides new insights into calcification responses of P. astreoides under changing environmental conditions and sheds light on the potential of corals to acclimate30,41,47,61,62. Using the geochemical proxies in combination with the bio-inorganic model brought forward by McCulloch et al. 30, we could explain 41% of the variability in coral growth rates along a Ωsw gradient. The variability which is not explained indicates that additional physiological and environmental processes contribute to the control of calcification rates in natural environments. This provides promising new avenues towards studying acclimation and adaptation potential of long-lived marine invertebrates such as corals.
Methods
Site description and coral core collection
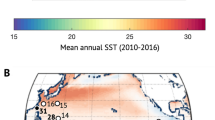
Cores from colonies of P. astreoides were collected at the ojos—natural springs of low-pH water—in the National Maritime Park at Puerto Morelos, Mexico (see refs. 11,43 for more details). Five cores were drilled in close proximity to the low pH discharge and seven cores were drilled from control sites outside the ojos discharge influence (~2–5 m away). After collection, cores were dried at 50 °C before further analysis. Water chemistry was measured at the different sites (summarised in Supplementary Table 1 and for more details see refs. 11,33,43,44,63,64) and used to calculate carbonate chemistry (see Supplementary Table 1). In general, corals were collected from sites that have similar light conditions, differ marginally in temperature (<1 °C lower at the ojos averaged over all seasons with temperatures cooler than ambient in summer and slightly warmer in winter), have consistently lower salinity (2–4 units lower than ambient), and are considerably different in Ωsw (Supplementary Table 1)11,44. We note that these submarine springs are not perfect analogues for future ocean acidification. Specifically, the conditions creating low-pH seawater at the ojos differ from those of the ocean acidification scenario as the high CO2 in the discharging water at the ojos is derived from brackish water that has interacted with soil and limestone. The spring water is characterised by lower pH, higher DIC, higher TA but similar calcium (Ca2+) concentration compared to the ambient conditions away from the spring influence. The corals at these ojos are constantly exposed to these discharging water (Supplementary Table 1), as discussed in detail in refs. 11,43,44, and they represent settings with persistent low Ωsw. In particular, because such conditions have persisted at the ojo discharge sites at least since the last deglaciation (~18,000 years ago65) the corals at these sites were exposed to low Ωsw for their whole life span, potentially allowing enough time for acclimation. Moreover, it is quite likely that strong selection processes have resulted in successful colonisation of the ojos by a fraction of the coral population that is better adapted to low pHsw and high CO2.
Water samples were also taken for seawater boron concentrations (measured on a ICP-MS Finnigan Element XR following Krupinski and colleagues66; ~430 ± 8 μM, with no difference between ojos and control) and a boron isotopic composition (δ11Bsw) of 39.15 (1sd = 0.12; n = 3) for the control site and 38.85 (1sd = 0.17; n = 5) for the low pH ojos. Boron isotopic samples were analysed on a Neptune multi-collector inductively coupled mass spectrometer at National Cheng Kung University, Taiwan, using the standard-sample-standard bracketing technique67. The boric acid standard IAEA-B-1 was used as the reference standard (e.g. 39.77‰) to determine the δ11B of the samples, reproducibility (±0.25‰).
Sample preparation and geochemical analysis
Collected coral cores were cut in half. One half was bleached for 24 h, thoroughly washed with milli-Q and dried overnight at 50 °C. Subsequently, the slab surfaces were carefully ground (Struers Silicon carbide grinding paper SiC 500–4000) and briefly polished (Struers DiaPor Dur 9 µm polishing suspension) in preparation for boron analysis using a Struers TegraPol-21 with TegraForce-5 head (Grinder and Polisher). The δ11B and B/Ca composition was measured simultaneous by laser ablation multi-collector inductively coupled plasma mass spectrometer (Thermo Fisher MC-ICP-MS AXIOM, connected to a UP193fx laser ablation system of New Wave Research, equipped with an excimer 193 nm laser). The measurement procedure followed Fietzke et al.68 and Wall et al.40 with slight modifications. Specifically, we used Multiplier and Faraday cups simultaneously to collect data for B10 and B11 (both on multiplier) as well as C12 (Faraday cup). This allows us to derive B/C and δ11B from the same skeletal material. Similar to previous work the cones were cleaned on a regular basis (every 2–4 days). The tubes going from the ablation cell to the plasma torch were checked for material deposition and cleaned by high flow rates overnight and/or mobilisation of the debris by increased flow rates transporting it out of the tubes. Prior to each measurement session the standard and samples were pre-ablated to remove surface contaminations (spot size used was one size bigger than during analysis). A standard-sample-bracketing method was used. The data of one measurement session contained 5–6 brackets. Both C12 and the variation of the standard (NIST SMR610) for each session were used to check for instrument stability and contaminations. Sessions were repeated when the standard drift was higher than the internal reproducibility of the standards (2 SD of the session on the standards). Twenty individual laser tracks (25 × 500 µm) were placed as close as possible to the edge of the skeletal section (expecting to mainly ablate fibres and avoid centres of calcification (COC)), far enough away to avoid ablation through the skeletal part. Yet COC areas may not have been completely avoided. To account for this we: (a) subsequently screened the individual tracks for abnormalities in C12 indicative of either ablation through the coral skeletal part (since the underlying skeletal depth is unknown from the surface view, this screening is completed afterwards) or increased organics and excluded this parts from analysis, and (b) aimed for 20 tracks of ∼25 × 500 × 20 µm on all individuals to have a representative δ11B dataset per individual. By this approach we expect to cover a representative sample set and minimise the natural intra-skeletal variability and cover similar proportions in each of the different corals (assuming that COC to fibre ratio in coral grown under various environmental conditions stays constant). The accuracy of our δ11B measurements has been checked by repeated analyses of Porites coral standard Jcp-1 and NIST SRM610, measured against a pellet of primary boron standard NBS951 (boric acid) (see Supplementary Fig. 2).
δ11B determination
The data reduction followed Fietzke et al.68. This yields one δ11B value per sample and session with an average precision of <1‰ (1 SD) for ∼1.7 µg of carbonate sample. A minimum of 15 and up to 20 values of δ11B spread over the core surface in the upper few mm of each coral colony (below the tissue, representing ∼1 year of growth) were measured to obtain a representative data set per sample. The data set reflects the high variability in δ11B for a single colony, and replicates were averaged afterwards to yield values that reflect the mean δ11B value, hence the mean internal calcification conditions (see below).
B/Ca determination
B/C elemental ratios have been determined simultaneously with the boron isotope ratios via LA–MC–ICP–MS. Boron isotope data (10B and 11B) have been collected using a pair of ion counters, while carbon (12C) had been determined using a Faraday cup. B/C data are based on the integrated boron intensities (10B + 11B) divided by the 12C intensity. The calibration (conversion from intensity ratios to concentration ratios) has been done using a natural L. pertusa coral sample covering a B concentration range of about 450–950 µmol/mol, which had been determined before using LA–ICP–MS relative to standard NIST-SRM610 using 43Ca as internal standard. This calibration procedure resulted in: B/C [µmol/mol] = 78,800 × B/C [cps/cps]; (cps—counts per second, ion beam intensity). We used stoichiometric ratio of C/Ca = 1 as approximation for natural carbonates and translated B/C ratios in B/Ca [µmol/mol] ratios.
δ11B as internal pHcf proxy
All δ11B values were translated into internal pHcf following Eq. (1) with a seawater δ11Bsw of 38.85 for ojo centres and 39.15 for control sites, a fractionation factor (αB) of 1.027269 and pK*B averaged for the two sites (see Supplementary Table 1).
Following the method in Trotter and colleagues24 the superimposed physiological pH control was calculated with the equation:
and related to the seawater aragonite saturation state (Ωsw) to quantify the extent of the physiological control on the internal pHcf.
We note here, that the local variability in carbonate chemistry at the ojos and hence, associated changes in pKB and seawater δ11B can add some uncertainty to the derived pHcf and overestimate or underestimate its actual value. To test the sensitivity to changes in pKB we used our dataset and recalculated pHcf values. We applied a range of seawater δ11Bsw that encompasses the average measured δ11Bsw per site but also seawater isotopic composition beyond this level ranging from 38.55‰ to 39.45‰ and recalculated pHcf (Supplementary Fig. 3a). This allowed us to decipher the combined role of site specific pKB and seawater δ11Bsw for a range of skeletal δ11B (Supplementary Fig. 3b). In general, the δ11B-derived pHcf decreases slightly with increasing seawater δ11B. Changes in seawater δ11Bsw in the corals surrounding will either over or underestimate pHcf and calculated changes in pHcf range from 0.019 to 0.023 pH units per 0.3 change in δ11Bsw (Supplementary Fig. 3c the average difference between our sites; or change from 0.056–0.065 for the entire seawater δ11Bsw range tested). Compared to the pHcf range (8.2–8.8) derived from individually measured skeletal δ11B values such changes are minor (Supplementary Fig. 3a; in contrast to the individual coral’s pHcf standard deviation of 0.04–0.13, Supplementary Table 2).
B/Ca as CO3 2− cf and DICcf proxy
All individual B/Ca data were used to estimate CO32−cf based on the δ11B-derived pHcf data and further used to calculate the DICcf following the approach of McCulloch et al.30. This allows to use the following simplified relationship to determine the CO32−cf concentration from B/Ca30:
and the distribution coefficient is determined for synthetic aragonite and follows the equation:
based on the internal pHcf29,30. Both pHcf and [CO32−]cf are then used to calculate DICcf.
Modelling calcification rate using internal pHcf and DICcf
Calcification rate (G) was calculated following McCulloch et al.19 IpHRAC model:
The calcification response was calculated with the temperature-dependent rate law constant k and reaction order constant n (applying the equations given in McCulloch et al.19: k = −0.0177*T2 + 1.47*T + 14.9 and n = 0.0628*T + 0.0985). The individual average temperature data for the different sites were used (Table S1).
For a sole pH-regulation-based model we used seawater DIC concentration DICsw that were measured at the different sites the individual corals were collected (see Table S1). Aragonite saturation state at the site of calcification (Ωcf) was calculated from pHcf and DICcf using seacarb. We first followed recent approaches19,20 by setting DICcf equivalent to double DICsw19. In a second step an advanced bio-inorganic model used both geochemically determined calcification parameters to calculate Ωcf. For the seacarb (R) calculations we used the measured average salinity and temperature for the different sites. We assumed [Ca]2+ concentrations that equals seawater values to calculate Ωcf.
Both modelled calcification rates were plotted against the measured calcification rates11, by converting them into relative rates and setting the control site as 1 (or 100%19).
Statistical analysis
Statistical analysis of the geochemical proxies and derived internal calcification conditions was performed by comparing the two treatment groups (control conditions vs. reduced aragonite saturation state at the centres of the ojos) using Welch’s t-test (unequal sample numbers). To understand how well the internal calcification conditions (Ωcf—a combined value of both internal pHcf and DICcf) can explain measured changes in net calcification rate we applied simple linear models regressing model-derived relative growth as a function of internal Ωcf. Similarly, changes in geochemical proxies as well as internal conditions were regressed to decipher correlation between these parameters and potential driving forces explaining changes in net calcification rate along the natural environmental seawater Ωsw gradient20.
Data analysis and visualisation was done with R Studio version 3.0.1 (R Development Core Team, 2015).
Data availability
All coral geochemical data and derived calcification conditions are available as Supplementary data file.
References
Fabricius, K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 1, 165–169 (2011).
De’ath, G., Lough, J. M. & Fabricius, K. E. Declining coral calcification on the Great Barrier Reef. Science 323, 116–119 (2009).
Albright, R. & Langdon, C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Chang. Biol. 17, 2478–2487 (2011).
Anthony, K. R. N., Kline, D. I., Diaz-Pulido, G., Dove, S. & Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17442–17446 (2008).
Venn, A. A. et al. Impact of seawater acidification on pH at the tissue–skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639 (2013).
Langdon, C. et al. Effect of calcium carbonate saturation state on calcifcation rate of an experimental coral reef. Glob. Biogeochem. Cycles 14, 639–654 (2000).
Jokiel, P. L. et al. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483 (2008).
Dove, S. G. et al. Future reef decalcification under a business-as-usual CO2 emission scenario. Proc. Natl Acad. Sci. USA 110, 15342–15347 (2013).
Albright, R. et al. Reversal of ocean acidification enhances net coral reef calcification. Nature 531, 362–365 (2016).
Albright, R. et al. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature https://doi.org/10.1038/nature25968 (2018).
Crook, E. D., Cohen, A. L., Rebolledo-vieyra, M., Hernandez, L. & Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl Acad. Sci. USA 110, 11044–11049 (2013).
Shamberger, K. E. F. et al. Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys. Res. Lett. 41, 499–504 (2014).
Barkley, H. C. & Cohen, A. L. Skeletal records of community-level bleaching in Porites corals from Palau. Coral Reefs 35, 1407–1417 (2016).
Goffredo, S. et al. Biomineralization control related to population density under ocean acidification. Nat. Clim. Chang. 4, 593–597 (2014).
Chan, N. C. S. & Connolly, S. R. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Chang. Biol. 19, 282–290 (2013).
Strahl, J. et al. Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comp. Biochem. Physiol. Part A 184, 179–186 (2015).
Castillo, K. D., Ries, J. B., Bruno, J. F. & Westfield, I. T. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B 281, 20141856 (2014).
Ries, J. B., Cohen, A. L. & McCorkle, D. C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134 (2009).
McCulloch, M., Falter, J., Trotter, J. & Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2, 623–627 (2012).
Holcomb, M. et al. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 (2014).
Al-Horani, F., Al-Moghrabi, S. M. & de Beer, D. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: active internal carbon cycle. J. Exp. Mar. Biol. Ecol. 288, 1–15 (2003).
Cai, W. et al. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Commun. 1–8 https://doi.org/10.1038/ncomms11144 (2016).
Comeau, S. et al. Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc. R. Soc. B 284, 20161669 (2017).
Trotter, J. et al. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163–173 (2011).
Hönisch, B. et al. Assessing scleractinian corals as recorders for paleo-pH: empirical calibration and vital effects. Geochim. Cosmochim. Acta 68, 3675–3685 (2004).
Rollion-Bard, C., Chaussidon, M. & France-Lanord, C. Biological control of internal pH in scleractinian corals: implications on paleo-pH and paleo-temperature reconstructions. C. R.-Geosci. 343, 397–405 (2011).
Georgiou, L. et al. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl Acad. Sci. USA 112, 13219–13224 (2015).
Krief, S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001 (2010).
Holcomb, M., DeCarlo, T. M., Gaetani, G. A. & McCulloch, M. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 437, 67–76 (2016).
McCulloch, M. T., D’Olivo, J. P., Falter, J., Holcomb, M. & Trotter, J. A. Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 8, 1–8 (2017).
Rivest, E. B., Comeau, S. & Cornwall, C. E. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Chang. Rep. 1–11 https://doi.org/10.1007/s40641-017-0082-x (2017).
Fabricius, K. E., De’ath, G., Noonan, S. & Uthicke, S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc. R. Soc. B 281, 20132479 (2014).
Hofmann, G. E. et al. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 (2011).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. A. Mechanisms of reef coral resistance to future climate change. Science (80-.) 344, 895–898 (2014).
Zoccola, D. et al. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 5, 1–11 (2015).
DeCarlo, T. M., Comeau, S., Cornwall, C. E. & McCulloch, M. T. Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proc. R. Soc. B 285, 20180564 (2018).
Ross, C. L., Schoepf, V., Decarlo, T. M. & McCulloch, M. T. Mechanisms and seasonal drivers of calcification in the temperate coral Turbinaria reniformis at its latitudinal limits. Proc. R. Soc. B 285, 20180215 (2018).
Ross, C. L., DeCarlo, T. M. & Mcculloch, M. T. Environmental and physiochemical controls on coral calcification along a latitudinal temperature gradient in Western Australia. Glob. Chang. Biol. 25, 431–447 (2019).
Allison, N. & Finch, A. A., EIMF. δ11B, Sr, Mg and B in a modern Porites coral: the relationship between calcification site pH and skeletal chemistry. Geochim. Cosmochim. Acta 74, 1790–1800 (2010).
Wall, M. et al. Internal pH regulation facilitates in situ long-term acclimation of massive corals to end-of-century carbon dioxide conditions. Nat. Publ. Gr. 1–7 https://doi.org/10.1038/srep30688 (2016).
Schoepf, V., Jury, C. P., Toonen, R. J. & McCulloch, M. T. Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc. Biol. Sci. 284, 20172117 (2017).
Tambutté, E. et al. Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat. Commun. 6, 7368 (2015).
Crook, E. D., Potts, D., Rebolledo-Yieyra, M., Hernandez, L. & Paytan, A. Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 31, 239–245 (2012).
Paytan, A. et al. Reply to Iglesias-Prieto et al.: combined field and laboratory approaches for the study of coral calcification. Proc. Natl Acad. Sci. USA 111, E302–E303 (2014).
Tambutte, E. et al. Calcein labelling and electrophysiology: insights on coral tissue permeability and calcification. Proc. R. Soc. B 279, 19–27 (2012).
McConnaughey, T. A. & Whelan, J. F. Calcification generates protons for nutrient and bicarbonate uptake. Earth-Sci. Rev. 42, 95–117 (1997).
Allison, N., Cohen, I., Finch, A. A., Erez, J. & Tudhope, A. W. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Commun. 5, 5741 (2014).
Comeau, S., Cornwall, C. E., DeCarlo, T. M., Krieger, E. & Mcculloch, M. T. Similar controls on calcification under ocean acidification across unrelated coral reef taxa. Glob. Chang. Biol. 24, 4857–4868 (2018).
Zoccola, D. et al. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora pistillata. Biochim. Biophys. Acta 1663, 117–126 (2004).
Barott, K. L., Perez, S. O., Linsmayer, L. B. & Tresguerres, M. Differential localization of ion transporters suggests distinct cellular mechanisms for calcification and photosynthesis between two coral species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R235–R246 (2015).
Tresguerres, M. et al. Cell Biology of Reef-Building Corals: Ion Transport, Acid/Base Regulation, and Energy Metabolism. In Acid-Base Balance and Nitrogen Excretion in Invertebrates (eds. Weihrauch, D. & O’Donnell, M.) 193–218 (Springer International Publishing, 2017).
Allemand, D., Tambutté, É., Zoccola, D. & Tambutté, S. Coral calcification, cells to reefs. In Coral Reefs: An Ecosystem in Transition SE-9 (eds. Dubinsky, Z. & Stambler, N.) 119–150 (Springer, Netherlands, 2011).
Martinez, A. et al. Species-specific calcification response of Caribbean corals after 2-year transplantation to a low aragonite saturation submarine spring. Proc. R. Soc. B 286, https://doi.org/10.1098/respb.20190572 (2019).
Chan, N. C. S., Wangpraseurt, D., Kühl, M. & Connolly, S. R. Flow and coral morphology control coral surface pH: implications for the effects of ocean acidification. Front. Mar. Sci. 3, 1–11 (2016).
Gold, Z. & Palumbi, S. R. Long-term growth rates and effects of bleaching in Acropora hyacinthus. Coral Reefs https://doi.org/10.1007/s00338-018-1656-3 (2018).
Morrow, K. M. et al. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J. 9, 894–908 (2015).
Anthony, K. R. N., Hoogenboom, M. O., Maynard, J. A., Grottoli, A. G. & Middlebrook, R. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Funct. Ecol. 23, 539–550 (2009).
Wall, C. B., Mason, R. A. B., Ellis, W. R., Cunning, R. & Gates, R. D. Elevated pCO2 affects tissue biomass composition, but not calcification, in a reef coral under two light regimes. R. Soc. Open Sci. 4, 170683 (2017).
Kerswell, A. P. & Jones, R. J. Effects of hypo-osmosis on the coral Stylophora pistillata: nature and cause of ‘ low-salinity bleaching’. Mar. Ecol. Prog. Ser. 253, 145–154 (2003).
Guo, W. Seawater temperature and buffering capacity modulate coral calcifying pH. Sci. Rep. 9, 1189 (2019).
Comeau, S., Cornwall, C. E. & McCulloch, M. T. Decoupling between the response of coral calcifying fluid pH and calcification to ocean acidification. Sci. Rep. 7, 1–10 (2017).
D’Olivo, J. P. & McCulloch, M. T. Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Sci. Rep. 7, 1–15 (2017).
Crook, E. D. et al. Recruitment and succession in a tropical benthic community in response to in-situ ocean acidification. PLoS ONE https://doi.org/10.1371/journal.pone.0146707 (2016).
Null, K. A. et al. Composition and fluxes of submarine groundwater along the Caribbean coast of the Yucatan Peninsula. Cont. Shelf Res. https://doi.org/10.1016/j.csr.2014.01.011 (2014).
Medina-Elizalde, M. & Rohling, E. J. Collapse of classic maya civilization related to modest reduction in precipitation. Science (80-.) 335, 956–959 (2012).
Krupinski, N. B., Russell, A. D., Pak, D. K. & Paytan, A. Core-top calibration of B/Ca in Pacific Ocean Neogloboquadrina incompta and Globigerina bulloides as a surface water carbonate system proxy. Earth Planet. Sci. Lett. 466, 139–151 (2017).
Wang, B.-S. et al. Direct separation of boron from Na- and Ca-rich matrices by sublimation for stable isotope measurement by MC-ICP-MS. Talanta 82, 1378–1384 (2010).
Fietzke, J. et al. Boron isotope ratio determination in carbonates via LA-MC-ICP-MS using soda-lime glass standards as reference material. J. Anal. Spectrom. 25, 1953 (2010).
Klochko, K., Kaufman, A. J., Yao, W., Byrne, R. H. & Tossell, J. A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 276–285 (2006).
Acknowledgements
The research was funded by the German Federal Ministry for Education and Research project BIOACID II (Consortium 3: Natural CO2-rich reefs as windows into the future: Acclimation of marine life to long-term ocean acidification and consequences for biogeochemical cycle, Grant number: 03F0655A), the Austrian Science Fund Schrödinger Fellowship (funding to M.W., FWF J3667-B25), and National Science Foundation (NSF) OCE-1040952, a University of California Institute for Mexico and the United States (UC-Mexus) grant (to A.P.), and NSF OCE-1041106. E.D.C. was funded through NSF-GFR and a EPA-STAR fellowships. All corals were collected under Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA) Permit DGOPA.00153.170111.-0051 and exported with a Convention on International Trade in Endangered Species (CITES) Permit MX52912.
Author information
Authors and Affiliations
Contributions
M.W., J.F. and A.P. designed the experimental analyses. E.D.C. collected the samples. M.W. prepared the samples. M.W. and J.F. analysed the samples. E.D.C. and A.P. provided background data. M.W. analysed data. M.W., J.F., A.P. and E.D.C. were involved in the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Communications thanks Thomas DeCarlo and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wall, M., Fietzke, J., Crook, E.D. et al. Using B isotopes and B/Ca in corals from low saturation springs to constrain calcification mechanisms. Nat Commun 10, 3580 (2019). https://doi.org/10.1038/s41467-019-11519-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-11519-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.