Abstract
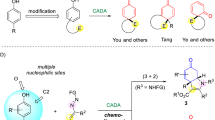
Asymmetric dearomatization reactions have recently emerged as a powerful tool for the rapid build-up of the molecular complexity. Chiral three-dimensional polycyclic molecules bearing contiguous stereogenic centers can be synthesized from readily available planar aromatic feedstocks. Here we report that an intermolecular asymmetric dearomatization reaction of α-naphthols bearing a tethered nucleophile at the C4 position of the naphthol ring is achieved by a chiral phosphoric acid. The reaction proceeds via a highly chemo- and regioselective aminative dearomatization/Michael addition sequence, affording a wide array of functionalized cyclic ketones in good yields (up to 93%) with excellent enantioselectivity (up to >99% ee). The catalyst loading can be reduced to 0.1 mol%. Preliminary mechanistic investigations identify that the enantioselectivity is established in the dearomatization step, while the Michael addition is the rate-limiting step. A working model accounting for the origin of the stereochemistry is proposed based on DFT calculations.
Similar content being viewed by others
Introduction
The demands for effective assembly of diverse molecular scaffolds are continuously growing along with the development of organic chemistry. Among various kinds of strategies that aim to rapidly increase molecular complexity, catalytic asymmetric dearomatization (CADA) reactions1,2,3,4,5,6,7,8,9,10,11,12 have recently received considerable attention of the synthetic community. They are capable of significantly enriching the chemical space by converting readily available planar aromatic compounds to diverse three-dimensional molecules bearing spiro or fused polycyclic skeletons with multiple stereogenic centers including quaternary ones.
Naphthols serve as important aromatic feedstocks in organic chemistry. However, the asymmetric dearomatization reactions of naphthols are relatively less explored compared with those of other electron-rich arenes like indoles13,14,15,16,17,18. With the oxidation system consisting of a chiral hypervalent iodine reagent and m-CPBA as the terminal oxidant19,20,21,22,23,24,25,26,27,28,29, or transition-metal catalysts that are capable of promoting single electron oxidation30,31, α- or β-naphthols could react with intra- or intermolecular nucleophiles to yield chiral functionalized cyclic ketones. Notably, the asymmetric dearomatization reactions under non-oxidative conditions are rather limited within β-naphthols32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52, and the corresponding reactions of α-naphthols are relatively rare. In 2015, Luan and coworkers reported an elegant Pd-catalyzed dynamic kinetic asymmetric transformation of 4-(2-bromoaryl)-α-naphthols with alkynes for the synthesis of chiral spirocyclic enones53. Sporadic examples of non-oxidative asymmetric dearomatization of α-naphthols via organocatalytic chlorination37, annulation54, Ir-catalyzed allylic alkylation38, or Pd-catalyzed cross coupling55,56,57 were disclosed. However, the dearomatization reactions in these examples generally relied on the pre-installation of an intramolecular electrophile (Fig. 1a). Notably, during the preparation of this manuscript, Shao and coworkers reported an intermolecular asymmetric allylic dearomatization reaction of α-naphthols at the C2 position by a chiral phosphoric acid58. However, highly efficient asymmetric dearomatization reactions of α-naphthols with intermolecular electrophiles still remain underdeveloped.
In 2017, we reported a Pd-catalyzed arylative dearomatization/aza-Michael addition sequence between aryl bromide and α-naphthols bearing a tethered nucleophile at the C4 position of the naphthol ring59. The exclusive chemo-, and regioselectivity favoring the C4 position among the multiple potential nucleophilic sites encouraged us to further probe the possibility of developing a highly enantioselective dearomatization of α-naphthols/Michael addition sequence. However, all attempts to realize such an asymmetric reaction under Pd catalysis have been failed in our hands. In this regard, we speculated that azodicarboxylates60,61,62,63,64,65,66 might be employed as suitable electrophilic amination reagents which are compatible with the dearomatization of electron-rich arenes in the presence of a chiral phosphoric acid (CPA)67,68,69,70.
Here, we accomplished the efficient synthesis of a wide array of functionalized cyclic ketones bearing two consecutive stereogenic centers in excellent diastereo- and enantioselectivity (Fig. 1b). The products are readily involved in further transformations towards various enantioenriched polycyclic molecules.
Results
Reaction development
Our studies commenced with the attempt of the reaction of α-naphthol 1a with 1.5 equivalents of diethyl azodicarboxylate (DEAD) (Table 1). In the presence of 10 mol% of chiral phosphoric acid C1, the target reaction proceeded smoothly in 1,2-dichloroethane (DCE) at 50 °C for 24 h, affording the dearomatized product 2a in 85% yield with 75% ee as a single trans diastereoisomer (Table 1, entry 1). The structure and absolute configuration of 2a (3R,4R) were established unambiguously by X-ray crystallographic analysis of an enantiopure sample. Interestingly, the Friedel–Crafts reaction at the C2 position was not observed. Systematic evaluation of a series of CPAs (C2–C7) was conducted (Table 1, entries 2–7). The results revealed that the catalysts have great influence on the reaction outcome. Notably, TRIP-CPA C2 gave the optimal results (90% yield and 99% ee, Table 1, entry 2). Utilizing SiPh3-derived catalyst C4 also led to comparable results with those of C2 (90% yield and 98% ee, Table 1, entry 4). On the other hand, 2a was obtained in the nearly racemic form (2% ee) when 4-NO2C6H4 derived C6 was employed (Table 1, entry 6). Although excellent enantioselectivity has been obtained, other reaction parameters were further investigated. Typical solvents including EtOAc, THF or toluene also well facilitated the reaction (Table 1, entries 8-10). When the temperature was lowered to 40 °C, prolonged time (36 h) was necessary to provide 2a in 88% yield (Table 1, entry 11). Similar results (85% yield and 99% ee) could be achieved in shorter time when elevating the reaction temperature to 60 °C (Table 1, entry 12). However, slightly decreased yield (77%) was observed when 1.1 equivalents of DEAD was used (Table 1, entry 13). The reactions proceeded well with reduced loading of C2 (≤1 mol %, Table 1, entries 14-17). The remarkably high catalytic efficiency was exemplified by the fact that 2a was afforded in 52% yield with 97% ee in the presence of 0.1 mol% of C2 at 80 °C (Table 1, entry 17). However, only complex mixture was obtained in the absence of a CPA catalyst (Table 1, entry 18).
Scope and limitation
With the optimal conditions in hands (Table 1, entry 2), we next explored the substrate scope (Fig. 2). The desired reactions of 1a with other azodicarboxylates all proceeded smoothly, leading to 2b–2d in high yields (88–89%). The N-protecting group of α-naphthol substrates could be switched to Ns, CO2Me or p-MeC6H4CO2. The corresponding products (2e–2g) could be obtained in 89–92% yields. The prolonged tether at the C4 position of the naphthol ring was well accommodated, affording the piperidine-fused product 2h in 93% yield. Besides nitrogen nucleophiles, the hydroxyl group could also work as the nucleophile. The tetrahydrofuran-fused product 2i was accomplished smoothly in 87% yield. Notably, when a malonate diester nucleophile was employed, only the dearomatization process occurred under the standard conditions. The target product 2j could be obtained (81% yield) after a subsequent Michael addition promoted by tBuOK.
On the other hand, various substituted α-naphthols underwent the desired dearomatization reactions. Electron-donating groups such as MeO and Me could be tolerated at the C6 to C8 positions (2k–2p, 91–93% yields). The C6 and C7 positions could accommodate halogen atoms including Cl and Br (2q–2s, 90–93% yields). Besides, when a CN group was installed at the C6 or C7 position, the dearomatization reaction delivered the desired products in high yields (2t, 93% yield; 2u, 91% yield). Gratifyingly, exceedingly high enantioselectivity was observed in all the above cases (2a–2u, 98 to >99% ee). However, when the substrate bearing a methyl group at the C2 position was subjected to the standard conditions, the reaction outcomes decreased apparently in terms of yield and enantioselectivity (2v, 34% yield and 78% ee). It should be noted that only complex mixtures were obtained when substrates bearing a substituent at the C5 position were utilized.
The dearomatization reaction of phenols is much more challenging compared with that of naphthols. In addition, by-products of Friedel–Crafts reactions are difficult to be avoided. To further expand the substrate scope of this aminative dearomatization/Michael addition sequence, several phenol analogs were tested (Fig. 3). Encouragingly, α-tetrahydronaphthol-derived substrate 3a participated the title reaction smoothly, delivering the desired product 4a in 90% yield with 96% ee, without the observation of Friedel–Crafts by-product. The reaction of sterically less demanding phenol substrate (2,3-Me2) also proceeded well. The corresponding product 4b was obtained in good yield (82%) but with rather decreased enantioselectivity (53% ee). Notably, a mixture of Friedel–Crafts-type byproducts became dominating for the substrates bearing one substituent (Me, MeO, or Br) at the C2 or C3 position. Compound 5 was isolated in 84% yield for the phenol substrate bearing no substituent at the C2 or C3 position.
Mechanistic studies
To shed light on the reaction mechanism, we first attempted to isolate the intermediate of this sequential reaction (Fig. 4). When the reaction of 1a was quenched within 30 min, only trace amount of 2a was observed (<5% NMR yield), while conjugated enone 6 was isolated in 86% yield with >99% ee. Compound 6 was found stable enough to be stored at −20 °C for weeks without apparent transformation into 2a or decomposition. Taking into consideration that much longer time (24 h) was required for most substrates to fully transfer into the final products, and the identical enantiopurity of 6 and that of 2a obtained under the standard conditions, we proposed that the stereochemistry of the whole reaction is established irreversibly in the aminative dearomatization step, whereas the following Michael addition is the rate-limiting step.
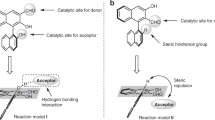
To explore the origin of the exceedingly high enantioselectivity, DFT calculations were then performed for the aminative dearomatization step (Fig. 5). The relative Gibbs free energy of TS-major, the transition state leading to the major product, was 2.0 kcal/mol lower than that of TS-minor, which was in qualitative agreement with the experimental results. The two prochiral faces at the C4 position of α-naphthol were well discriminated by the chiral phosphoric acid. In TS-major, the Si-face of the C4 position was attacked, which allowed the intact benzene ring of α-naphthol in distal to the chiral pocket. Whereas in TS-minor, strong steric congestion was developed due to the Re-face attack and the concomitant approximation of the intact benzene ring to one TRIP group of C2. These computational results corresponded well with the fact that substituents were tolerated at the C6 to C8 positions of α-naphthol (2k–2u), but not at the C2 position (2v). In addition, a fused saturated skeleton that is sterically roughly comparable with a benzene ring should be beneficial for the high enantioselectivity (4a), but sterically less demanding 2,3-Me2 groups were not that effective (4b).
Transition states. Optimized structures and relative Gibbs free energies (in kcal/mol) of the transition states of the aminative dearomatization step (ωB97XD/def2-TZVPP//B97D/6-31G**). The catalysts are presented with the van der Waals model. The substrates are presented with the stick model. The forming C–C bonds are shown in yellow dash lines. The transferring protons are shown in green spheres. The intact benzene rings of α-naphthols are shown in pink
Product transformations
To highlight the practicality and synthetic utility of this method, a gram-scale reaction of 1a with DEAD was performed under the standard reaction conditions. 2a was obtained in 87% yield (1.35 g) with 99% ee. In addition, several functional group transformations of 2a were demonstrated (Fig. 6). Tertiary alcohol 7 was delivered in 91% yield when treated with NaBH4. The ketone group could also be hydrogenated to methylene group under the catalysis of palladium on charcoal, leading to 8 in 78% yield. In addition, the N–N bond could be cleaved by treating 2a with ethyl bromoacetate and cesium carbonate. The corresponding product 9 was afforded in 52% yield. Notably, no erosion of enantiomeric purity was observed in all these transformations.
Transformations of 2a. Reaction conditions for synthesis of 7: 2a (0.2 mmol), NaBH4 (2.0 mmol) in MeOH (4 mL) at 0 °C. Reaction conditions for synthesis of 8: 2a (0.2 mmol), Pd/C (10%, 50 mg), HCl (conc. 20 μL) in MeOH (4 mL) under H2 (1 atm) at room temperature. Reaction conditions for synthesis of 9: 2a (0.2 mmol), Cs2CO3 (0.26 mmol), BrCH2CO2Et (0.22 mmol) in CH3CN (4 mL) at 50 °C
Discussion
In conclusion, we have achieved a chiral phosphoric acid-catalyzed dearomatization reaction of α-naphthols bearing a tethered nucleophile at the C4 position of the naphthol ring. Preliminary mechanistic investigations confirmed that the reaction proceeded via two steps, a stereochemistry-determining aminative dearomatization followed by a rate-limiting Michael addition. The reaction occurred under mild conditions, affording a wide array of polycyclic ketones in good yields with excellent enantioselectivity. Besides, the reaction features high catalytic efficiency and diverse transformations of the products. A working model accounting for the origin of the stereochemical induction was proposed based on DFT calculations.
Methods
Representative procedure
To a flask containing a mixture of 1a (0.1 mmol) and (S)-C2 (7.5 mg, 0.01 mmol) under argon was added a solution of the corresponding azodicarboxylate (0.15 mmol) in anhydrous 1,2-dichloroethane (2 mL). The reaction was stirred at 50 °C until TLC showed complete consumption of the starting material. The reaction mixture was cooled to room temperature, quenched with NaHCO3 (aq., 10 mL) and extracted with CH2Cl2 (3 × 15 mL). The combined organic layer was washed with brine, separated, dried over Na2SO4 and filtrated. After the solvent was removed under reduced pressure, the residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1/6 to 1/2) to afford 2a.
Data availability
The X-ray crystallographic coordinates for product 2a have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession code 1888563. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this work, including experimental procedures, compound characterization data, and computational details, are available within the article and its Supplementary Information files.
References
Pape, A. R., Kaliappan, K. P. & Kündig, E. P. Transition-metal-mediated dearomatization reactions. Chem. Rev. 100, 2917–2940 (2000).
Roche, S. P. & Porco, J. A. Jr. Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 50, 4068–4093 (2011).
Zhuo, C.-X., Zhang, W. & You, S.-L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 51, 12662–12686 (2012).
Zhuo, C.-X., Zheng, C. & You, S.-L. Transition-metal-catalyzed asymmetric allylic dearomatization reactions. Acc. Chem. Res. 47, 2558–2573 (2014).
Ding, Q., Zhou, X. & Fan, R. Recent advances in dearomatization of heteroaromatic compounds. Org. Biomol. Chem. 12, 4807–4815 (2014).
Zheng, C. & You, S.-L. Catalytic asymmetric dearomatization by transition-metal catalysis: a method for transformations of aromatic compounds. Chem 1, 830–857 (2016).
Manoni, E., De Nisi, A. & Bandini, M. New opportunities in the stereoselective dearomatization of indoles. Pure Appl. Chem. 88, 207–214 (2016).
Chen, J.-B. & Jia, Y.-X. Recent progress in transition-metal-catalyzed enantioselective indole functionalizations. Org. Biomol. Chem. 15, 3550–3567 (2017).
Wu, W.-T., Zhang, L. & You, S.-L. Recent progress on gold-catalyzed dearomatization reactions. Acta Chim. Sin. 75, 419–438 (2017).
Wertjes, W. C., Southgate, E. H. & Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 47, 7996–8017 (2018).
An, J. & Bandini, M. Gold-catalyzed dearomatization reactions. Chimia 72, 610–613 (2018).
You, S.-L. Ed. Asymmetric Dearomatization Reactions (Wiley-VCH, Weinheim, Germany, 2016).
Quideau, S., Pouységu, L. & Deffieux, D. Oxidative dearomatization of phenols: why, how and what for? Synlett 467-495 (2008).
Pouységu, L., Deffieux, D. & Quideau, S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron 66, 2235–2261 (2010).
Pouységu, L. et al. Hypervalent iodine-mediated oxygenative phenol dearomatization reactions. Tetrahedron 66, 5908–5917 (2010).
Wu, W.-T., Zhang, L. & You, S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 45, 1570–1580 (2016).
Sun, W., Li, G., Hong, L. & Wang, R. Asymmetric dearomatization of phenols. Org. Biomol. Chem. 14, 2164–2176 (2016).
Flores, A., Cots, E., Bergès, J. & Muñiz, K. Enantioselective iodine(I/III) catalysis in organic synthesis. Adv. Synth. Catal. 361, 2–25 (2019).
Dohi, T. et al. A chiral hypervalent iodine(III) reagent for enantioselective dearomatization of phenols. Angew. Chem. Int. Ed. 47, 3787–3790 (2008).
Quideau, S. et al. Asymmetric hydroxylative phenol dearomatization through in situ generation of iodanes from chiral iodoarenes and m-CPBA. Angew. Chem. Int. Ed. 48, 4605–4609 (2009).
Uyanik, M., Yasui, T. & Ishihara, K. Enantioselective Kita oxidative spirolactonization catalyzed by in situ generated chiral hypervalent iodine(III) species. Angew. Chem. Int. Ed. 49, 2175–2177 (2010).
Dohi, T. et al. Asymmetric dearomatizing spirolactonization of naphthols catalyzed by spirobiindane-based chiral hypervalent iodine species. J. Am. Chem. Soc. 135, 4558–4566 (2013).
Uyanik, M., Yasui, T. & Ishihara, K. Hydrogen bonding and alcohol effects in asymmetric hypervalent iodine catalysis: enantioselective oxidative dearomatization of phenols. Angew. Chem. Int. Ed. 52, 9215–9218 (2013).
Volp, K. A. & Harned, A. M. Chiral aryl iodide catalysts for the enantioselective synthesis of para-quinols. Chem. Commun. 49, 3001–3003 (2013).
Bosset, C. et al. Asymmetric hydroxylative phenol dearomatization promoted by chiral binaphthylic and biphenylic iodanes. Angew. Chem. Int. Ed. 53, 9860–9864 (2014).
Murray, S. J. & Ibrahim, H. Asymmetric Kita spirolactonisation catalysed by anti-dimethanoanthracene-based iodoarenes. Chem. Commun. 51, 2376–2379 (2015).
Zhang, D.-Y., Xu, L., Wu, H. & Gong, L.-Z. Chiral iodine-catalyzed dearomatizative spirocyclization for the enantioselective construction of an all-carbon stereogenic center. Chem. Eur. J. 21, 10314–10317 (2015).
Uyanik, M., Yasui, T. & Ishihara, K. Chiral hypervalent organoiodine-catalyzed enantioselective oxidative spirolactonization of naphthol derivatives. J. Org. Chem. 82, 11946–11953 (2017).
Hempel, C., Maichle-Mössmer, C., Pericàs, M. A. & Nachtsheim, B. J. Modular synthesis of triazole-based chiral iodoarenes for enantioselective spirocyclizations. Adv. Synth. Catal. 359, 2931–2941 (2018).
Oguma, T. & Katsuki, T. Iron-catalyzed dioxygen-driven C–C bond formation: oxidative dearomatization of 2-naphthols with construction of a chiral quaternary stereocenter. J. Am. Chem. Soc. 134, 20017–20020 (2012).
Oguma, T. & Katsuki, T. Iron-catalysed asymmetric tandem spiro-cyclization using dioxygen in air as the hydrogen acceptor. Chem. Commun. 50, 5053–5056 (2014).
Zhuo, C.-X. & You, S.-L. Palladium-catalyzed intermolecular asymmetric allylic dearomatization reaction of naphthol derivatives. Angew. Chem. Int. Ed. 52, 10056–10059 (2013).
Zheng, J., Wang, S.-B., Zheng, C. & You, S.-L. Asymmetric dearomatization of naphthols via a Rh-catalyzed C(sp2)–H functionalization/annulation reaction. J. Am. Chem. Soc. 137, 4880–4883 (2015).
Yang, D. et al. Intermolecular enantioselective dearomatization reaction of β-naphthol using meso-aziridine: a bifunctional in situ generated magnesium catalyst. Angew. Chem. Int. Ed. 54, 2185–2189 (2015).
Yang, D. et al. Application of a C–C bond-forming conjugate addition reaction in asymmetric dearomatization of β-naphthols. Angew. Chem. Int. Ed. 54, 9523–9527 (2015).
Wang, S.-G. et al. Asymmetric dearomatization of β-naphthols through a bifunctional-thiourea-catalyzed Michael reaction. Angew. Chem. Int. Ed. 54, 14929–14932 (2015).
Yin, Q. et al. Organocatalytic asymmetric chlorinative dearomatization of naphthols. Chem. Sci. 6, 4179–4183 (2015).
Cheng, Q., Wang, Y. & You, S.-L. Chemo-, diastereo-, and enantioselective iridium-catalyzed allylic intramolecular dearomatization reaction of naphthol derivatives. Angew. Chem. Int. Ed. 55, 3496–3499 (2016).
Wang, L. et al. MgII-mediated catalytic asymmetric dearomatization (CADA) reaction of β-naphthols with dialkyl acetylenedicarboxylates. Chem. Eur. J. 22, 8483–8487 (2016).
Zhu, G. et al. Chiral phosphoric acid catalyzed asymmetric oxidative dearomatization of naphthols with quinones. Org. Lett. 18, 5288–5291 (2016).
Tu, H.-F., Zheng, C., Xu, R.-Q., Liu, X.-J. & You, S.-L. Iridium-catalyzed intermolecular asymmetric dearomatization of β-naphthols with allyl alcohols or allyl ethers. Angew. Chem. Int. Ed. 56, 3237–3241 (2017).
Shen, D., Chen, Q., Yan, P., Zeng, X. & Zhong, G. Enantioselective dearomatization of naphthol derivatives with allylic alcohols by cooperative iridium and Brønsted acid catalysis. Angew. Chem. Int. Ed. 56, 3242–3246 (2017).
Zhang, Y. et al. Catalytic asymmetric hydroxylative dearomatization of 2-naphthols: synthesis of lacinilene derivatives. Chem. Sci. 8, 6645–6649 (2017).
Ge, S. et al. Chiral N,N’-dioxide/Sc(OTf)3 complex-catalyzed asymmetric dearomatization of β-naphthols. Chem. Commun. 53, 11759–11762 (2017).
Li, X.-Q. et al. Asymmetric arylative dearomatization of β-naphthols catalyzed by a chiral phosphoric acid. Chem. Eur. J. 23, 5381–5385 (2017).
An, J. et al. Gold-catalyzed dearomatization of 2-naphthols with alkynes. Chem. Eur. J. 23, 17473–17477 (2017).
Liu, X. et al. Construction of vicinal all-carbon quaternary stereocenters enabled by a catalytic asymmetric dearomatization reaction of β-naphthols with 3-bromooxindoles. ACS Catal. 8, 10888–10894 (2018).
Wang, P. et al. Asymmetric dearomative halogenation of β-naphthols: the axial chirality transfer reaction. Adv. Synth. Catal. 360, 401–405 (2018).
Wang, Y.-F. et al. Asymmetric brominative dearomatization of naphthols catalyzed by chiral copper complexes. Adv. Synth. Catal. 360, 2285–2290 (2018).
Wang, L. et al. Diversiform reactivity of naphthols in asymmetric dearomatization or O-alkylation reactions with aziridines. Adv. Synth. Catal. 360, 4491–4496 (2018).
Han, L., Wang, H. & Luan, X. Pd(II)-Catalyzed [3+2] spiroannulation of α-aryl-β-naphthols with alkynes via a C–H activation/dearomatization approach. Org. Chem. Front 5, 2453–2457 (2018).
Wang, J.-J., Yang, H., Gou, B.-B., Zhou, L. & Chen, J. Enantioselective organocatalytic sulfenylation of β-naphthols. J. Org. Chem. 83, 4730–4738 (2018).
Yang, L. et al. Palladium-catalyzed dynamic kinetic asymmetric transformation of racemic biaryls: axial-to-central chirality transfer. J. Am. Chem. Soc. 137, 4876–4879 (2015).
Zhu, G. et al. Catalytic kinetic resolution of spiro-epoxyoxindoles with 1-naphthols: switchable asymmetric tandem dearomatization/oxa-Michael reaction and Friedel–Crafts alkylation of 1-naphthols at the C4 position. ACS Catal. 8, 1810–1816 (2018).
Rousseaux, S., García-Fortanet, J., Del Aguila Sanchez, M. A. & Buchwald, S. L. Palladium(0)-catalyzed arylative dearomatization of phenols. J. Am. Chem. Soc. 133, 9282–9285 (2011).
Bai, L. et al. Palladium(0)-catalyzed intermolecular carbocyclization of (1,n)-diynes and bromophenols: an efficient route to tricyclic scaffolds. Angew. Chem. Int. Ed. 55, 6946–6950 (2016).
Zhao, G., Xu, G., Qian, C. & Tang, W. Efficient enantioselective syntheses of (+)-dalesconol A and B. J. Am. Chem. Soc. 139, 3360–3363 (2017).
Yang, B. et al. Organocatalyzed intermolecular asymmetric allylic dearomatization of both α- and β-naphthols. Org. Lett. 21, 330–334 (2019).
Xu, R.-Q., Gu, Q. & You, S.-L. Construction of the benzomesembrine skeleton: palladium(0)-catalyzed intermolecular arylative dearomatization of α-naphthols and subsequent aza-Michael reaction. Angew. Chem. Int. Ed. 56, 7252–7256 (2017).
List, B. Direct catalytic asymmetric α-amination of aldehydes. J. Am. Chem. Soc. 124, 5656–5657 (2002).
Brandes, S., Bella, M., Kjærsgaard, A. & Jørgensen, K. A. Chirally aminated 2-naphthols–organocatalytic synthesis of non-biaryl atropisomers by asymmetric Friedel–Crafts amination. Angew. Chem. Int. Ed. 45, 1147–1151 (2006).
Matsubara, R. & Kobayashi, S. Catalytic asymmetric amination of enecarbamates. Angew. Chem. Int. Ed. 45, 7993–7995 (2006).
Zhang, Z. & Antilla, J. C. Enantioselective construction of pyrroloindolines catalyzed by chiral phosphoric acids: total synthesis of (−)-debromoflustramine B. Angew. Chem. Int. Ed. 51, 11778–11782 (2012).
Wang, S.-G., Yin, Q., Zhuo, C.-X. & You, S.-L. Asymmetric dearomatization of β-naphthols through an amination reaction catalyzed by a chiral phosphoric acid. Angew. Chem. Int. Ed. 54, 647–650 (2015).
Nan, J. et al. Direct asymmetric dearomatization of 2-naphthols by scandium-catalyzed electrophilic amination. Angew. Chem. Int. Ed. 54, 2356–2360 (2015).
Lian, X., Lin, L., Wang, G., Liu, X. & Feng, X. Chiral N,N’-dioxide-scandium(III)-catalyzed asymmetric dearomatization of 2-naphthols through an amination reaction. Chem. Eur. J. 21, 17453–17458 (2015).
Akiyama, T. Stronger Brønsted Acids. Chem. Rev. 107, 5744–5758 (2007).
Terada, M. Binaphthol-derived phosphoric acid as a versatile catalyst for enantioselective carbon–carbon bond forming reactions. Chem. Commun. 4097-4112 (2008).
Yu, J., Shi, F. & Gong, L.-Z. Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles. Acc. Chem. Res. 44, 1156–1171 (2011).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Acknowledgements
We thank Ministry of Science and Technology of China (2016YFA0202900), National Natural Science Foundation of China (21772219, 21821002), Science and Technology Commission of Shanghai Municipality (16XD1404300, 18QA1404900, 16490712200), Chinese Academy of Sciences (XDB20000000, QYZDYSSWSLH012) and the Youth Innovation Promotion Association (2017302) of CAS for generous financial support.
Author information
Authors and Affiliations
Contributions
Z.-L.X. and R.-Q.X. initially discovered the title reaction. Z.-L.X. conducted the reaction development. Z.-L.X. and C.Z. performed the mechanistic studies. Z.-L.X., C.Z. and S.-L.Y. drafted and revised the manuscript. S.-L.Y. directed the whole project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Communications thanks Xiaoming Feng and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xia, ZL., Zheng, C., Xu, RQ. et al. Chiral phosphoric acid catalyzed aminative dearomatization of α-naphthols/Michael addition sequence. Nat Commun 10, 3150 (2019). https://doi.org/10.1038/s41467-019-11109-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-11109-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.